 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
The present study compared whole grain teff flours grown in Ethiopia (ETF) and South Africa (STF) for their chemical composition (proximate, mineral, and amino acid) and pasting, thermal, and functional properties. The proximate composition of flours was determined using methods of the European Commission Regulation (152/2009). Atomic Spectrometer, ion-exchange chromatography, Rapid Visco-Analyzer (RVA), and Differential Scanning Calorimetry (DSC) were used to measure minerals, amino acids, pasting, and thermal properties, respectively. The inter-relationships of the measured attributes were determined using Pearson correlation and principal component analysis (PCA). Significant (p < .05) differences in most of the evaluated properties were found between ETF and STF. However, several significant (p < .01) correlations were observed among the measured attributes of flours as revealed by Pearson correlation and PCA. Significantly (p < .05) higher contents of moisture (11.2 g/100 g flour), protein (13.3 g/100 g flour), and zinc (34.6 mg/kg) were found in STF, whereas significantly (p < .05) higher contents of calcium (1306 mg/kg) and iron (363 mg/kg) were perceived in ETF. The ETF was observed to have higher foam stability (71 mL/100 mL initial foam), water absorption capacity (1.1 g/g), oil absorption capacity (0.9 g/g), and swelling power (6.4 g/g) as compared to STF. Observations from thermal and pasting properties revealed that onset, peak and end temperatures, trough, final and set back viscosities, including peak time (6.3 min), and pasting temperature (90 °C) were found to be higher in the case of STF. In conclusion, findings of this study would contribute to the selection of teff for the development of healthier food products to meet the current consumer preferences .
INTRODUCTION
Teff (Eragrostis tef) is an attractive source of gluten-free flour, and it is an indigenous plant in Ethiopia and Eritrea. Recently, it has been growing widely in India, Australia, Canada, United States, and South Africa. Teff is an annual cereal having the smallest grain size[Citation1] with a mass nearly 0.7 g/100 g of wheat grain,[Citation2] which makes difficult to remove its bran .[Citation1] Hence, teff is processed to whole grain flour;[Citation3] as a result, its flour has a high fiber content. Teff is advantageous to improve the hemoglobin level in the human body and supports to prevent the prevalence of anemia and diabetes. Moreover, it is an important cash crop next to coffee and a source of income for local farmers in Ethiopia and makes approximately 500 million USD per year .[Citation4] Ethiopia is one of the largest teff producers in the world to have adopted teff harvests for basic needs. Teff accounts for the first cereal in the area planted, followed by maize and sorghum, whereas in production, it is next to maize .[Citation5] Teff covers approximately 26 % of the annual harvests and 31 % of the cereals .[Citation6]
The hydrolysis results of teff starch (simple sugars) and protein (amino acids) are essential for the production of fermented beverages .[Citation7] Two-third of the daily protein intake in Ethiopia covers as a result of consuming teff products .[Citation6] Teff has well-balanced essential amino acids, including lysine, which is a trace amino acid in most cereals. Regular intake of teff products helps to minimize the risk of anemia because teff is a good source of calcium and iron .[Citation8] In Ethiopia, teff flour is used for the production of a range of traditional foods such as injera, kitta, and tela .[Citation9] Recently, it has become important in the healthy food market and has been used to produce various food items such as cake, cookies, biscuit, sourdough, extruded product, malt for gluten free lactic acid fermented beverage, and bread. The interests in teff utilization are growing because of its good nutritional profile and gluten-free nature. Moreover, there is a great potential to familiarize teff to the other parts of the world for the production of healthy food and beverage products. Therefore, teff has been preferred for the utilization of gluten-free plus nutritious food products .[Citation10]
However, the global utilization and market value of teff are not good enough as compared to other common cereals (wheat, maize, and rice) because of scientific knowledge gap on its performances .[Citation11] The details of teff functional properties and its nutritional values are found scarcely in the literature .[Citation12] Particularly, the recently explored teff variety in Ethiopia called Dagme (DZ-Cr 438) needs intensive research on its quality attributes such as nutritional composition and processing properties. Overall, with this inadequate knowledge and information of teff characteristics, it is difficult to utilize teff for the development of diverse food products .[Citation13] Accordingly, the need to examine the performances of teff flour on its different characteristics could improve its utilization, market value, and consumer preferences. In succession with considering these, the present study aimed to determine and compare the chemical composition (proximate, mineral, and amino acid), color, and pasting, thermal, and functional properties of whole-grain teff flours grown in Ethiopia and South Africa. Information generated from this study would contribute to the selection of teff for the development of suitable products to meet various consumer preferences in elsewhere.
Materials and Methods
Samples
Whole grain teff (Eragrostis tef) (30 kg) was collected from the Debrezeit Agricultural Research Center, Debrezeit, Ethiopia, in December 2019. Its variety name is Dagme (DZ-Cr-438). The grain was milled using attrition disc mill, which is commonly used for teff grinding commercially in Ethiopia. The other whole grain teff flour sample (25 kg) was collected from Teff-shop de, Manuel Boesel, Homburger Str.49a, 61191 Rosbach v. d. height, Germany.
Compositional Examination of ETF and STF
Moisture contents of teff flour samples were examined using an automatic electronic moisture analyzer (KERN MLS-D, KERN & Sohn GmbH, Germany). Crude protein (Kjeldahl, N x 6.25), fat (Soxhlet, Solvent extraction), and fiber (Gerhardt Fibretherm) contents of ETF and STF were determined according to the methods described by the European Commission Regulation (EC) No. 152/2009 EU,[Citation14] while the ash content was determined using AACC method 08‐01.01 .[Citation15] Amino acid and mineral contents of ETF and STF were determined using the methods described by European Commission Regulation (EC) No. 152/2009 EU .[Citation14] Ion-exchange liquid chromatography (Thermo Scientific IC, type Integrion-RFIC, Thermo Fisher Scientific Inc., USA) and Atomic Spectroscopy (ICP-MS NexION 300X, PerkinElmer, Inc., Waltham, USA) were used to analyze amino acids and minerals, respectively. Studies were conducted in duplicate.
Pasting properties of ETF and STF
Pasting properties of ETF and STF were determined according to AACC method 76–21.02[Citation15] using a Rapid Visco-Analyzer (RVA-4 Standalone, Newport Scientific, Australia) via standard method 1. The TCW3 software was used as a control. Teff flour-water suspensions were held at 50 °C for 1 min, then heated for 10 min at 95 °C, and finally cooled to 50 °C for 2 min. The volume of distilled water added and sample weights were calculated based on 14 % moisture content. Pasting profiles of flours were determined using suspensions (8 % w/w, 28 g total weight). The parameters registered during analyses by RVA include peak viscosity, trough viscosity, breakdown viscosity, final viscosity, setback viscosity (mPa s), peak time (min.), and pasting temperature (°C). Triplicate determinations were carried out.
Thermal properties of ETF and STF
Thermal properties of ETF and STF were determined using differential scanning calorimetry (DSC) (DSC 6000, Perkin-Elmer, U.S.A) by the method of A-S Hager, M Czerny, J Bez, E Zannini, and EK Arendt .[Citation16] Approximately 5 to 9 µg of flour sample was weighed into a DSC aluminum pan, and 10 to 16 µL of distilled water was added: water ratio of 1:2. The aluminum pan was hermetically sealed and allowed to stand at least for an hour to reach moisture equilibration. The covered pans were heated from 25 °C to 105 °C under nitrogen gas at a heating rate of 5 °C min−1 to gelatinize the flour samples. An empty aluminum pan was used as a reference, and the calorimeter was calibrated with indium. Onset temperature, peak temperature, end temperature, and enthalpy of gelatinization were determined using Perkin-Elmer Pyris Software. Measurements were made in triplicate.
Functional properties of ETF and STF
Foaming properties, absorption capacities of water and oil, and swelling power were examined by the method of W Klunklin and G Savage .[Citation17] Foaming properties: In 100 mL of graduated cylinder, about 2 g of flour sample was mixed with 50 mL of distilled water at 30 °C. Then, the suspension was shaken vigorously for 5 min to form a foam. The foam volume (mL) after 30 s shaking was expressed as foam capacity. The volume after 1 h was used as an indicator of foam stability and expressed as a percentage of initial foam volume,
where VO is the initial foam volume in mL and V1 is the foam volume in mL after 1 h. Water and oil absorption capacities: About 10 mL of distilled water or corn oil added to 1 g of flour sample in a centrifuge tube. The suspension was stirred using a vortex mixer and then centrifuged (2200xg for 25 min). The separated water and oil were removed with a pipette and weighed. Absorption capacities were expressed as g of water or oil absorbed per g of sample. Swelling power: About 1 g of flour sample mixed with 10 mL of distilled water in a test tube. The suspension was heated in a shaking water bath set at 80 °C for 30 min. Then, tubes were cooled to room temperature and centrifuged (870xg for 15 min). Finally, the paste was weighed to determine the swelling power of the flour. Swelling power was expressed as g of paste per g of sample. Each measurement was analyzed in triplicate.
Particle size distribution and Falling number determination of ETF and STF
Particle size distribution of ETF and STF was determined according to AACC method 55–60.01[Citation15] using a sieve shaker (Test sieve JEL 200 shaker, Retsch GmbH, Germany). Sieves with screen openings of 0.8, 0.5, 0.315, 0.224, 0.16, and 0.1 mm were used. Approximately 100 g of sample flour and 0.2 g of aerosol were mixed well in the mixing vessel. The empty weight of all sieves with the bottom were weighted, and a cube was placed on each sieve. The sieves were placed on top of each other according to the sieve size opening. Then, samples were placed on the top sieve and put on the lid, and the complete arrangement was firmly clamped into the test sieve shaker. After a sieving time of 10 min, individual sieves were weighed, and the weight of the sieve fraction was determined by deducting the empty weight and expressed as a percentage of flour in each particle size range. Measurements were performed in duplicate.
The Falling number was examined according to the AACC method 56‐81.04[Citation15] using a Falling number machine (Falling Number 1400, Perten Instruments AB, Sweden). Based on the 14 % moisture content, 6.70 g of ETF and 6.78 g of STF were mixed separately with 25 mL of distilled water in a Falling number tube. A rubber stopper was used for shaking the sample holder at up and down positions until contents become well mixed. A viscometer stirrer is used to scrape down the slurry coating of the upper part of the tube and scrape all slurry from the stopper. Finally, the tube and viscometer stirrer were placed into a water bath within 30–60 s. The tube was kept in a boiling water bath for 5 s and stirred for 55 s, and then the machine recorded the time for the stirrer to fall from top to bottom of the tube. Falling number reading was the sum of 5 s of standing in boiling water, 55 s of stirring, and time for the stirrer to fall. Results are the mean of four experiments.
Color of ETF and STF
Color of ETF and STF was determined by RGB (Red, Green, and Blue) values. The RGB values were examined using ImageJ 1.52a software. This software was developed in 2018 by the National Institute of Health, USA. Images were taken in a professional photo studio using a Sony FE 2/28 camera. The control was remote software. Duplicate examinations were performed.
Statistical Analysis
All measurements were performed at least in duplicate. The mean and standard deviation of each measured attribute were calculated using Microsoft excel 2016. The t-test was used to calculate to verify significant (p < .05) differences in means. The means ± standard deviations are reported. Principal component analysis and Pearson correlation analyses of the results were performed using Minitab 18.1 statistical software (2017Minitab Inc., State College, PA, USA).
RESULTS AND DISCUSSION
Compositional Examination of ETF and STF
Proximate: The proximate analysis of ETF and STF is presented in . Proximate analysis shows dominant nutrients and is an important criterion to determine nutritional values and quality of food, which helps the development of specific food products .[Citation18] The proximate composition of flours from ETF and STF revealed significant (p < .05) differences except for their crude fiber contents. A higher moisture content of STF (11.2 g/100 g flour) was found as compared to ETF (10.2 g/100 g flour). The moisture content of ETF was in close agreement with 9.92–10.90 g/100 g flour,[Citation19] 10.3 g/100 g flour,[Citation20] and 9.9 g/100 g flour,[Citation21] which were observed in other teff varieties. The moisture content of flour is an important quality parameter, and usually, the low moisture content of flour is advantageous for increasing the storage stability. According to Ref. Citation22, less than 14 g/100 g flour moisture content of flour can resist microbial growth, which is important to have a long shelf life. Therefore, moisture contents observed in ETF and STF, which fall between 10 and 12 g/100 g flour, are within the acceptable range for safe storage. This, in turn, helps for further processing without risk of microorganism contamination.
Table 1. Proximate and mineral composition of ETF and STF
The ash content of ETF (2.5 g/100 g flour) was found to be significantly (p < .05) higher than that found in STF (1.7 g/100 g flour). The ash content shows the measurement of mineral matter present in the flour .[Citation18] Thus, ETF could have higher mineral matter than STF. The ash content of ETF was found to be in good agreement within the range of 2.00–2.78 g/100 g flour reported by L Yegrem, M Yimam, T Kore, and W Abebe[Citation19]; however, the ash content of STF was found to be the lowest. The protein content observed in ETF (9.5 g/100 g flour) was significantly (p < .05) lower than that observed in STF (13.3 g/100 g flour). The fat content found in ETF (3.3 g/100 g flour) was significantly (p < .05) higher than that found in STF (2.9 g/100 g flour). The carbohydrate content (CHO) (72.4 g/100 g flour) observed in ETF was also significantly higher than that observed in STF (68.4 g/100 g flour). The CHO observed in ETF and STF was lower than 83.4 g/100 g flour .[Citation23] Crude fiber contents acquired in ETF and STF were not significantly (p < .05) different. In general, most tested proximate parameters of ETF and STF were found to have significant differences; this variation might be due to environmental conditions, soil type, fertilizers used, and varieties.
Mineral: The minerals analysis of the two varieties of ETF and STF is presented in . Minerals are necessary constituents of the human diet because they serve as cofactors for many physiological and metabolic processes. Significantly (p < .05) higher contents of calcium (1306 mg/kg) and iron (363 mg/kg) were observed in ETF as compared to calcium (1142 mg/kg) and iron (58 mg/kg) observed in STF, whereas a significantly (p < .05) higher zinc content (34.6 mg/kg) was observed in STF than 24.1 mg/kg observed in ETF. The higher calcium content of 1543 mg/kg in other teff variety has been reported .[Citation16] The highest iron content (363 mg/kg) was found in ETF as compared to 76.3 mg/kg[Citation10] and 85.3 mg/kg,[Citation24] which have been reported in other teff varieties.
According to the United States Department of Agriculture (USDA),[Citation12] the highest contents of calcium, iron, and zinc in teff were reported to be 1800, 117, and 74.2 mg/kg, respectively. In this study, lower values of calcium and zinc were observed as compared to the USDA reports. However, iron observed in ETF was found to be much higher (more than three times) than the USDA reports. The appropriate Recommended Dietary Allowance (RDA) values of calcium, iron, and zinc for females aged 31–50 are 1000, 18, and 8 mg/day, respectively. With the same age category, the RDA values of calcium, iron, and zinc for males are 1000, 8, and 11 mg/day, respectively .[Citation25] Therefore, the required daily intake of iron, calcium, and zinc could be achieved by taking an appropriate teff-based food products.
Amino acid: The amino acid compositional analysis of the two varieties of ETF and STF is presented in . Samples of ETF and STF were used for quantitative analysis of 18 amino acids (AAs). The AAs are necessary biological components needed in the human body that could be essential and nonessential .[Citation26] The AAs compositional information definitely shows the first stage for every food protein’s nutritional evaluation.[Citation27] The concentration of individual amino acids was highly associated with their protein content. All essential amino acids need for human nutrition was found in ETF and STF with considerable amounts, which show good protein quality. Among the eighteen examined AAs, crude protein observed in ETF contained approximately 41.8 g/100 g of protein of essential amino acids and 58.2 g/100 g of protein of nonessential amino acids. Correspondingly, crude protein observed in STF had 44.7 g/100 g of protein of essential amino acids and 55.3 g/100 g of protein of nonessential amino acids. Glutamine was the highest nonessential amino acid observed in both ETF and STF. Valine, which usually improves muscle building, boosts exercise performance, decreases weakness,[Citation27] was the maximum essential amino acid exhibited in both flours of ETF and STF. Lysine is an essential amino acid, which exists in a limited amount in most cereals,[Citation16] was found to be higher in ETF and STF. Overall, significantly (p < .05) higher essential amino acids were investigated in STF as compared to ETF, which could reflect its nutritional benefit.
Table 2. Amino acid composition of ETF and STF
Pasting properties of ETF and STF
The pasting curves of ETF and STF are presented in , and their pasting parameters are shown in . Significant (p < .05) differences were observed in all measured pasting characteristics of flours from ETF and STF. Trough (1278.67 mPa.s), final (2418 mPa.s), and setback (1139 mPa.s) viscosities, including pasting temperature (90 °C), and peak time (6.3 min) were found to be higher in STF (), whereas higher peak (1622 mPa.s) and breakdown (584 mPa.s) viscosities were observed in ETF (). Peak viscosity is a measure of water holding capacity of starch in terms of swollen granule resistance. It also reflects the beginning of granule disruption when the granule structure can no longer support continuous swelling .[Citation28] Hence, higher peak viscosity observed in ETF shows its higher water swelling capacity. However, lower peak viscosity (1444 mPa.s) of STF perceived its lower water swelling capacity. Breakdown viscosity measures starch’s ability to resist thermal pasting. Flour with higher breakdown viscosity has lower ability to withstand heating .[Citation29] During breakdown, granules disrupted and linear molecules leach out into solution .[Citation30]
Table 3. Pasting parameters of flours from ETF and STF
Figure 1. Pasting curves of ETF and STF; ETF, teff flour grown in Ethiopia; STF, teff flour grown in South Africa; Temp, temperature.
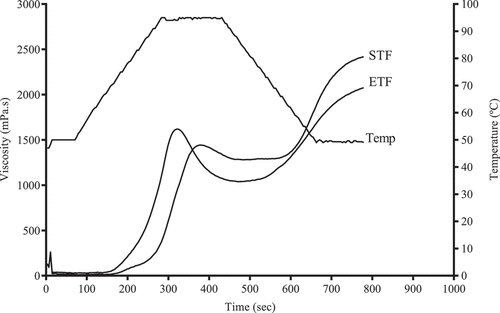
Final viscosity indicates the ability of material to form a viscous paste. It is determined by the retrogradation tendency of soluble amylose upon cooling .[Citation18] Higher final viscosity of STF indicates its potential of making a rigid gel structure in cooling as compared to ETF. Setback viscosity is the increase in viscosity of a starch paste during cooling, which determines the paste gel building capability .[Citation31] This could be due to the entanglement of amylose molecules. Amylopectin is relatively less likely to reorganize at the time of retrogradation than amylose. Amylopectin retrogradation occurs very slowly, which results in slower staling and softer products .[Citation32] Therefore, STF has a higher degree of recrystallization of gelatinized starch in time of cooling as compared to ETF.
Pasting temperature indicates the minimum temperature required to cook .[Citation33] Higher pasting temperature of the flour is an indication of its thermal resistance at the start of gelatinization. In turn, higher pasting temperature shows lower temperature required to cook the flour .[Citation18] Lower pasting temperature of ETF (74.0 °C) was observed than that of STF (90 °C) (). Higher pasting temperature of STF indicates its higher resistance to swelling and rupturing. This was verified by its lower swelling capacity as compared to ETF. Overall, both flours from ETF and STF revealed a gradual increase in viscosity with an increase in temperature. This is due to loss of water from the exuded amylose by granules as they swell .[Citation33] Moreover, viscosity increase after cooling to 50 °C could be attributed to the formation of a viscous paste between amylose molecules. Lower final viscosity (1767 mPa.s) and higher peak time (8.5 min) have been reported in the DZ-99-01 teff variet .[Citation23]
Intercorrelations were observed among the measured thermal and pasting parameters. A positive correlation of To, Tp, and TE with FV, TV, PT, and Pti and a negative correlation with PV have been observed in Pearson correlation analysis (p < .01, ). In addition, PV and To, Tp, and TE values went in opposite directions on the PCA loading plot, suggesting a negative correlation between them (). Findings of pasting properties showed that STF has higher retrogradation ability and needs longer cooking time as compared to ETF. Consequently, its bakery products (bread, cookie, noodles, etc) might be staled quickly as compared to ETF.
Table 4. Thermal and Functional properties of ETF and STF
Table 5. RGB and hex code values with approximate color names of ETF and STF
Table 6. Variables examined using principal component analysis (PCA)
Table 7. Pearson correlation coefficients between various proximate profiles and functional, thermal, and pasting properties of flours from ETF and STF
Thermal properties of ETF and STF
Thermal analysis results () disclosed that onset (65.9 °C), peak (71.6 °C), and end temperatures (78. 5 °C) were higher in the case of STF. An opposite trend was observed for enthalpy of gelatinization, i.e., STF (1.6 J/g) and ETF (2 J/g). The differences in peak temperature and enthalpy of gelatinization between ETF and STF were not significant (p < .05). Onset temperature shows the first measurable melting of the starch granules, and peak temperature indicates the highest energy transfer .[Citation28] Similar onset (66 °C) and peak temperatures (71 °C) have been reported .[Citation34] The differences in gelatinization temperatures observed in ETF and STF could be a result of deviation of starch granule distribution and starch structure .[Citation33] Lower gelatinization enthalpy of STF might be due to effects of nonstarch components in the flours such as protein and lipid. The presence of protein and fiber in the flour could be decreasing the availability of water for starch gelatinization. Onset and peak temperatures were negatively correlated with fat, CHO, and PV and positively correlated with protein, fiber, PT, and FV (p < .01), as analyzed by Pearson correlation ().
Functional properties of ETF and STF
depicts the functional properties of ETF and STF. Foam stability (71 mL/100 mL initial foam), water absorption capacity (1.1 g/g), oil absorption capacity (0.9 g/g), and swelling power (6.4 g/g) observed in ETF were significantly (p < .05) higher than those of STF. However, higher foam capacity (9.8 mL) was found in STF as compared to ETF. Foam capacity is the ability of flour to adsorb quickly on the air-liquid interface during bubbling, whereas foam stability is the flour ability to form a cohesive viscoelastic film by intermolecular interactions .[Citation35] Higher foam capacity obtained in STF might be due to its higher protein content (13.3 g/100 g flour) than that of ETF (9.5 g/100 g flour). The presence of protein in the flour might cause a lowering of surface tension at the water-air interface. The lower foam stability observed in STF shows its limitation to form a strong continuous cohesive film around air bubbles in foam. The PCA analysis showed a positive correlation of foam capacity with protein and a negative correlation with foam stability (). Good foam capacity and stability of flours are desired attributes for intended utilization in the production of various baked products such as bread .[Citation36] Uniform distributions of air bubbles in the structure of foods allow volatilization of flavors with increased palatability .[Citation37] Foaming properties are important factors in the bread making process since they influence gas retention and extension of gas cells during kneading and proofing .[Citation38] Foaming properties have an effect on consistency and appearance of foods. Wheat flour (well-known for bread making) has a higher foam stability (94 mL/100 mL)[Citation39] than ETF (71 mL/100 mL) and STF (47 mL/100 mL). Therefore, despite its gluten free, teff flour-based bread would be dense and firm as compared to wheat flour-based bread.
Water absorption capacity reports the flour’s ability to absorb and retain water under a centrifugal gravity force .[Citation35] Flour with high water absorption capacity has more hydrophilic constituents .[Citation33] Accordingly, higher water absorption capacity observed in ETF showed higher amounts of hydrophilic polysaccharide. Additionally, higher water absorption capacity might be an indication of weaker binding forces. It reflects higher amylose in ETF flour granules. Oil absorption capacity is a physical entrapment of oil within the starch structure and protein nonpolar side chains. It affects flavor and mouthfeel and indicates the emulsifying ability of the flour. Similar oil absorption capacity of rice flour and starch was reported .[Citation35] Oil absorption capacity of ETF and STF showed a significantly (p < .01) positive correlation with water absorption capacity and a negative correlation with the Falling number ( and ). Water absorption capacity is an important attribute in the development of viscous foods, i.e., soups, sauces, dough, and baked products as well as sausages,[Citation40] making ETF a more suitable ingredient than STF in gluten-free formulation. Water absorption capacity is the desired water volume to be mixed with flour to make a dough before it becomes excessively sticky to process. Usually, water absorption capacity levels vary from 1.5–1.6 g water/g flour in standard white bread formula, 4–9 g water/g flour in the artisan-type Ciabatta formula, and 1–1.2 g water/g flour in a cookie formula .[Citation36] Water absorption capacities of ETF and STF ranged from 1–1.1 g water/g flour, which falls within the range of cookie formula. This shows that flours from ETF and STF can be used to make cookie. However, if bread is prepared using flours from ETF and STF, the bread quality will be firm and dense, has low volume, and stales quickly because of their underwater absorption capacity. Therefore, ETF and STF needs blending of flour, which have higher water absorption capacity for the production of better bread quality. Oil absorption capacities examined in ETF (1.1 g oil/g flour) and STF (1 g oil/g flour) are very low as compared to wheat flour oil absorption (5.4 g oil/g flour)[Citation41] and (3.97–7.52 g oil/g flour) .[Citation42] These low oil absorption capacities of ETF and STF could be important in the production of low oil uptake frying batters and other fried products in which low oil uptake is a desirable attribute.
Swelling power is the widening ability of the flour particles because of water absorption .[Citation43] Lower swelling power observed in STF shows its strong lipid-amylose complexes (higher inhibitor of swelling) as compared to ETF .[Citation17] In addition, this could be attributed to the presence of more fibrous materials in ETF that possesses more swelling power than STF. The lower swelling power of STF was confirmed by its higher pasting temperature as compared to ETF. The lower result of swelling power (3.1 g/g) was reported in the DZ-99-01 teff variety .[Citation23] The ETF might be preferred over STF to prepare food products where better flavor and mouthfeel are required because of its higher oil absorption capacity.
Particle size distribution and Falling number of ETF and STF
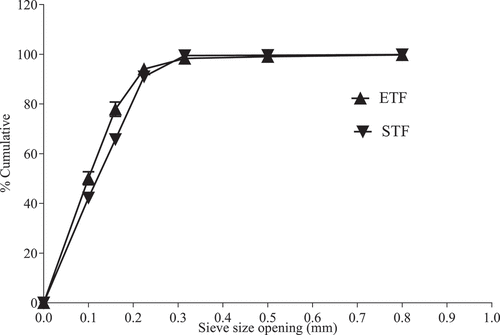
The particle size distribution of flours from ETF and STF is shown in . The particle size of flours is a critical factor for determining its usefulness and end-product quality .[Citation44] Particles of flours observed from ETF and STF did not show significant (p < .05) differences. Particles of flour from ETF were distributed within sieve size ranges of 224–315 µm, 160–224 µm, and 100–160 µm with analogous size ranges held at approximately 4, 16, and 28 g/100 g flour. Also, about 50 g/100 g of the flour particles were distributed within the sieve size range of < 100 µm. Similarly, particles of flour from STF were distributed within sieve size ranges 224–315 µm, 160–224 µm, and 100–160 µm with the corresponding sieve size ranges held at approximately 8, 25, and 23 g/100 g flour. Another 42 g/100 g flour of the particles were distributed within the sieve size range of < 100 µm.
Figure 2. Particle size distribution curve of flours from ETF and STF; STF, teff flour grown in South African; ETF, teff flour grown in Ethiopian.
The Falling numbers of ETF and STF did not show significant (p < .05) differences. Its measurement helps for the evaluation of grain α‐amylase activity. Falling number between 300 and 450 s or higher is desired. However, the lower falling number that shows that grain-sprouting damage is not preferred. The Falling numbers observed for ETF (436 s) and STF (462 s) were within the desired region. Therefore, flour samples of ETF and STF used in this study were at good condition.
RGB color values of ETF and STF
The RGB color values of the two flour varieties of ETF and STF are presented in . The hexadecimal color code #E8DFCF obtained in ETF symbolizes a very light shade of brown color. In RGB, color model #E8DFCF comprises 90.98 % red, 87.45 % green, and 81.18 % blue. In HSL (hue, saturation, and lightness), color space #E8DFCF has a hue of 38°, 35 % saturation, and 86 % lightness. In addition, in HSV (hue, saturation, and value), color space #E8DFCF has hue of 38°, 11 % saturation, and 91 % value. This color has an approximate wavelength of 576.85 nm. The relative percentage of red, green, and blue in the ETF image was, respectively, 35.04, 33.69, and 31.27 %. In addition, the hexadecimal color code #E5D8C8 was observed in STF, which shows a light shade of brown color. In RGB, color model #E5D8C8 comprises 89.8 % red, 84.71 % green, and 78.43 % blue. In HSL, color space #E5D8C8 has a hue of 33°, 36 % saturation, and 84 % lightness. Moreover, in the color space, HSV #E5D8C8 has a hue of 51°, a saturation of 9 %, and a value of 86 %. This color has an approximate wavelength of 579.07 nm. The relative percentage of red, green, and blue in STF image was, respectively, 35.50, 33.49, and 31.01 %.
Statistical correlation of ETF and STF quality attributes
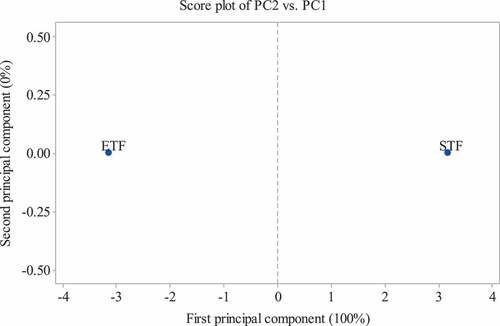
The variables subjected to principal component analysis (PCA) are listed in , and the results of the analysis are shown in and 4. The PCA plots provide an overview of the similarities and differences between the flours of ETF and STF and the inter-relationships among the measured attributes. The distance between the position of ETF and STF on the score plot is directly proportional to the degree of difference or similarity between them (). The total variabilities of the evaluated attributes were explained by the first principal component ( and 4). The score plot showed that ETF and STF were located at the far left and right, respectively (). The loading plot provides information about correlations between the measured chemical compositions and functional, thermal, and pasting properties of ETF and STF ().
Figure 3. Loading plot of first principal component (PC1) and second principal component (PC2) describing the variation among the different attributes of flours from ETF and STF. A thick solid line and a second line very close to it indicate two attributes that are highly correlated.
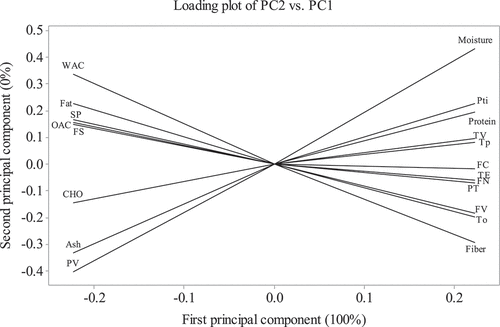
Figure 4. Score plot of first principal component (PC1) and second principal component (PC2) describing the variation between flours from ETF and STF.
The attributes whose curves are close to each other on the loading plot are positively correlated, whereas those whose curves went in opposite directions are negatively correlated. For instance, positive correlations of ash with PV and CHO and negative correlations of protein with FS and WAC can be observed in the loading plot (). Additionally, the correlations of the measured attributes from the Pearson correlation analysis could be observed in .
CONCLUSIONS
Flours from ETF and STF differed significantly in their contents of proximate, mineral, and amino acid, including in their pasting, thermal, and functional properties. This variation can be due to environmental conditions, soil types, varieties, and agronomic practices. All essential amino acids were observed in ETF and STF with a higher protein content of STF. The most abundant essential and nonessential AAs observed in both flours of ETF and STF were valine and glutamine, respectively. The iron content observed in ETF was much higher than that of STF. The higher pasting temperature and lower swelling power observed in STF verify its higher resistance to swelling and rupturing. Higher thermal temperatures found in STF can be the result of its higher amylopectin. The examination of Pearson correlation and PCA revealed the presence of significant inter-relationships among proximate and pasting, thermal, and functional properties of flours from ETF and STF. Finally, findings of this study would contribute to the selection of teff for the development of healthier food products to meet the current diverse consumer preferences.
Acknowledgments
The authors thank Mr. Herbert Goetz (Department of Process Analytics and Cereal Science, Institute of Food Science and Biotechnology, University of Hohenheim, Garbenstr. 23, 70599 Stuttgart, Germany) for his technical support during this research work. The authors declare that there is no conflict of interest.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Galassi, E.; Taddei, F.; Ciccoritti, R.; Nocente, F.; Gazza, L. Biochemical and Technological Characterization of Two C4 Gluten‐free Cereals: Sorghum Bicolor and Eragrostis Tef. Cereal Chem. 2020, 97(1), 65–73. DOI: 10.1002/cche.10217.
- Gebru, Y. A.; Hyun-Ii, J.; Young-Soo, K.; Myung-Kon, K.; Kwang-Pyo, K. Variations in Amino Acid and Protein Profiles in White versus Brown Teff (Eragrostis Tef) Seeds, and Effect of Extraction Methods on Protein Yields. Foods. 2019, 8(6), 202. DOI: 10.3390/foods8060202.
- Assefa, Y.; Emire, S.; Villanueva, M.; Abebe, W.; Ronda, F. Influence of Milling Type on Tef Injera Quality. Food Chem. 2018, 266, 155–160. DOI: 10.1016/j.foodchem.2018.1005.1126.
- Mekonen, S.; Ambelu, A.; Spanoghe, P. Reduction of Pesticide Residues from Teff (Eragrostis Tef) Flour Spiked with Selected Pesticides Using Household Food Processing Steps. Heliyon. 2019, 5(5), e01740. DOI: 10.01016/j.heliyon.02019.e01740.
- Lee, H. Teff, a Rising Global Crop: Current Status of Teff Production and Value Chain. The Open Agriculture J. 2018, 121, 185–193. DOI:10.2174/1874331501812010185.
- Satheesh, N.; SW, F. Review on Structural, Nutritional and Anti-nutritional Composition of Teff (Eragrostis Tef) in Comparison with Quinoa (Chenopodium Quinoa Willd.). Cogent Food Agric. 2018, 4(1), 1546942.
- Gebremariam, M. M.; Zarnkow, M.; Becker, T. Teff (Eragrostis Tef) as a Raw Material for Malting, Brewing and Manufacturing of Gluten-free Foods and Beverages: A Review. J. Food Sci. Technol. 2014, 51(11), 2881–2895. DOI: 10.1007/s13197-012-0745-5.
- Do Nascimento, K.; Paes, S.; de Oliveira, I. R.; Reis, I. P.; Augusta, I. M. Teff: Suitability for Different Food Applications and as a Raw Material of Gluten-free, a Literature Review. J. Food Nutr. Res. 2018, 6, 74–81.
- Alemneh, S. T.; Emire, S. A.; Hitzmann, B. Teff-Based Probiotic Functional Beverage Fermented with Lactobacillus Rhamnosus and Lactobacillus Plantarum. Foods. 2021, 10(10), 2333. DOI: 10.3390/foods10102333.
- Zhu, F. Chemical Composition and Food Uses of Teff (Eragrostis Tef). Food Chem. 2018, 239, 402–415. DOI: 10.1016/j.foodchem.2017.06.101.
- Sharma, K.; Chauhan, E. S. Nutritional Composition, Physical Characteristics and Health Benefits of Teff Grain for Human Consumption: A Review. Pharm. Innovation J. 2018, 7(10), 3–7.
- Koubová, E.; Sumczynski, D.; Šenkárová, L.; Orsavová, J.; Fišera, M. Dietary Intakes of Minerals, Essential and Toxic Trace Elements for Adults from Eragrostis Tef L.: A Nutritional Assessment. Nutrients. 2018, 10(4), 479. DOI: 10.3390/nu10040479.
- Golmohamadi, A.; Yazdi, R. A. N.; Kita, K. 2020. Teff Could Contribute to the Sustainable Goals of United Nation’s Goals in Low-income Areas of Middle East and North Africa. Open Science J 5(1) DOI: 10.23954/osj.v5i1.2221.
- EU: Commission Regulation (EC) No 152/2009 of 27 January 2009. Laying down the Methods of Sampling and Analysis for the Official Control of Feed. Off. J. Eur. Union. 2009, 54, 1–130.
- AACC: American Association of Cereal Chemists Approved Methods of Analysis, 11th ed.; Cereals & Grains Association: St. Paul, MN, U.S.A, 1999.
- Hager, A.-S.; Czerny, M.; Bez, J.; Zannini, E.; Arendt, E. K. Starch Properties, in Vitro Digestibility and Sensory Evaluation of Fresh Egg Pasta Produced from Oat, Teff and Wheat Flour. J. Cereal Sci. 2013, 58(1), 156–163. DOI: 10.1016/j.jcs.2013.03.004.
- Klunklin, W.; Savage, G. Physicochemical, Antioxidant Properties and in Vitro Digestibility of Wheat–purple Rice Flour Mixtures. Int. J. Food Sci. Technol. 2018, 53(8), 1962–1971. DOI: 10.1111/ijfs.13785.
- Mir, N. A.; Gul, K.; Riar, C. S. Physicochemical, Pasting and Thermal Properties of Water Chestnut Flours: A Comparative Analysis of Two Geographic Sources. J. Food Process. Preserv. 2015, 39(6), 1407–1413. DOI: 10.1111/jfpp.12359.
- Yegrem, L.; Yimam, M.; Kore, T.; Abebe, W. Comparative Analysis of Proximate and Mineral Composition of Released Tef (Eragrostis Tef (Zucc.) Trotter) Varieties in Ethiopia. Academic Research Journal of Agricultural Science and Research . 2019, 372–379. DOI:10.14662/ARJASR12019.14110.
- Heiru, M.; Bultosa, G.; Busa, N.; Yildiz, F. Effect of Grain Teff, Finger Millet and Peanut Blending Ratio and Processing Condition on Weaning Food Quality. Cogent Food Agric. 2019, 5(1), 1671116. DOI: 10.1080/23311932.2019.1671116.
- Mesías, M.; Morales, F. J. Effect of Different Flours on the Formation of Hydroxymethylfurfural, Furfural, and Dicarbonyl Compounds in Heated Glucose/flour Systems. Foods. 2017, 6(2), 14. DOI: 10.3390/foods6020014.
- Hayma, J.;. AD31E the Storage of Tropical Agricultural Products; Agromisa Foundation, Wageningen, 2003.
- Abebe, W.; Collar, C.; Ronda, F. Impact of Variety Type and Particle Size Distribution on Starch Enzymatic Hydrolysis and Functional Properties of Tef Flours. Carbohydr. Polym. 2015, 115, 260–268. DOI: 10.1016/j.carbpol.2014.08.080.
- Hager, A.-S.; Wolter, A.; Jacob, F.; Zannini, E.; Arendt, E. K. Nutritional Properties and Ultra-structure of Commercial Gluten Free Flours from Different Botanical Sources Compared to Wheat Flours. J. Cereal Sci. 2012, 56(2), 239–247. DOI: 10.1016/j.jcs.2012.1006.1005.
- Trumbo, P.; Yates, A. A.; Schlicker, S.; Poos, M. Dietary Reference Intakes: Vitamin A, Vitamin K, Arsenic, Boron, Chromium, Copper, Iodine, Iron, Manganese, Molybdenum, Nickel, Silicon, Vanadium, and Zinc. J. Am. Diet. Assoc. 2001, 101(3), 294–301. DOI: 10.1016/S0002-8223(01)00078-5.
- Adebiyi, J. A.; Obadina, A. O.; Adebo, O. A.; Kayitesi, E. Comparison of Nutritional Quality and Sensory Acceptability of Biscuits Obtained from Native, Fermented, and Malted Pearl Millet (Pennisetum Glaucum) Flour. Food Chem. 2017, 232, 210–217. DOI: 10.1016/j.foodchem.2017.1004.1020.
- Barakat, H. Nutritional and Rheological Characteristics of Composite Flour Substituted with Baobab (Adansonia Digitata L.) Pulp Flour for Cake Manufacturing and Organoleptic Properties of Their Prepared Cakes. Foods. 2021, 10(4), 716. DOI: 10.3390/foods10040716.
- Zhong, F.; Li, Y.; Ibáñez, A. M.; Oh, M. H.; McKenzie, K. S.; Shoemaker, C. The Effect of Rice Variety and Starch Isolation Method on the Pasting and Rheological Properties of Rice Starch Pastes. Food Hydrocolloids. 2009, 23(2), 406–414. DOI: 10.1016/j.foodhyd.2008.02.003.
- Wang, H.; Yang, Q.; Gao, L.; Gong, X.; Qu, Y.; Feng, B. Functional and Physicochemical Properties of Flours and Starches from Different Tuber Crops. Int. J. Biol. Macromol. 2020, 148, 324–332. DOI: 10.1016/j.ijbiomac.2020.1001.1146.
- Ilowefah, M.; Chinma, C.; Bakar, J.; Ghazali, H. M.; Muhammad, K.; Makeri, M. Fermented Brown Rice Flour as Functional Food Ingredient. Foods. 2014, 3(1), 149–159. DOI: 10.3390/foods3010149.
- Gayin, J.; Abdel-Aal, E.-S. M.; Manful, J.; Bertoft, E.; Marcone, M.; Ragaee, S. Physical, Cooking and Thermal Properties of African Rice (Oryza Glaberrima) and Its Starch Digestibility in Vitro. LWT. 2017, 75, 481–487. DOI: 10.1016/j.lwt.2016.09.023.
- Mezgebe, A. G.; Taylor, J.; de Kock Hl. Influence of Waxy (High Amylopectin) and High Protein Digestibility Traits in Sorghum on Injera Sourdough-Type Flatbread Sensory Characteristics. Foods. 2020, 9(12), 1749. DOI: 10.3390/foods9121749.
- Kaur, M.; Singh, N. Studies on Functional, Thermal and Pasting Properties of Flours from Different Chickpea (Cicer Arietinum L.) Cultivars. Food Chem. 2005, 91(3), 403–411. DOI: 10.1016/j.foodchem.2004.1006.1015.
- Wolter, A.; Hager, A.-S.; Zannini, E.; Arendt, E. K. In Vitro Starch Digestibility and Predicted Glycaemic Indexes of Buckwheat, Oat, Quinoa, Sorghum, Teff and Commercial Gluten-free Bread. J. Cereal Sci. 2013, 58(3), 431–436. DOI: 10.1016/j.jcs.2013.09.003.
- Falade, K. O.; Semon, M.; Fadairo, O. S.; Oladunjoye, A. O.; Orou, K. K. Functional and Physico-chemical Properties of Flours and Starches of African Rice Cultivars. Food Hydrocolloids. 2014, 39, 41–50. DOI: 10.1016/j.foodhyd.2013.11.002.
- Awuchi, C. G.; Igwe, V. S.; Echeta, C. K. The Functional Properties of Foods and Flours. Int J Advanced Academic Res 2019, 5(11), 139–160.
- Mune, M. A. M.; Sogi, D. S. Emulsifying and Foaming Properties of Protein Concentrates Prepared from Cowpea and Bambara Bean Using Different Drying Methods. Int. J. Food Prop. 2016, 19(2), 371–384.
- Horstmann, S. W.; Foschia, M.; Arendt, E. K. Correlation Analysis of Protein Quality Characteristics with Gluten-free Bread Properties. Food Funct. 2017, 8(7), 2465–2474. DOI: 10.1039/C7FO00415J.
- Nawaz, H.; Shad, M. A.; Mehmood, R.; Rehman, T.; Munir, H. Comparative Evaluation of Functional Properties of Some Commonly Used Cereal and Legume Flours and Their Blends. Int J Food and Allied Sci. 2015, 1(2), 67–73. DOI: 10.21620/ijfaas.2015267-73.
- Granito, M.; Guerra, M.; Torres, A.; Guinand, J. Efecto Del Procesamiento Sobre Las Propiedades Funcionales de Vigna Sinensis. Interciencia. 2004, 29(9), 521–526.
- Chandra, S.; Singh, S.; Kumari, D. Evaluation of Functional Properties of Composite Flours and Sensorial Attributes of Composite Flour Biscuits. J. Food Sci. Technol. 2015, 52(6), 3681–3688. DOI: 10.1007/s13197-014-1427-2.
- Punia, S.; Sandhu, K. S.; Siroha, A. K. Difference in Protein Content of Wheat (Triticum Aestivum L.): Effect on Functional, Pasting, Color and Antioxidant Properties. J. Saudi Soc. Agric. Sci. 2019, 18(4), 378–384. DOI: 10.1016/j.jssas.2017.12.005.
- Pellegrini, M.; Lucas-Gonzales, R.; Ricci, A.; Fontecha, J.; Fernández-López, J.; JA, P.-Á.; Viuda-Martos, M. Chemical, Fatty Acid, Polyphenolic Profile, Techno-functional and Antioxidant Properties of Flours Obtained from Quinoa (Chenopodium Quinoa Willd) Seeds. Ind. Crops Prod. 2018, 111, 38–46. DOI: 10.1016/j.indcrop.2017.10.006.
- Jan, R.; Saxena, D.; Singh, S. Comparative Study of Raw and Germinated Chenopodium (Chenopodium Album) Flour on the Basis of Thermal, Rheological, Minerals, Fatty Acid Profile and Phytocomponents. Food Chem. 2018, 269, 173–180. DOI: 10.1016/j.foodchem.2018.07.003.
