?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
It is difficult to develop instant coconut sugar and honey powders. Agglomeration could be used to increase the instant properties (physical and functional properties). The objective of this study was to determine the physical and functional properties of coconut sugar powder and honey powder agglomerated with rewetting method using polyvinylpyrrolidone (PVP). PVP concentrations used are 1% and 5% compared to nonagglomerated powder as a control. Agglomeration process using PVP decreased the hygroscopic rate, accelerated dissolving time and wettability, decreased bulk, and tapped densities, which led to improvements in flowability and cohesiveness of powder. Color analysis showed an increase in color intensity for brightness (L*) and redness (a*), but a decrease in the yellowness (b*). The use of 5% PVP resulted in better physical and functional properties of powder than 1% PVP, except for the hygroscopic rate, bulk and tapped densities.
Introduction
Coconut sap and honey are food in fluid form with high sugar content. The main sugar content of coconut sap is sucrose (12.30–17.40%)[Citation1] and the anhydrous sucrose has a glass transition temperature (Tg) of 62°C.[Citation2] Unlike coconut sap, the main sugar content in honey is fructose (38–40%) and glucose (33–35%) with glass transition temperature of anhydrous fructose and glucose (Tg) of 5oC and 31oC, respectively.[Citation3]
The processing of coconut sap and honey into powder form can increase the practicality in serving because it becomes easier to pack and carry, extend shelf life due the low water content, increase economic value, simplify the packaging and distribution process, and make the taste and flavor more distinctive.[Citation4] The process of making coconut sugar powder and honey powder could be done with several drying methods, one of them is vacuum drying method.
Vacuum drying is a process in which materials are dried in a reduced pressure environment or vacuum. According to the ideal gas law, at lower absolute pressure (vacuum condition), water can evaporate in a lower temperature than at normal conditions (1 atm pressure).[Citation5] Vacuum oven is a tool used for vacuum drying. According to V. Kutovoy, L. Nikolaichuk, and V. Slyesov,[Citation6] the results of vacuum drying method have a better texture, taste, and nutritional content because the material is not damaged by the use of high temperatures. This drying method is suitable for materials that have sensitivity with high temperature because it can reduce the level of damage to the appearance and nutritional content of food ingredients.[Citation7]
Drying process of materials with high sugar content material such as coconut sap and honey have problems with stickiness and unstable obtained amorphous products. This occurs because materials with high sugar content have low glass transition temperatures. Glass transition temperature (Tg) can be increased, by adding coatings or filler materials that have high stability and glass transition temperature, such as maltodextrin.
Maltodextrin can reduce stickiness in powder with high sugar content by increasing the glass transition temperature (Tg) of solids.[Citation8] However, the resulting product had high hygroscopicity and been difficult to dissolve due to lump formation. High hygroscopicity is caused by the amorphous structure, which dominates the molecular structure of the product after vacuum drying.[Citation9] It is necessary to modify the surface of the coconut sugar powder and honey powder by forming granules through the agglomeration process to reduce hygroscopicity so the formation of lump when powder is dissolved in water can be avoided.
Agglomeration is a process to enlarge particle size of materials. Agglomeration is usually used to improve powder properties that are associated with handling and reconstitution such as wettability, dispersibility, and solubility.[Citation10] The agglomeration process can be done by spraying a binding solution into the particles until they get wet and collide with other particles to form aggregates.[Citation11] This type of agglomeration is called as rewetting agglomeration and most agglomeration technique used.[Citation12]
Polyvinylpyrrolidone (PVP) is a binding material that can maintain the compactness and durability of granules. PVP is easily soluble in water, ethanol, and chloroform.[Citation13] The concentration of PVP as a binder is 0.5–5%.[Citation14] PVP is usually added in tablet as disintegrant agent to make the tablet easily dispersed in water.[Citation14] The use of PVP concentrations that are too low will produce weak-link granules, whereas the use of PVP concentrations that are too high will produce hard granules. Therefore, a research that varies the concentration of PVP from lowest to highest needs to be carried out to find the right concentration. The purpose of this study was to determine the physical and functional properties of coconut sugar powder and honey powder agglomerated with rewetting method using polyvinylpyrrolidone (PVP).
Materials and methods
Materials
Coconut sap was obtained from Pangandaran, Jawa Barat, Indonesia. Honey (Apis dorsata) was obtained from a forest in Riau, Indonesia. The properties of both raw materials were presented as below (). Further ingredients were Polyvinylpyrrolidone or PVP K-30 was obtained from JH Nanhang Life Science Co., Ltd, distilled water, ethanol 96%, maltodextrin with dextrose equivalent (DE) of 18–20 was obtained from Qin Huang Dao Lihua Starch Co., Ltd., China, and silica gel.
Table 1. Properties of coconut sap and honey (Apis dorsata)
Sample preparation
Coconut sugar dan honey powder was produced using Vacuum Drying Method. Coconut sap and honey were filtered and then tested for total dissolved solids (°Brix) using Atago Pal-alpha digital pocket refractometer (Atago Co., Ltd., Japan). Total dissolved solids of coconut sap and honey were used as a reference for calculating the amount of maltodextrin needed. Maltodextrin was added to the coconut sap and honey solution with a ratio of maltodextrin: total solid of honey and coconut solution is 1:1 (w:w; solid/solid). Maltodextrin was weighed according to a predetermined weight and then dissolved in distilled water using magnetic stirrer on a Thermo Scientific CIMAREC stirring hot plates (Thermo Fisher Scientific Inc, Massachusetts, US) at 40°C. Dissolution was carried out until maltodextrin was completely dissolved, marked by the solution becoming clear. Then the coconut sap or honey was then mixed with a solution of maltodextrin, where maltodextrin functioned as a coating material, with a ratio of maltodextrin and honey or coconut sap solid of 1:1 (w:w), in a beaker glass and dissolved using a magnetic stirrer on a hot plate stirrer at 40°C for 15 minutes. The solutions of either coconut sap (20% total solid) or honey (40% total solid) were put into a silicone container with a thickness of ± 3 mm and then dried at a temperature of 70°C and a vacuum pressure of 25 inHg (Pabsolute = 4,921 inHg) for 6–7 hours using B-One VOV-50 vacuum drying oven (China). Drying was done to eliminate the water content in the samples to the maximum extent so that dry samples in powder form were produced. Size reduction process was carried out on dry samples using a grinder, then sieved with an 80 mesh sieve.
Rewetting agglomeration
Rewetting agglomeration was carried out to enlarge the size of coconut sugar and honey powder using Polyvinylpyrrolidone or PVP as a binder. PVP solution was prepared by dissolving PVP in ethanol 96% with concentration of PVP 1% and 5%. The PVP solution was then filled into a spray bottle and then sprayed into the coconut sugar and honey powder with a ratio of coconut sugar or honey powder to PVP solution 2:1 (w:w). Spraying was done evenly while stirring was continued until the entire surface of coconut sugar and honey powder became wet. The Samples were put into a silicone container with a thickness of ± 3 mm, then dried at 60°C with a pressure of 5 inHg for 2–3 hours using B-One VOV-50 vacuum drying oven (China). Drying was done to remove ethanol 96% solution, which was previously used as a solvent for PVP. The size of the agglomerated coconut sugar powder and honey powder were reduced using a grinder and then packed using plastic. The powders were stored at ±23°C in a desiccator contains silica gels for further analysis.
Measurement of hygroscopic rate
Hygroscopic rate was determined according to the method described by Nurhadi, Andoyo, Mahani, & Indiarto.[Citation5] An amount of 0.5 g of the powder sample was put into a desiccator with RH of 70%. The increase in weight was checked every 60 minutes for 240 minutes. The slope obtained from the relationship between water content (db) and time was calculated.
Measurement of dissolving time
Dissolving time was determined according to the method described by GEA Niro Research Laboratory (2005).[Citation15] Beaker glass containing 50 mL of distilled water was placed on the stirrer. An amount of 1 g of the powder sample was dissolved into the distilled water using a stirrer at speed dial position 4 to create a homogeneous solution. The time taken for the sample to dissolve completely in water was recorded.
Measurement of bulk and tapped densities
Bulk and tapped densities were determined according to the method described by Szulc & Lenart.[Citation16] An amount of 25 g of the powder sample was inserted slowly into a measuring cylinder. Bulk density is the ratio between the weight of the sample and the initial volume of the sample shown on the measuring cylinder. Samples were tapped 100 times using a volume pressure OMRON H3BA-N solid state timer (OMRON corporation, Japan). Tapped density is the ratio between the weight of the sample and the final volume of the sample shown on the measuring cylinder after 100 taps. Bulk density is expressed in ρL and tapped density is expressed in ρT.
Measurement of flowability and cohesiveness
Flowability and cohesiveness were determined according to the method described by Jinapong et al.[Citation10] Classification of powder flowability was determined based on Carr index (CI).
Classification of powder cohesiveness was determined based on Hausner ratio (HR).
Measurement of wettability
Wettability was determined according to the method described by Atomizer.[Citation17] One hundred milliliters of distilled water was poured into a 250 mL beaker glass. A glass funnel was placed over the beaker glass. A pestle was placed inside the funnel so that it blocked the lower opening. An amount of 0.5 g of the powder sample was placed around the pestle. As the pestle was lifted, the time was counted using a stopwatch. The final time was the time required for all powder particles to get wet and penetrate the surface of the water.
Polarized light microscopy analysis
Light microscopy with polarized light was done to all samples using Olympus BX51 (Olympus Corporation, Tokyo, Japan). The image was captured and processed with Jenoptik C14 Imagic camera.[Citation18]
Color analysis
Color analysis was carried out using Konica Minolta Chromameter CR-400 (Konica Minolta, Inc, Japan). Color of the powders was determined using CIELab system (L*a*b) described by Yam & Papadakis.[Citation19] L* value determines lightness or darkness, a* determine redness or greenness, while b* determines yellowness or blueness.
Results and discussion
Hygroscopic rate
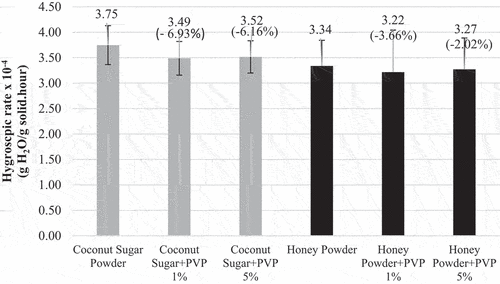
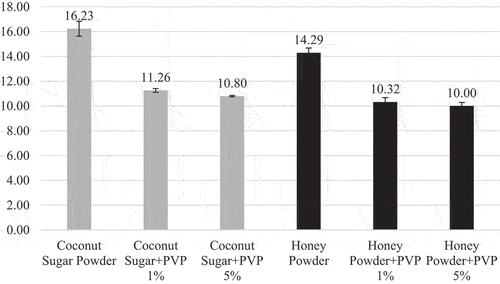
Hygroscopic rate is commonly used to explain the interaction of a material with water.[Citation20] Hygroscopic rate is influenced by the rate and amount of water absorbed by the powder from the air.[Citation21] The hygroscopic rate of coconut sugar powder and honey powder are shown in . Coconut sugar agglomerate and honey agglomerate had a lower hygroscopic rate than non-agglomerated powder. This is due to initial water content of the powder. Initial water content of coconut sugar powder is 2.91% (wb), coconut sugar agglomerate PVP 1% is 4.19% (wb), coconut sugar agglomerate PVP 5% is 4.06% (wb), honey powder is 2.61% (wb), honey agglomerate PVP 1% is 3.57% (wb), and agglomerate PVP 5% is 3.46% (wb). Powder with low initial water content has a high water absorption rate so it is more hygroscopic. Nonagglomerated powder has low water content caused by high and long drying processed (70°C, 6–7 hours). The high drying rate resulted in powder with higher amorphous structure.[Citation22,Citation23] According to Nurhadi et al.[Citation24] products with amorphous structure are more hygroscopic compared to products with crystalline structure. Agglomerated powder has higher initial water content caused by rewetting process using 96% ethanol which still contains 4% water. Nevertheless, the second drying in agglomeration process was done using lower temperature (60°C).
As given in agglomeration process decreased 6.16–6.93% hygroscopic rate of coconut sugar powder, whereas in the honey powder, agglomeration process decreased 2.02–3.66% hygroscopic rate. This might due to the main component of coconut sugar and honey, as well as the structure of the resulting powder particles. Coconut sap contains 18–20% sucrose.[Citation25] Sucrose is a non-reducing sugar that can be formed into a crystalline structure during drying process. The sucrose in the agglomerated coconut sugar powder forms a crystalline structure partially (). Crystalline structure has a denser (compact) bond and not hygroscopic.[Citation26,Citation27] Honey contains reducing sugar, which is 28–35% glucose and 32–41% fructose.[Citation25] According to a study conducted by Indahyanti, Kamulyan, and Ismuyanto[Citation28] reducing sugar content forms an amorphous structure that is easier to absorb water.
Figure 2. Polarized light microscopy of (a) coconut sugar powder PVP 1% agglomerates and(b) coconut sugar powder PVP 5% agglomerates.
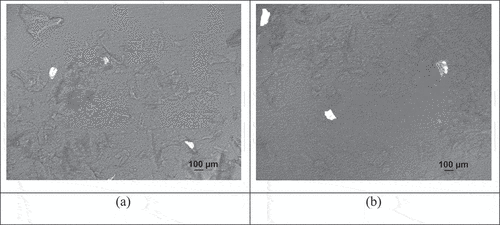
Based on , non-agglomerated coconut sugar powder, non-agglomerated honey powder, agglomerated honey powder PVP 1%, and agglomerated honey powder PVP 5% are composed of amorphous structure. However, agglomerated coconut sugar powder PVP 1% and agglomerated coconut sugar powder PVP 5% are composed of amorphous and crystalline structures partially. Crystalline structures of agglomerated coconut sugar powder PVP 1% and 5% were formed due to the addition of 96% ethanol which can dissolve sucrose in coconut sugar powder until it is concentrated to form a saturated sucrose solution, which then forms a crystalline structure. Sucrose can form crystalline structure.[Citation29]
An increase in the concentration of PVP binder increased the hygroscopic rate of agglomerated coconut sugar powder and honey powder although the increase is not significant (). This occurred due to PVP characteristic, which is soluble in water.[Citation14] Increasing the concentration of PVP added causes more amount of PVP to be concentrated by air so that the formation of sucrose through saturation becomes less. Coconut sugar powder and honey powder agglomerated with 1% and 5% PVP binder have hygroscopic rate values that are not significantly different because the water content that can dissolve PVP binder is only 4%.
Dissolving time
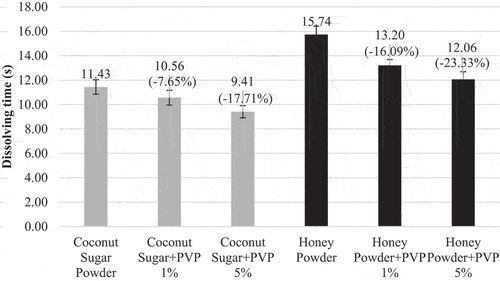
shows the dissolving time of coconut sugar powder and honey powder. Agglomerated coconut sugar powder and honey powder have shorter dissolving time than nonagglomerated powder. Same results were found with agglomerated whole milk/sugar mixture powders.[Citation30] The particles of either coconut sugar powder or honey powder particles of either was joined one another, bounded by PVP, than produced much larger particle size when the agglomeration process occurred. The larger particle size accelerated the powder material to sink into the water so they can dissolve rapidly and not clot on the surface of water.
From , dissolving time of coconut sugar powder and honey powder decreases with the increase of PVP concentration on agglomeration process. This result comply with Jinapong et al.[Citation10] that stated the agglomerate become easily dispose in the line the amount of binder added. Moreover, higher PVP concentration forms stronger agglomerates particle bonds and lower porosity. The agglomerates powder with lower porosity result in shorter dissolving time.[Citation31] Coconut sugar powder has shorter dissolving time than honey powder. This occurred because the agglomeration process makes some part of sucrose in the coconut sugar powder formed a crystalline structure. Crystalline structure has a dense formation so that the porosity values is low. According to Lee & Yoo[Citation31] the dissolving time of agglomerates depends on its porosity, agglomerates with lower porosity produce shorter dissolving time. Honey powder formed amorphous particle completely which made it easier to clump when dissolved in water so that the dissolving time of honey powder is longer than coconut sugar powder.
Bulk and tapped densities
As shown on , the bulk and tapped densities of the nonagglomerated coconut sugar powder and honey powder were higher than those of the agglomerated powder. This is due to the formation of a porous structure during the agglomeration process, which involves occluded air. The results are in accordance to the findings of Samborska, Sokołowska, & Szulc,[Citation32]where nonagglomerated honey powder has a higher bulk and tapped densities than agglomerated honey powder. This can be interpreted mainly as the effect of size enlargement. In general, the larger the particle size is, the lower density will be.[Citation30] shows that the more amount of PVP binder used increase the bulk and tapped densities, although the increase is not significant. This results are in accordance with the research that is carried out by Szulc & Lenart[Citation16] on agglomeration process of dairy powder, which indicates that the more high concentration of binder used, the more high- value bulk and tapped densities of the agglomerates were produced.
Table 2. The glass transition temperatures (Tg) of amorphous samples
Flowability and cohesiveness
Flowability is known as relative movement of bulk particles to other particles in the vicinity along the surface of container wall.[Citation33] Cohesiveness is defined as the attraction between larger particles when compared to the weight of the particle itself. One of the standard methods for evaluating flowability and cohesiveness of a particulate system is to calculate Carr Index (CI)[Citation34] and Hausner Ratio (HR).[Citation35] As given in , vacuum dried coconut sugar powder indicates a bad flowability. This is due to the fact that coconut sugar powder contains some sticky substance, which is hygroscopic and it absorbs water from surrounding atmosphere easily. Agglomeration process using PVP binder improved coconut sugar powder from bad (35–45) to fair (20–35) flowability. During agglomeration process, the fine particles such as coconut sugar powders join or bind with one another, resulting in an agglomerates much larger in size than the original material.[Citation36] The larger particle size of a powder, the better the flowability.[Citation37] Same results were found in wheat flour,[Citation38] honey powder,[Citation32] and baby foods powder.[Citation39] The study showed that agglomeration process improves food powder properties such as flowability.
Table 3. Bulk and tapped densities analysis of coconut sugar and honey powder
Wettability
The effect of agglomeration process using PVP as a binder on coconut sugar powder and honey powder are shown in . It can be seen that agglomeration process increase the wettability of coconut sugar powder and honey powder. Increased wettability is shown by the shorter time needed for the entire surface of coconut sugar powder and honey powder to be penetrated by the water. Non- agglomerated coconut sugar powder and nonagglomerated honey powder are difficult to be penetrated by the water, it forms lumps on the surface because it has a high cohesiveness. High cohesiveness will cause powder particles to stick and form a solid bridge into the mound on the surface of the water.[Citation40] Based on wettability of coconut sugar powder and honey powder with higher PVP binder concentration is better than lower PVP binder concentration. That might be due to lower cohesiveness of agglomerates with higher PVP binder concentration.
Table 4. Flowability and cohesiveness characteristics (Carr index, CI, and Hausner ratio, HR) of coconut sugar powder and honey powder
Table 5. Wettability of coconut sugar powder and honey powder
Color intensity
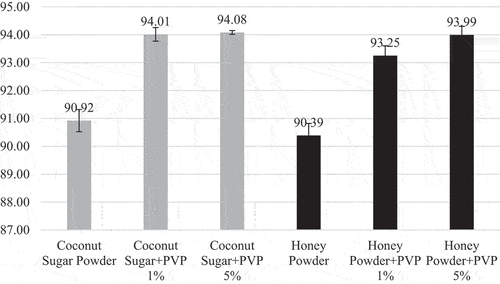
Color intensity evaluation was carried out to find out the effect of agglomeration using PVP as a binder to the color intensity of coconut sugar powder and honey powder. The color of agglomerated and nonagglomerated powder seemed not different visually (). Degree of lightness to darkness stated by L* value that ranges from 0 (black) to 100 (white). As shown on , agglomerated coconut sugar powder and honey powder using PVP as a binder shows a higher brightness than nonagglomerated powder. Furthermore, higher PVP concentration also contribute to higher L* value. The brighter color was obtained due to the addition of polyvinylpyrrolidone which has white color.[Citation14]
Figure 4. Color intensity of (a) coconut sugar powder vacuum drying, (b) coconut sugar powder PVP 1% agglomerates, (c) coconut sugar powder PVP 5% agglomerates, (d) honey powder vacuum drying, (e) honey powder PVP 1% agglomerates, (f) honey powder PVP 5% agglomerates.

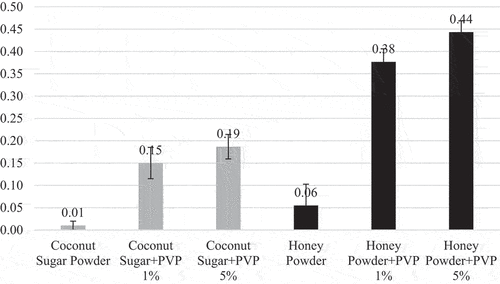
Degree of redness to greenness (a* value) and degree of yellowness to blueness (b* value) is presented in . Agglomeration process increased redness (+a*) but decreased yellowness (+b*) of coconut sugar powder and honey powder. Additionally, Increasing PVP will increase redness but decrease yellowness. However, a* values in all analyzed powders are close to zero, which means white color. The color change was due to double heating on rewetting agglomeration process that can cause Maillard reaction, which happens between reducing sugars and amino acids in food which can produce brown redness and yellowness pigments.[Citation41]
Conclusion
Agglomeration process using PVP as a binder on coconut sugar powder and honey powder decreased the hygroscopicity rate, accelerated dissolving time and wettability, decreased bulk, and tapped densities, which led to improvements in flowability and cohesiveness of powder, increased color intensity for brightness (L *) and redness value (a *), but decreases the yellowness value (b *). The use of 5% PVP resulted in better physical and functional properties of powder than 1% PVP, except for the hygroscopicity rate, bulk, and tapped densities. This study offers a better understanding of agglomeration effect on coconut sugar powder and honey powder using PVP as a binder. Such information can be used as an alternative process to produce coconut sugar powder and honey powder with desired properties.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Santoso, H. B. Pembuatan Gula Kelapa; Penerbit Kaninus: Yogjakarta, 1993.
- Roos, Y. H.; Drusch, S. Phase Transitions in Foods, Second. Oxford: Academic Press. 2015.
- Umesh Hebbar, H.; Rastogi, N. K.; Subramanian, R. Properties of Dried and Intermediate Moisture Honey Products: A Review. Int. J. Food Prop. 2008, 11, 804–819. DOI: 10.1080/10942910701624736.
- Crysse, Z.; Widyastuti, E.; Hadi, S. W. PEMBUATAN GULA SEMUT KELAPA (KAJIAN pH GULA KELAPA DAN KONSENTRASI NATRIUM BIKARBONAT) Making Coconut Palm Sugar. J. Pangan dan Agroindustri. 4(1), 109–119 . 2016.
- Nurhadi, B.; Mahani, R. A.; Indiarto, R. Study the Properties of Honey Powder Produced from Spray Drying and Vacuum Drying Method. Int. Food Res. J. 2012, 19(3), 907–912.
- Kutovoy, V.; Nikolaichuk, L.; Slyesov, V. The Theory Of Vacuum Drying. Int. Dry. Symp. 2004, A, p. 26627 .
- Orikasa, T.; Koide, S.; Okamoto, S.; Imaizumi, T.; Muramatsu, Y.; Takeda, J.-I.; Shiina, T.; Tagawa, A. Impacts of Hot Air and Vacuum Drying on the Quality Attributes of Kiwifruit Slices. J. Food Eng. 2014, 125, 51–58. DOI: 10.1016/j.jfoodeng.2013.10.027.
- Shi, Q.; Fang, Z.; Bhandari, B. Effect of Addition of Whey Protein Isolate on Spray-Drying Behavior of Honey with Maltodextrin as a Carrier Material. Dry. Technol. 2013, 31, 1681–1692. DOI: 10.1080/07373937.2013.783593.
- Nurhadi, B.; Nurhasanah, S. Sifat Fisik Bahan Pangan; Widya Padjadjaran: Bandung, 2010.
- Jinapong, N.; Suphantharika, M.; Jamnong, P. Production of Instant Soymilk Powders by Ultrafiltration, Spray Drying and Fluidized Bed Agglomeration. J. Food Eng. 2008, 84(2), 194–205. DOI: 10.1016/j.jfoodeng.2007.04.032.
- Tan, H. S.; Salman, A. D.; Hounslow, M. J. Kinetics of Fluidized Bed Melt granulation-II: Modelling the Net Rate of Growth. Chem. Eng. Sci. 2006, 61, 3930–3941. DOI: 10.1016/j.ces.2006.01.005.
- Barbosa-Canovas, G. V.; Ortega-Rivas, E.; Juliano, P.; Yan, H. Food Powders: Physical Properties, Processing, and Functionality. 2005, 180–181.
- Lachman, L.; Lieberman, H. A.; Kaning, J. L. Teori Dan Praktek Farmasi Industri Edisi III. 2008.
- Rowe, R. C.; Sheskey, P. J.; Fenton, M. E. Handbook of Pharmaceutical Excipients: Pharmaceutical Excipients. 2012, 611.
- Budeanu, A.;; GEA Niro Research Laboratory. A 6 A -powder Dispersibility IDF Method GEA Niro Method No. A 6 A. J. Clean. Prod. 2005, 13, 89–97. DOI: 10.1016/j.jclepro.2003.12.024.
- Szulc, K.; Lenart, A. Surface Modification of Dairy Powders: Effects of Fluid-bed Agglomeration and Coating. Int. Dairy J. 2013, 33(1), 55–61. DOI: 10.1016/j.idairyj.2013.05.021.
- Atomizer, A. N. No Title; De Forenede Trykkerier A/S: Copenhagen, 1978.
- Maidannyk, V.; McSweeney, D. J.; Hogan, S. A.; Miao, S.; Montgomery, S.; Auty, M. A. E.; McCarthy, N. A. Water Sorption and Hydration in Spray-dried Milk Protein Powders: Selected Physicochemical Properties. Food Chem. 2020, 304, 125418. DOI: 10.1016/j.foodchem.2019.125418.
- Yam, K. L.; Papadakis, S. E. A Simple Digital Imaging Method for Measuring and Analyzing Color of Food Surfaces. J. Food Eng. 2004, 61, 137–142. DOI: 10.1016/S0260-8774(03)00195-X.
- Newman, A. W.; Reutzel‐Edens, S. M.; Zografi, G. Characterization of the “Hygroscopic” Properties of Active Pharmaceutical Ingredients. J. Pharm. Sci. 2008, 97(3), 1047–1059. DOI: 10.1002/jps.21033.
- Murikipudi, V.; Gupta, P.; Sihorkar, V. Efficient Throughput Method for Hygroscopicity Classification of Active and Inactive Pharmaceutical Ingredients by Water Vapor Sorption Analysis. Pharm. Dev. Technol. 2013, 18, 348–358. Doi:10.3109/10837450.2011.618947.
- Starzak, M.; Mathlouthi, M. Formation of Amorphous Sugar in the Syrup Film - a Key Factor in Modelling of Industrial Sugar Drying. Food Chem. 2010, 122, 394–409. DOI: 10.1016/j.foodchem.2009.07.033.
- Nurhadi, B.; Sukri, N.; Sugandi, W. K.; Widanti, A. P.; Restiani, R.; Noflianrini, Z.; Rezaharsamto, B.; Herudiyanto, M. Comparison of Crystallized Coconut Sugar Produced by Traditional Method and Amorphous Coconut Sugar Formed by Two Drying Methods: Vacuum Drying and Spray Drying. Int. J. Food Prop. 2018, 21(1), 2339–2354.
- Nurhadi, B.; Roos, Y. H. Influence of Anti-caking Agent on the Water Sorption Isotherm and Flowability Properties of Vacuum Dried Honey Powder. J. Food Eng. 2017, 210, 76–82. DOI: 10.1016/j.jfoodeng.2017.04.020.
- DeMan, J. M. Principles of Food Chemistry, Third Edit; Aspen Publishers, Inc: Gaithersburg, Maryland, 1999.
- Bhesh, B.; Nidhi, B.; Min, Z.; Pierre, S. Spray Drying for Food Powder Production; Cambridge, UK: Woodhead Publishing Limited, 2013.
- Indahyanti, E.; Kamulyan, B.; Ismuyanto, B. Optimasi Konsentrasi Garam Bisulfit Pada Pengendalian Kualitas Nira Kelapa. J. Penelit. Saintek. 2014, 19(1), 1–8.
- Indahyanti, E.; Kamulyan, B.; Ismuyanto, B. Optimasi Konsentrasi Garam Bisulfit Pada Pengendalian Kualitas Nira Kelapa. J. Penelit. Saintek. 2014.
- Potter, N.; Hotchkiss, J. Food Science, Fifth; New York, USA: Chapman and Hall, 1995; Vol. 147 no. 5.
- Chever, S., Mejean S, Dolivet A, Mei F, Den Boer CM, Le Barzic G, Jeantet R, Schuck P. Agglomeration during Spray Drying: Physical and Rehydration Properties of Whole Milk/sugar Mixture Powders. LWT - Food Sci. Technol. 2017, 83, 33–41. DOI: 10.1016/j.lwt.2017.05.002.
- Lee, H.; Yoo, B. Agglomerated Xanthan Gum Powder Used as a Food Thickener: Effect of Sugar Binders on Physical, Microstructural, and Rheological Properties. Powder Technol. 2020, 362, 301–306. DOI: 10.1016/j.powtec.2019.11.124.
- Samborska, K.; Sokołowska, P.; Szulc, K. Diafiltration and Agglomeration as Methods to Improve the Properties of Honey Powder Obtained by Spray Drying. Innov. Food Sci. Emerg. Technol. 2017, 39, 33–41. DOI: 10.1016/j.ifset.2016.10.002.
- Peleg, M. Flowability of Food Powders and Methods for Its Evaluation — A Review. J. Food Process. Eng. 1977, 1, 303–328. DOI: 10.1111/j.1745-4530.1977.tb00188.x.
- Carr, R. L. Classifying Flow Properties of Solids. Chem. Eng. 1965, 72, 69–72.
- Hausner, H. H. Friction Conditions in a Mass of Metal Powder. Int. J. Powder Metall 4 (3): 7–13 . 1967.
- Ortega-Rivas, E. Handling and Processing of Food Powders and Particulates. 2005. p. 92.
- Fitzpatrick, J. Food Powder Flowability. 2005, 247–258.
- Rondet, E.; Cuq, B.; Cassan, D.; Ruiz, T. Agglomeration of Wheat Powders by a Novel Reverse Wet Agglomeration Process. J. Food Eng. 2016, 173, 92–105. DOI: 10.1016/j.jfoodeng.2015.10.046.
- Szulc, K.; Lenart, A. Effect of Agglomeration on Flowability of Baby Food Powders. J. Food Sci. 2010, 75, E276–E284. DOI: 10.1111/j.1750-3841.2010.01634.x.
- Erick. “Pemecahan Masalah Inkonsistensi Proses Penakaran Pada Mesin Pengemas Bumbu Pelezat Serbaguna Di Pt Unilever Indonesia Tbk., Cikarang,” 2010. [Online]. Available: https://repository.ipb.ac.id/jspui/bitstream/123456789/59810/1/F10eri.pdf.
- BeMiller, J. N. Carbohydrate Chemistry for Food Scientists. 2018. p. 25.