ABSTRACT
This study aimed to introduce minced common carp (Cyprinus caprio L.) meat as a nutritional valuable by-product from carp filleting. This research was focused on fatty acid composition and considered its sensitivity to oxidation during frozen storage (−20°C). Additionally, copper chloride was used to magnify possible oxidation reactions. A better understanding of minced carp meat quality and deterioration during frozen storage could help to promote the usage of this underestimated by-product. The utilization of this by-product for human consumption could be a useful way to increase the carp aquaculture economy and sustainability. The fatty acid profile showed a favorable content of essential fatty acids, as well as a beneficial ratio of ω-3/ω-6 polyunsaturated fatty acids. Furthermore, during 4 months of frozen storage (−20°C), no significant changes were detected in fatty acid composition. Negligible changes were observed in the oxidation of lipids and proteins. The nutritional value and storage stability of minced carp meat was shown.
Introduction
Fish are an important source of essential fatty acids (FA) and valuable proteins, both of which are considered important for human health.[Citation1] Fish are a particularly important source of essential fatty acids – α-linolenic (LNA, 18:3 ω-3), linoleic (LA, 18:2 ω-6) and nutritionally important ω-3 long-chain polyunsaturated fatty acids (PUFA), eicosapentaenoic acid (EPA, 20:5 ω-3) and docosahexaenoic acid (DHA, 22:6 ω-3).[Citation2] EPA and DHA are known to be crucial in the nervous system of children in prenatal development. They have positive effects on the cardiovascular system and prevent obesity and metabolic syndrome.[Citation2–4] Besides, it is not only the content of ω-3 PUFA itself but the ω-3/ω-6 ratio in the diet that is of importance. Since the ω-6 and ω-3 PUFA are metabolized by the same enzymes, a high proportion of dietary ω-6 PUFA will lead to increased ω-6 metabolites. In many cases, these possess opposite, and therefore non-beneficial, effects over ω-3 metabolites.[Citation5] Therefore, in general, a diet with an ω-3/ω-6 ratio higher than 0.25 is recommended.[Citation5,Citation6]
Common carp (Cyprius caprio L.) is considered one of the most cultured and highly consumed fish species in Central Europe. Carp produced in earthen ponds are rich in ω-3 PUFA, based on natural feed such as benthos and plankton.[Citation7,Citation8] Furthermore, carps possess ∆-6 desaturase – which is crucial for synthesizing longer chain PUFA from essential 18:3 ω-3.[Citation9] This enzymatic equipment permits the production of ω-3 enriched carp in semi-intensive production systems by the fish feed modulation.[Citation7,Citation8] Nowadays, minced carp meat is an underutilized by-product of carp filleting. The flesh that remains on the skeleton of the carp after filleting is mechanically separated and minced. This mince is a rich source of lipids, proteins and other essential components; hence it can be used as raw material for other products with increased nutritional value.[Citation10] However, due to the prejudice of it being oxidized very easily and the resulting rancid off-flavor, its utilization for products for human consumption is rather low. Similar minced products such as fish sausage, cakes, patties, balls and pastes from other fish species also exist and are successfully used. These fish species include Atlantic horse mackerel (Trachurus trachurus), sea bass (Dicentrarchus labrax), gilthead seabream (Sparus aurata) and rainbow trout (Oncorhynchus mykiss).[Citation11,Citation12]
This study aimed to evaluate the lipid and fatty acid composition of fresh carp minced meat and its stability during frozen storage at −20°C in relation with regards to lipids and proteins oxidation. Also, a comparison of normally stored and oxidation-induced minced meat was performed. We assumed that carp meat, with its high-fat content as a minced product, would oxidize much faster than whole fillets. Earlier trials have used 90 days of storage for similar products from various species[Citation11,Citation12] and the recommended frozen storage time for whole fatty fish is 3–6 months.[Citation13] Therefore, in our study, we assessed the oxidative stability of lipids and proteins of meat during 4 months of frozen storage.
Materials and methods
Chemicals
The Boron trifluoride-methanol solution~10% (~1.3 M); for GC derivatization, LiChropur™ was obtained from Supelco, and the other chemicals from Sigma-Aldrich: Hexane HPLC Plus, for HPLC, GC, and residue analysis, ≥95%; Methanol suitable for HPLC, gradient grade, suitable as ACS-grade LC reagent, ≥99.9% 1-Propanol suitable for HPLC, ≥99.9% Diethyl ether suitable for HPLC, ≥99.9%, inhibitor-free; Acetic acid glacial, ACS reagent, ≥99.7%; Copper(I) chloride ≥99.995% trace metals basis; Sodium sulfate ACS reagent, ≥99.0%, anhydrous, powder; Sodium chloride ACS reagent, ≥99.0%.
Sample preparation and storage
The common carp (Cyprinus caprio L.) were reared in ponds for 3–4 years, supplemented with cereals (wheat and barley), and subsequently slaughtered at an average weight of 2.2–3.5 kg by a local fisherman’s company (Rybařství Chlumec nad Cidlinou, a.s.). Fish were handled according to the law. Two dozen fish were then skinned and filleted, the rest of the corpse was used for meat separation and minced by the BAADER 603 device (Baader, Lubeck, Germany); all of which took place in the local fisherman’s company. The obtained minced carp meat was mixed and partitioned to 12 packages (200 g ± 10 g) for starting point and to 24 packages of 200 g ± 10 g for freezing experiment at −20°C. 6 samples were used per variant. The samples were packed into the common polypropylene zip bags 18 × 25 cm in size and 50 µm in thickness and the air was removed before freezing. To get a better estimation of the ongoing oxidation processes, in half of the samples lipid oxidation was induced by adding 10 µg of copper chloride per 1 g of the sample. The copper chloride was added to the samples before analyses (fresh samples) or freezing, and each sample was mixed to provide equal distribution of copper chloride through the sample. Samples of both the normal (N) and induced (IND) groups were analyzed as performed (n = 6) at the beginning of the experiment (time/month 0; fresh sample), and after 2 and 4 months of storage at −20°C.
Lipid classes and fatty acid analyses
The lipid extraction was performed according to the method of Hara and Radin[Citation14] with slight modification.[Citation15] In brief, Ultra Turrax (T25, Janke and Kunkel, IKA Werke, Germany) was used for the homogenization of an approximately 2 g sample in 10 ml of hexane-isopropanol (3:2), and 6 ml of Na2SO4 (6.67%) were added to the obtained homogenates and mixed. After centrifugation, the upper lipid phase was transferred into pre-weighed tubes and subsequently evaporated under nitrogen. The lipid content was quantified gravimetrically.
Thin-layer chromatography (TLC) was used to investigate the lipid class (LC) composition. The analysis was performed according to the method of Olsen[Citation16] with minor changes. TLC plates (20x10 cm; Silicagel 60; 0.20 mm layer, Merck, Darmstadt, Germany) were employed as a stationary phase and 25 ml hexane: diethyl ether: acetic acid (85:15:1, v/v) as mobile phase. CAMAG TLC Sampler 4, Twin Through Chamber 20x20, and development chamber (All Camag, Switzerland) devices were used to perform the lipid classes separation – for details see Mraz and Pickova.[Citation15] Identification of the lipid classes was performed by comparison with an external standard (TLC 18–4A, Nu-Chek Prep, Elysian, USA).
For FA analyses, methylation of total lipids was performed according to Appelqvist.[Citation17] FA composition was analyzed by gas chromatography (GC) (Trace Ultra FID; Thermo Scientific, Milan, Italy) on a BPX-70 50 m fused silica capillary column (id. 0.22 mm, 0.25 μm film thickness, SGE, USA) – for details see Mraz and Pickova.[Citation15] The peaks were identified by comparing the retention times of samples to those of the standard mixture GLC-68-A (Nu-Chek Prep, Elysian, USA).
Lipid and protein oxidation analysis
Lipid oxidation was analyzed as thiobarbituric acid reactive substances (TBARS), based on the method described by Miller.[Citation18] Results were expressed as equivalents to malondialdehyde (MDA) in µg/g. Protein oxidation was assessed as a carbonyl content which was measured spectrophotometrically according to the method of Oliver, Ahn[Citation19] at 370 nm (Infinite M200, Tecan).
Statistics and calculations
Averages, standard deviations and FA percentages were calculated in MS Excel, and statistical analyses were performed using the Statistica CZ 12 software package. One-way analysis of variance (ANOVA) and Tukey’s HSD for determination of differences among treatment and within different time points was performed (p ≤ .05). Furthermore, all measured attributes of fish meat quality in this study – lipids, fatty acid content and lipids and proteins oxidation level – were evaluated by multivariate statistical analysis. The lipid classes ratio and FAs content stood as variables. Time, MDA, and carbonyl values were taken as non-nominal ecological variables. The obtained data of LC and FA profile, lipid and protein oxidation values were statistically evaluated using the statistics software CANOCO 4.5 (Biometrics, Plant Research International, Wageningen UR, Netherlands) for the DCA, PCA, RDA, and Monte-Carlo permutation test analyses – for details see Schneedorferova, Tomcala.[Citation20] The Monte-Carlo permutation test was used for statistical significance determination.
Results
Lipid composition and the oxidative ratio of fresh minced carp meat
The main LC in common carp mince were triacylglycerols (TAGs), reaching almost 70% of total lipid content, followed by phospholipids (PLs) and free fatty acids (FFAs) (). The level of essential fatty acid 18:2 ω-6 was more than 7% and the level of 18:3 ω-3 reached almost 2%. Together, the EPA and DHA were around 2% of the total detected FAs (.). Additionally, the ω-3/ω-6 ratio was 0.63 ± 0.05 (.), the MDA value was 0.05 ± 0.01 µg/g (), and the recorded level of carbonyl was 4.20 ± 0.93 mM/g ().
Table 1. Fatty acid composition (%) of the total identified fatty acids of minced carp meat during the frozen storage period
Figure 1. Amount (%) of lipid classes in minced carp during the storage period (n = 6). The different uppercase letters indicate significant differences (p ≤ .05) within a treatment (N – normal or IND – induced) between time points for the same treatment. PL – polar lipids, DAG – diacylglycerols, Chol – cholesterols, FFA – free fatty acids, TAG – triacylglycerols.
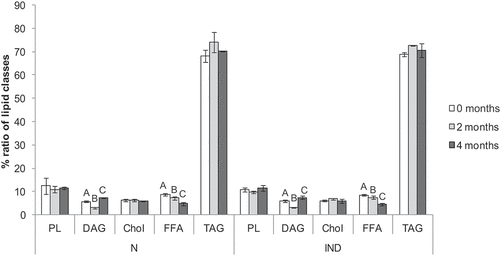
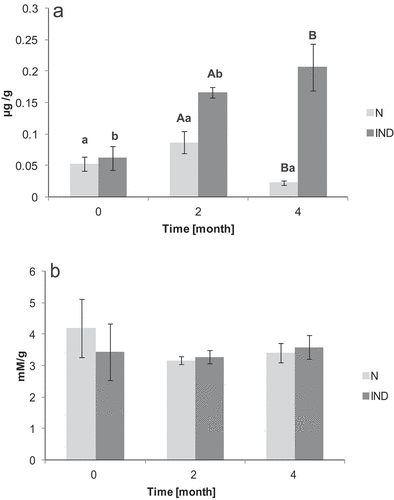
Figure 2. Amount of MDA (µg/g; panel A) and carbonyl (mM/g; panel B) in minced carp during the storage period (n = 6). An uppercase letters represent significant differences between N and IND treatments at the same time point (p ≤ .05). Lowercase letters represent differences between time points for the same treatment (p ≤ .05).
Effect of time in standard storage conditions
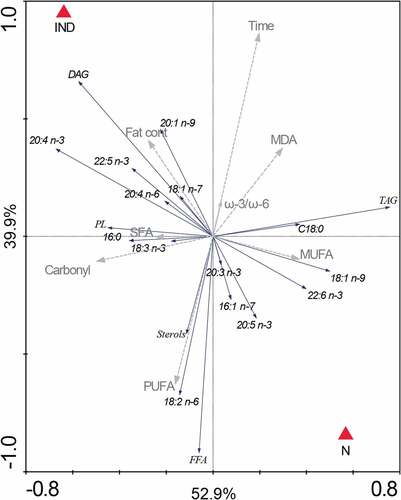
RDA analysis of all monitored attributes did not reveal differences between the obtained dataset from the starting point and after 4 months of storage at −20°C (P = .335). Although the statistical analysis did not reveal significant differences in overall data, some particular values show substantial differences. For instance, the fluctuation of diacylglycerols (DAGs) content, which drops after 2 months of storage from 5.80 ± 0.31% to 3.11 ± 0.1% and subsequently rises to 7.35 ± 0.07% after 4 months of storage (). Also apparent is a reduction of FFAs content from 8.74 ± 0.46% to 7.22 ± 0.15%, and 4.81 ± 0.60% after 2 and 4 months respectively. The content and ratio of the particular FA remained unchanged throughout the experiment (.), as well as the amount of carbonyl (). Therefore no significant protein oxidation occurred. On the other hand, the MDA values increased significantly after 2 months of storage but decreased afterward to an even lower level than in fresh minced carp meat ().
Comparison of normal and oxidation induced storage
All obtained data were used for the multivariate statistical analysis. The RDA revealed a significant difference between the normal stored (N) and oxidation induced (IND) group (P = .001) ().The diagram in . represents a 2D model of spatial data distribution explaining 52.9% of data variables on the x-axis and 39.9% on the y-axis. The red triangles are for a particular carp minced meat treatment. The gray dashed arrows are for non-nominal environmental variables and the blue solid for the classical variables. The direction of the arrow and its length determine the degree of influence of a particular variable on the data segregation (in our case triangles). The diagram clearly shows that the main non-nominal variable is Time. The influence of storage time mainly shifts the data obtained from IND samples. The rise of MDA values follows the Time arrow in the case of IND (). The opposite position of the FFA arrow against the Time arrow shows the drop of FFA in both N and IND, but although it is higher on the IND group (for details see . and ). In both variants, the amount of DAG drops after the 2 months storage period, but in the 4th month, a raise was detected – mainly in the IND group (). The influence of an oxidative agent, copper chloride, is supported not only by the MDA values (an increase from 0.06 to 0.27 µg/g MDA) () but also by the changes in the amounts of 18:2 ω-6. demonstrates a decreasing trend of the amount of this essential fatty acid after 4 months of storage in oxidatively induced samples. On the other hand, the level of 18:2 ω-6 in N samples remains stable. There was no significant difference between N and IND groups in carbonyl levels over the period [from time 0 (P = .18) to 2nd month (P = .86) and 4th month (P = .37)] ().
Discussion
The total fat content of fresh minced carp meat was around 10%, which is comparable to the muscle fat content of common carp (Cyprinius carpio) coming from semi-intensive systems and fed cereals or pellets.[Citation21] As expected, with fatty fish like carp, the main lipid class were TAGs followed by PLs. A similar lipid class ratio was also reported for common carp fillets by Mraz and Pickova[Citation15] and Hematyar, Masilko.[Citation22] Sterol levels were found to be between 5.9–7.3% of the lipid fraction, which was slightly lower than those found by Zajic, Mraz.[Citation21] The content of FFA in the fresh meat varied from 7.2–8.3% of total lipids. These values were higher than those found in the total lipid fraction in whole fillets of common carp (3.2% and 4.7%),[Citation22] but can be explained by the higher enzymatic activity in the minced flesh compared to the whole fillet. For example, Siddaiah, Reddy[Citation23] found 7.7% FFA of total lipids in mince from silver carp (Hypophthalmicthys molitrix).
One of the most valuable nutritional benefits provided by fish meat is a high content of essential fatty acids and the ratio of ω-3/ω-6 PUFAs.[Citation6] Previous studies dealing with the FA composition of common carp fillets have shown favorable amounts of essential fatty acids, PUFAs and the ω-3/ω-6 ratio.[Citation15,Citation22,Citation24] In the case of essential fatty acids and PUFAs, the recorded data in our study are comparable. However, the ω-3/ω-6 ratio from minced carp meat shows lower results in comparison to the fillets – varying from 0.87 to 1.25 in previous studies.[Citation21,Citation22,Citation24] The explanation for this is provided by the Mraz and Pickova study,[Citation15] which focuses on the comparison of a different part of the common carp body. The recorded ω-3/ω-6 ratio in white muscles was 1.01 ± 0.2. On the other hand, the value in the abdominal wall was significantly lower (0.47 ± 0.09). Since the minced meat is a combination of both of these flesh types, our results fully align with the previously obtained data. Based on these data, we can consider that the found ω-3/ω-6 ratio was higher than 0.25, and the EPA+DHA content was approximately 2% – pointing to a good nutritional value of fresh minced carp meat.
In our study, the storage time did not affect fat content. This result is similar to those found in studies by Majumdar, Deb[Citation25] which dealt with minced meat of silver carp (Hypophthalmichtys molitrix), and Hematyar, Masilko[Citation22] which focused on common carp (Cyprius caprio) fillets. Interestingly, in our current study, the FFA showed a decrease after 2 and 4 months of storage. In general, it would be expected that the FFA content increases progressively due to the enzymatic activity. For example, Majumdar, Deb[Citation25] and Reddy and Bhandary[Citation26] found increasing values of FFA in washed and unwashed mince from silver carp after just 30 days of frozen storage. However, the decrease found in the current study could be due to faster oxidation of the released FFA by mincing compared to their rise by enzymatic degradation of glycerolipids. The fluctuating levels of DAG class from 5.8% to 3.1% and subsequently to 7.35% during the storage is enigmatic. The previous study only shows a decreasing trend of DAG class in common carp fillets.[Citation22]
The FA composition did not change during 4 months of storage, indicating low lipid oxidation. However, some trends could be observed (). Actually, no changes in FA composition were reported by Hematyar, Masilko[Citation22] in common carp fillets during the storage at −20°C for 6 months. Also, for minced meat from horse mackerel (Trachurus trachurus)[Citation12] and rainbow trout (Oncorhynchus mykiss),[Citation11] only minor changes of FA composition during 90 days of frozen storage were found. However, in minced meat from sea bream (Dicentrarchus labrax) and sea bass (Sparus aurata), a significant decrease in the amount of PUFAs was found,[Citation11] indicating different storage stability for minced meat from different species. The species-dependent thermal stability of PUFA was also reported by Schneedorferova, Tomcala.[Citation20]
In the N group, the MDA level increased significantly between the starting time and 2 months. However, after that, the MDA values decreased. A very similar phenomenon was observed by Majumdar, Deb[Citation25] in minced silver carp (Hypophthalmichtys molitrix) and by Hematyar, Masilko[Citation22] in common carp (Cyprius caprio) fillet. The reason for this might be similar to the FFA – a further reaction or decomposition of the MDA. Primary and secondary oxidation products will react further to more stable volatile and nonvolatile compounds.[Citation1,Citation27]
Similar to our results, Hematyar, Masilko[Citation22] showed almost no changes in MDA levels in common carp fillets. On the other hand, in minced silver carp meat, the MDA level increases by more than double – from 0.42 to 1.23 µg/g – during 6 months of storage.[Citation25] Earlier it was shown that an oxidation value of around 2 µg/g is connected with an off-flavor which can be detected by the human senses.[Citation28] Therefore, we can conclude that our recorded MDA values always stayed below the suggested detectable levels throughout the storage period.
Also, with protein oxidation, an unexpected trend of decrease carbonyls during the storage period after 2 months was found. Whereas at the end of the storage time, values were similar to the start levels. In general, an increase in protein oxidation during storage has been observed for common carp fillet[Citation22] and minced Atlantic mackerel (Scomber scombrus).[Citation29,Citation30] The trend for decreasing carbonyls could be due to a reaction of the protein carbonyls with other cellular molecules during storage for instance carbohydrates, lipids and nucleophilic compounds – which has been discussed earlier for Atlantic mackerel mince.[Citation29] Nevertheless, as the values at the end of storage time after 4 months were similar to the values at the start we can conclude that protein oxidation was not an issue during frozen storage of minced carp meat.
Most of the trends which could be observed in the N group become more eminent or even significant in the IND group. The most descriptive attribute, in this case, is the level of lipid oxidation, which was 4.5 times higher after 4 months of storage time. The very same results were achieved by the application of copper chloride to the cuttlefish (Sepia pharaonis) paste during the freeze-thaw cycles[Citation31] and during the salting time of cod (Gadus morhua).[Citation32] All these results show that copper chloride represents a good tool for enhancing lipid oxidation in fish products.
Conclusion
In opposition to earlier findings on mechanically separated fish mince from gilthead sea bream and sea bass, our results show that carp mince has higher storage stability and lower sensitivity to oxidation and enzymatic degradation, i.e. despite a higher fat content. The mince showed to contain more than 9% of essential FA, approximately 2% of combined EPA and DHA and had an ω-3/ω-6 ratio of around 0.6. These findings indicate that minced carp meat is a good source of the mentioned compounds. Besides this, the FA composition was not affected by the frozen storage, thus the meat seems to be a very good candidate for further processing for human consumption. A normally unobservable start of oxidation already in the early storage period described by the significantly increased MDA level could be seen in the IND group. Hence inappropriate storage conditions could be detected by MDA levels. Determination of the FA content focused on ω-3/ω-6 ration, essential fatty acids, PUFAs and the oxidation stability of minced carp meat is the first step to prove the nutritional value of this underestimated by-product. Furthermore, introducing minced carp meat and its products to the food industry could increase the economic sustainability of carp aquaculture.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Samples, S. Oxidation and Antioxidants in Fish and Meat from Farm to Fork. In Food Industry; Muzzalupo, I., Ed.; Tech: Rijeka Croatia, 2013; pp 115–144.
- Williams, C. M. Dietary Fatty Acids and Human Health. Annales De Zootechnie. 2000, 49(3), 165–180. DOI: 10.1051/animres:2000116.
- Adamkova, V.; Kacer, P.; Mraz, J.; Suchanek, P.; Pickova, J.; Králova Lesná, I.; Skibova, J.; Kozak, P.; Maratka, V. The Consumption of the Carp Meat and Plasma Lipids in Secondary Prevention in the Heart Ischemic Disease Patients. Neuroendocrinol. Letters. 2011, 32, 17–20.
- Calder, P. C.; Yaqoob, P. Understanding Omega-3 Polyunsaturated Fatty Acids. Postgraduate Med. 2009, 121(6), 148–157. DOI: 10.3810/pgm.2009.11.2083.
- Simopoulos, A. P. The Importance of the Ratio of Omega-6/omega-3 Essential Fatty Acids. Biomed. Pharmacother. 2002, 56(8), 365–379. DOI: 10.1016/S0753-3322(02)00253-6.
- Simopoulos, A. P. Evolutionary Aspects of Diet, Essential Fatty Acids and Cardiovascular Disease. Eur. Heart J. Suppl. 2001, 3(D), D8–D21. DOI: 10.1016/S1520-765X(01)90113-0.
- Mraz, J.; Zajic, T.; Pickova, J. Culture of Common Carp (Cyprinus Carpio) with Defined Flesh Quality for Prevention of Cardiovascular Diseases Using Finishing Feeding Strategy. Neuroendocrinol. Letters. 2012, 33, 60–67.
- Mraz, J.; Máchová, J.; Kozák, P.; Pickova, J. Lipid Content and Composition in Common Carp - Optimization of N-3 Fatty Acids in Different Pond Production Systems. J. Appl. Ichthyol. 2012, 28(2), 238–244.
- Zheng, X.; Seiliez, I.; Hastings, N.; Tocher, D. R.; Panserat, S.; Dickson, C. A.; Bergot, P.; Teale, A. J. Characterization and Comparison of Fatty Acyl Delta 6 Desaturase cDNAs from Freshwater and Marine Teleost Fish Species. Comp. Biochem. Physiol. B-Biochem. Mol. Biol. 2004, 139(2), 269–279.
- Sampels, S.; Zajic, T.; Mraz, J. Increasing the Omega-3 Content of Traditional Meat Products by the Addition of an Underutilised By-Product from Fish Processing. Czech J. Food Sci. 2015, 33(5), 431–440. DOI: 10.17221/35/2015-CJFS.
- Secci, G.; Borgogno, M.; Lupi, P.; Rossi, S.; Paci, G.; Mancini, S.; Bonelli, A.; Parisi, G. Effect of Mechanical Separation Process on Lipid Oxidation in European Aquacultured Sea Bass, Gilthead Sea Bream, and Rainbow Trout Products. Food Control. 2016, 67, 75–81. DOI: 10.1016/j.foodcont.2016.02.033.
- Secci, G.; Borgogno, M.; Mancini, S.; Paci, G.; Parisi, G. Mechanical Separation Process for the Value Enhancement of Atlantic Horse Mackerel (Trachurus Trachurus), a Discard Fish. Innovative Food Sci. Emerg. Technol. 2017, 39, 13–18. DOI: 10.1016/j.ifset.2016.10.018.
- Boyer, R.; McKinney, J. Food Storage Guidelines for Consumers; Boyer, R, ed; Virginia Cooperative Extension: Virginia, 2018.
- Hara, A.; Radin, N. S. Lipid Extraction of Tissues with a Low Toxicity Solvent. Anal. Biochem. 1978, 90(1), 420–426. DOI: 10.1016/0003-2697(78)90046-5.
- Mraz, J.; Pickova, J. Differences between Lipid Content and Composition of Different Parts of Fillets from Crossbred Farmed Carp (Cyprinus Carpio). Fish Physiol. Biochem. 2009, 35(4), 615–623. DOI: 10.1007/s10695-008-9291-5.
- Olsen, R. E.; Henderson, R. J. The Rapid Analysis of Neutral and Polar Marine Lipids Using Double-development Hptlc and Scanning Densitometry. J. Exp. Marine Biol. Ecol. 1989, 129(2), 189–197. DOI: 10.1016/0022-0981(89)90056-7.
- Appelqvist, L. A. Rapid Methods of Lipid Extraction and Fatty Acid Methyl Ester Preparation for Seed and Leaf Tissue with Special Remarks on Preventing Accumulation of Lipid Contaminants. Arkiv for Kemi. 1968, 28(6), 551-+.
- Miller, D. D. Food Chemistry, a Laboratory Manual; Wiley Interscience: New York, 1998.
- Oliver, C. N.; Ahn, B. W.; Moerman, E. J.; Goldstein, S.; Stadtman, E. R. Age-related-changes in Oxidized Proteins. J. Biol. Chem. 1987, 262(12), 5488–5491.
- Schneedorferova, I.; Tomcala, A.; Valterova, I. Effect of Heat Treatment on the N-3/n-6 Ratio and Content of Polyunsaturated Fatty Acids in Fish Tissues. Food Chem. 2015, 176, 205–211. DOI: 10.1016/j.foodchem.2014.12.058.
- Zajic, T.; Mraz, J.; Sampels, S.; Pickova, J. Fillet Quality Changes as a Result of Purging of Common Carp (Cyprinus Carpio L.) With Special Regard to Weight Loss and Lipid Profile. Aquaculture. 2013, 400, 111–119. DOI: 10.1016/j.aquaculture.2013.03.004.
- Hematyar, N.; Masilko, J.; Mraz, J; Sampels, S. Nutritional Quality, Oxidation, and Sensory Parameters in Fillets of Common Carp (Cyprinus Carpio L.) Influenced by Frozen Storage (−20 Degrees C). J. Food Process. Preserv. 2018, 42(5).
- Siddaiah, D.; Sagar Reddy, G. V.; Raju, C. V.; Chandrasekhar, T. C. Changes in Lipids, Proteins and Kamaboko Forming Ability of Silver Carp (Hypophthalmichthys Molitrix) Mince during Frozen Storage. Food Res. Int. 2001, 34(1), 47–53.
- Tenyang, N.; Tiencheu, B.; Tonfack Djikeng, F.; Morfor, A. T.; Womeni, H. M. Alteration of the Lipid of Red Carp (Cyprinus Carpio) during Frozen Storage. Food Sci. Nutr. 2019, 7(4), 1371–1378.
- Majumdar, R. K.; DEB, S.; Dhar, B.; Priyadarshini, B. M. Chemical Changes in Washed Mince of Silver Carp (Hypophthalmichthys Molitrix) during Frozen Storage at −20°C with or without Cryoprotectants. J. Food Process. Preserv. 2013, 37(5), 952–961.
- Reddy, A. M.; Bhandary, M. H. Biochemical and Sensory Acceptability Changes of Fish Cutlet Prepared from Filleting Waste of Reef Cod (Epinephelus Chlorostigma) during Frozen Storage. J. Food Process. Preserv. 2015, 39(4), 369–375. DOI: 10.1111/jfpp.12241.
- Undeland, I.; Ekstrand, B.; Lingnert, H. Lipid Oxidation in Minced Herring (Clupea Harengus) during Frozen Storage. Effect of Washing and Precooking. J. Agric. Food Chem. 1998, 46(6), 2319–2328. DOI: 10.1021/jf9707795.
- Younathan, M. T.; Watts, B. M. Relationship of Meat Pigments to Lipid Oxidation. Food Technol. 1959, 24(6), 728–734.
- Babakhani, A.; Farvin, K. H. S.; Jacobsen, C. Antioxidative Effect of Seaweed Extracts in Chilled Storage of Minced Atlantic Mackerel (Scomber Scombrus): Effect on Lipid and Protein Oxidation. Food Bioprocess. Technol. 2016, 9(2), 352–364. DOI: 10.1007/s11947-015-1630-9.
- Baron, C. P.; Kjærsgård, I. V. H.; Jessen, F.; Jacobsen, C. Protein and Lipid Oxidation during Frozen Storage of Rainbow Trout (Oncorhynchus Mykiss). J. Agric. Food Chem. 2007, 55(20), 8118–8125.
- Thanonkaew, A.; Benjakul, S.; Visessanguan, W.; Decker, E. A. The Effect of Metal Ions on Lipid Oxidation, Colour and Physicochemical Properties of Cuttlefish (Sepia Pharaonis) Subjected to Multiple freeze-thaw Cycles. Food Chem. 2006, 95(4), 591–599.
- Lauritzen, K. Quality of Salted Cod (Gadus Marhus) as Influenced by Raw Mateial and Salt Composition. In Norwegian College of Fishery Science; University of Tromso: Tromso. p. 61, 2004.