ABSTRACT
Response surface methodology was used to optimize the fermentation factors of blackened jujube vinegar (BJV). Physicochemical properties, antioxidant activity, sensory evaluation, and volatile compounds were studied with the methods of high-performance liquid chromatography (HPLC) and gas chromatography-mass spectrometry (GC-MS). The results indicated that the optimal fermentation conditions was the acetic acid inoculation amount of 15%, the fermentation temperature of 30.44°C, and the initial alcohol content of 6.45% vol. During fermentation processing, there was a delay in the start of BJV fermentation and the fermentation rate was lower than red jujube vinegar (RJV). There was a significant difference in cyclic adenosine monophosphate (cAMP) content between these two (P < .05), and BJV is 3.55 times that of RJV. The content of polysaccharides in BJV and RJV decreased significantly and the trend was the same. The 5-Hydroxymethylfurfural (5-HMF) content of BJV decreased rapidly with the process and reached a very low level finally. The antioxidant activity of BJV was significantly higher than that of RJV in terms of 2,20 -azinobis (3-ethylbenzothiazoline6-sulfonicacid) (ABTS) and ferric reducing antioxidant power (FRAP) (P < .05). From sensory evaluation, the aroma and taste of BJV were better than those of RJV. There were no significant differences in the categories and quantities of volatile compounds between them. Volatile acid of BJV was less than that of RJV with the value of 72.91% and 81.15%, respectively, meanwhile, volatile esters of BJV (19.11%) was significantly higher than that of RJV (9.28%), for these reasons, sensory of BJV was softer, richer ester fragrance, and RJV was more stimulating.
Introduction
Jujube (Ziziphus Jujuba Mill.) is one of the most distinctive national fruits in China, accounting for more than 98% of the world’s planting area and output and nearly 100% of world trade.[Citation1,Citation2] Jujube is rich in vitamins, polysaccharides, organic acids, amino acids, minerals, and cyclic adenosine, which make jujubes have a variety of health effects, such as antioxidant, anti-inflammatory, anti-cancerous, anti-insomnia.[Citation3–6] Consequently, jujubes and their products have been popular due to their high nutritional value and medicinal properties.[Citation7] However, the research and development of high value-added products of jujubes is in the initial stage, and the utilization rate of raw materials in the jujube processing industry is still low.[Citation8]
Blackened jujube, obtained from red jujube by a blackening processing at high humidity and temperature,[Citation9] has been considered as an innovative processed product in recent years after the popularity gained from the development of black garlic.[Citation10,Citation11] After the blackening process, the appearance and chemical composition of the jujube changed significantly. The content of sucrose decreased and reducing sugars, such as fructose and glucose increased. meanwhile, the content of functional components, such as total phenolic, 5-hydroxymethylfurfural (5-HMF), triterpene acids (include ursolic acid, oleanolic acid, and betulinic acid) and amino acids increased.[Citation9,Citation10] Besides, the increase of 2-acetylfuran, furfural and their derivatives provided caramel flavor to blackened jujube. Compared with red jujube, blackened jujube has a higher nutritional value.[Citation10]
Furthermore, natural foods and additives fermented by microorganisms are becoming more and more popular.[Citation12] At present, many fermentation technologies have been applied to develop novel jujube products, such as probiotic juice[Citation13,Citation14] and vinegar,[Citation15,Citation16] as good alternatives to the fresh fruit while retaining the functional characteristics of the raw material. In addition, vinegar contains many organic acids and functional ingredients from raw materials, which make it have antioxidant, antitumor, antibacterial, antidiabetic, and other health effects.[Citation8,Citation17] At present, the fermentation of red jujube vinegar has been widely studied, including the fermentation process, functional components, flavor, antioxidant capacity, and bioactivities.[Citation15,Citation16,Citation18–21] But the fermentation of blackened jujube vinegar (BJV) and related reports are very few. Actually, blackened jujubes are more suitable for acetic acid fermentation with a unique flavor than red jujubes due to their high sugar content and better nutritional composition,[Citation22] which makes the BJV contain the double nutritional value of vinegar and blackened jujube.
At present, the fermentation technology of fruit vinegar has been widely studied,[Citation23–25] however, the one-step fermentation method applied in fruit vinegar production is not well explored. Compared to the traditional “two-step fermentation method” which involves the process of alcohol fermentation and acetic acid fermentation, the ‘one-step’ fermentation has simple operation, short fermentation period, low contamination rate of bacteria, and it is convenient to control the parameters in the fermentation process. In the meantime, it can better retain the original fruit aroma.[Citation26–28] The fermentation process of fruit vinegar is accompanied by the reduction and increase of certain volatile gas components, polysaccharides, phenols, acids, and other substances. The changes of these substances determine the taste, flavor, and antioxidant activity of fruit vinegar. It is very important to study the changes of physical and chemical properties during fermentation.[Citation29] In this study, Black jujube vinegar (BJV) was produced by fermenting blackened jujube through one-step fermentation method. The aims of this work were to: 1) optimize the fermentation factors; 2) study the changes of physicochemical properties during the fermentation of jujube vinegar; and 3) compare the antioxidant activity and volatile compounds of BJV and RJV. The development of blackened jujube vinegar is highly innovative, which improves its commodity value and is of great significance to the promotion of high value-added products in the jujube processing industry.
Materials and methods
Reagents
Acetobacter aceti (HuNiang 1.01) was obtained from Shanghai Brewing Research Institute (Shanghai, China). 2,2-azinobis (3-ethylbenzothia zone-6-sulfonic acid) diammonium salt (ABTS•+) was from Hefei Bomei Biological Technology Co. LTD (Anhui, China). 2,2’-diphenyl-1-picrylhydrazyl (DPPH•) was obtained from Shanghai Hualan Chemical Technology Co. Ltd. (Shanghai, China). 6-hydroxy-2,5,7,8-tetramethylchroman-2-carboxylic acid (Trolox) was obtained from Shanghai Yuanye Bio-Technology Co., Ltd. China (Shanghai, China). 3’,5’-cyclic AMP was obtained from Shanghai Yuanye Bio-Technology Co., Ltd. China (Shanghai, China). Acetonitrile and methanol were both of HPLC grade and were purchased from Shandong Yu Wang He Tianxia New Material Co. Ltd., (Yucheng, China). In this study, unless otherwise specified, all other chemicals used were analytical grade.
Chemicals and materials
The Chemicals used in this experiment are shown in . Red jujube (cultivar Huizao) was purchased from market of Taian, Shandong, China and produced from Xinjiang, China. Blackened jujube was processed following the method of Gao et al.[Citation9] The main composition of the red jujube and blackened jujube samples was listed in .
Table 1. Contents of the ingredients in samples of red jujube and blackened jujube
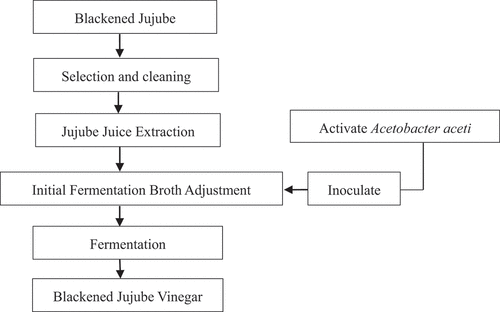
Fermentation process of BJV
The fermentation process of BJV was explained below and shown in . The washed blackened jujube was soaked in four times distilled water, in a water bath (HH-6, Jiangsu Ronghua Instrument Co., Ltd.) and heat it up to 45°C. Then, the mixture was allowed to stand for 4 h after the pectinase (20 mL/100 kg) was added. The jujube juice was raised to 80°C for sterilization. When the juice cooled down to 30°C, it was added SO2 and make it 0.05%, and adjusted the initial alcohol contents to 6% vol with edible alcohol. Then, the initial fermentation broth was inoculated with Acetobacter aceti. The broth was placed in an incubator (101–2ES, Beijing Yongguangming Medical Instrument Factory, China) at constant temperature and humidity. During the fermentation time, the broth had been kept ventilated and the air volume is 1:0.1.
Experimental design
Thorough acetic acid fermentation of the juice can obtain the fruit vinegar with the largest total acid content and a comprehensive understanding of the fermentation process. We have identified three factors that significantly affect the total acid content in single-factor experiments as the inoculation amount of Acetobacter aceti (HuNiang 1.01) (A), the content of initial alcohol (B), and fermentation temperature (C). This three adopted in the response surface experiment according to the Box-Behnken design principles, acetic acid yield (Y) was the response value. Response surface design with 3 factors and 3 levels was carried out. The optimal acetic acid fermentation parameters were obtained by regression equation. The fermentation process repeated three times. Factors and levels of response surface design showed in .
Table 2. Factors and levels of Box-Behnken design
Total acid content
The content of total acid measured referred to Chinese standard of Method for analysis of hygienic standard of vinegar (GB/T 5009.41–2003). Briefly, 10 mL sample was diluted 10 times in a 100 mL volumetric flask, 20 mL of the diluent and 60 mL deionized water placed in a 200 mL beaker which put on a magnetic stirrer, then titrated with NaOH standard solution [c(NaOH) = 0.050 mol/L] until the pH meter indicated pH 8.2, recorded the consumption (mL) of the NaOH standard titration solution in samples and minors the consumption in blank, the content of total acid was calculated (g/100 mL).
cAMP
The methods of determination of cAMP were referred to Sun et al.[Citation10] with slight modification in sample preparation, and the process is as follows, the vinegar (10.0 mL) was dried with lyophilizer (ALPHA2-4, Henan Brother Instrument Equipment Co., China.), then the sample was dissolved to volume of 10.0 mL. The solution (10.0 mL) was placed in an ultrasound (KQ2200DE, Jiangsu Kunshan Co., China) for 30 min at 60°C. The solution was at 66.7 Hz for 5 min, then the supernatant was separated and filtered through a 0.22 μm PTFE.
5-HMF
The methods of determination of 5-HMF were referred from Gao et al.[Citation9] with slight modification in sample preparation, and the process is as follows, the vinegar (20.0 mL) was dried with lyophilizer (ALPHA2-4, Henan Brother Instrument Equipment Co., China.), and 0.734 g sample was homogenized with 10 mL distilled water. Then, the solution was separated and filtered through a 0.22 μm PTFE filter for testing.
Crude polysaccharides
The methods of extraction and determination of polysaccharides were referred to Zhao[Citation30] et al. with slightly modified. Specifically, 15.0 mL of samples were mixed with 60 mL of ethanol. The mixture was centrifuged (TG16, Changsha Yingtai Co., China) at 50 Hz for 5 min, the supernatant was separated, and the residue was washed with ethanol (80%) for 3 times. Then, the residue was dissolved with water and the solution was filled to a constant volume into 250 mL volumetric flask. 0.2 mL sample solution was mixed with 2 mL phenol (50 g/L) and 8 mL concentrated sulfuric acid. The mixture was determined at 485 nm after 20 min interval in a boiling water bath.
DPPH scavenging activity
The DPPH scavenging activity was evaluated according to the method described by Wang[Citation31] et al. with minor modifications. Briefly, 0.1 mL of the standard Trolox solution (0.00 ~ 0.30 mg/mL) or vinegar samples were mixed with 5 mL of 0.10 mmol/L DPPH (dissolved in ethanol), and then, the mixture was kept in the dark for 30 min and the absorbance at 517 nm was measured. The results were expressed as mg Trolox equivalent/mL (mg TE/mL).
ABTS scavenging activity
The ABTS scavenging ability experiment was carried out according to the method of Re[Citation32] et al. Briefly, ABTS solution was prepared by mixing 10 mL of 7.00 mmol/L ABTS solution with 10 mL of 2.45 mmol/L K2S2O8 solution. This mixture was allowed to stand for 12–16 h at room temperature in the dark until reaching a stable oxidative state. The ABTS solution was diluted in 80% ethanol to an absorbance of 0.700 ± 0.050 at 734 nm. 5 mL of the ABTS solution and 0.2 mL of the standard Trolox solution (0.00–0.10 mg/mL) or vinegar samples were mixed, and the absorbance was measured at 734 nm after 8 min interval with a blank solution. The results were expressed as mg Trolox equivalent/mL (mg TE/mL).
Ferric ion reducing antioxidant activity
The Ferric ion reducing antioxidant activity (FRAP) was carried out following the procedure described by Feng et al.[Citation33] with slight modifications. Specifically, 0.6 ml sample solution or the Trolox standard solution (0.00–0.50 mg/mL) was mixed with 2.5 ml 0.20 mol/L phosphate buffer solution and 2.5 ml potassium ferricyanide solution (1% W/V). The mixture was incubated at 50°C in a water bath for 20 min. Then, 2.5 mL trichloroacetic acid (10% W/V) solution was added to the mixture, shaken, and centrifuged. 2.5 mL of the supernatant was mixed with 2.5 mL of distilled water and 2.5 mL of ferric chloride solution (0.1% W/V). The absorbance of the mixture was measured at 700 nm after 10 min. The results were expressed as mg Trolox equivalent/mL (mg TE/mL).
Sensory evaluation
The quantitative sensory evaluation was performed following the procedures described by Tao[Citation34] et al. with some modifications. A well-trained sensory panel consisting of 11 assessors majoring at food science and technology (six females/five males aged between 24 and 42 years) were invited for descriptive sensory analysis. Before the sensory evaluation, the assessors attended a discussion and a training session (1 h each). The vinegar was evaluated in four aspects: color, aroma, taste, and appearance. Then, the panel was trained on the same samples. The evaluation index of sensory is listed in .
Table 3. Sensory score of jujube-vinegar
During training and evaluation, approximately 15 mL jujube vinegar were randomly served to assessors at room temperature. Water was provided for palate cleansing for the assessors. Training and evaluation were conducted in accordance with the International Organization for Standardization (I.S.O., 1993), and carried out in a sensory laboratory fulfilling the requirements provided by the American Society for Testing and Materials.
Volatile gas components
The methods of samples processing were referred to Butkhup et al.,[Citation35] and the methods of determination of volatile compounds were referred to Sun.[Citation36]
Statistical analysis
All the experiments were performed in triplicate. The single factor experimental data was carried out using Origin 8.0. Response surface experimental data were carried out using Design-Expert. V8.0.6.1. Duncan’s multiple-comparison tests were conducted at significance levels of α = 0.05 or α = 0.01 using SPSS 17.0 (SPSS, Inc., Chicago 1 L, USA).
Results and discussion
Model establishment and variance analysis
The experimental design and results of Box-Behnken were shown in . The regression equation of acetic acid yield (acidity Y) to independent variables inoculation amount (A), fermentation temperature (B) and initial alcohol content (C) was obtained as following Eqs. (1).
Table 4. Factors and levels of Box-Behnken experiments design
Y = 4.99 + 0.26*A + 0.045*B + 0.084*C + 0.080*A*B + 0.14*A*C + 0.095*B*C-0.15*A2-0.38*B2-0.27*C2(1)
It can be seen from the equation that the coefficients of the quadratic term were all negative, so the total acid content had a maximum point, fermentation conditions can be optimized for analysis. It can be seen from the results of the analysis of variance of the regression model in , the P < .0001 of the response surface model indicates that it was extremely significant. The lack of fit (P = .5457 > 0.05) was not significant, indicating that the model fitted well with the experimental values. The correction coefficient R2Adj = 0.9404 indicated that only 5.96% of the variation cannot be explained by the regression equation. The CV value was 1.82, indicating that the model equation can well reflect the true experimental value. The correlation coefficient R2 = 97.39%>90% showed that there was a high correlation between the predicted value and the actual value. The model can be used to analyze and predict acidity production.
Table 5. Analysis of variance of regression model
The P values of coefficient A and C of the model equation were all less than 0.05, indicating that the inoculation amount and the initial alcohol content had significant effects on the results, and the order of the effects of each factor of the first term on the total acid content was A (inoculation amount) > C (initial alcohol content) > B (temperature). The quadratic terms A2, B2, and C2 were all significant, indicating that the three factors of Acetobacter aceti inoculation amount, fermentation temperature, and initial alcohol degree were not simply linear with the response value, and the response surface effect was significant.
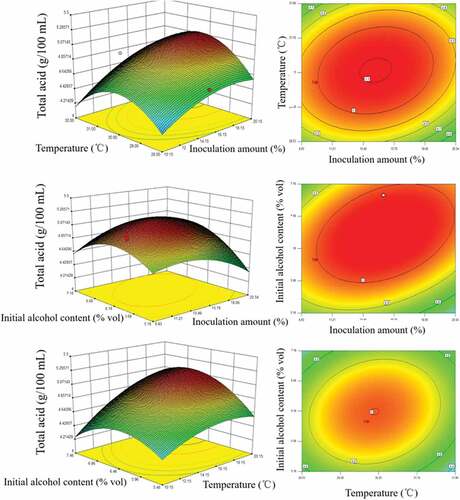
Analysis of interaction of factors
is a response surface curve diagram of the influence of various factors on the total acid content of BJV, and the interaction of various factors in the acetic acid fermentation process can be noticed. The contour shape reflected the strength of the interaction between the factors. It can be seen from the contour lines that the shapes of AC, BC, and AB were all oval, indicating that the Acetobacter aceti inoculation amount (A), fermentation temperature (B), and initial alcohol content (C) had complex effects on the fermentation system. Both had significant interaction. Among them, the interaction between the amount of inoculation and the initial alcohol level was highly obvious, which was consistent with the results of the analysis of variance.
Verification test
Through response surface analysis, the best process conditions for BJV fermentation were obtained as follows: inoculation amount 15%, fermentation temperature 30.44°C, and initial alcohol content 6.45% vol. The theoretical maximum acidity predicted by the model was 5.16 g/100 mL. The total acid content of BJV fermented under these conditions measured was 5.10 g/100 mL, which was in good agreement with the prediction model, indicating that the BJV fermentation process optimized by response surface was feasible and had practical applications value.
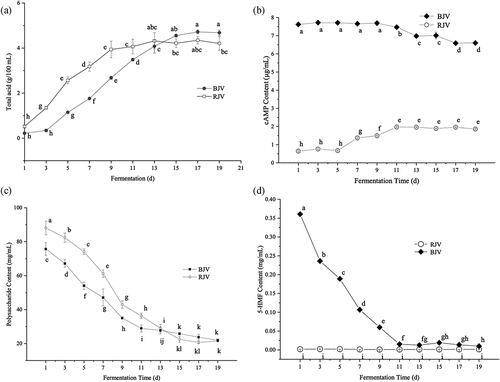
Changing comparison of total acids content on BJV and RJV
According to ), at the beginning of fermentation for 1–5 days, the total acid content of BJV increased rapidly, and the fermentation rate decreased on the fifth day. After 9 days of fermentation, the total acid content was stable with a value of 4.1 ± 1.5 g/100 mL in the end. However, there was a delay in the start of the fermentation of BJV fermentation. It was possible that the fermentation inhibitors, such as 5-HMF contained in BJV delayed the fermentation rate.[Citation37] Then, the acidity rose slowly within 1 to 3 days. The fermentation rate reached the highest on 7 to 11 days, and total acid content was maintained stable at 4.5 ± 1.2 g/100 mL after 15 days of fermentation. The fermentation rate was slower than that of RJV, however, total acid content exceeded that of RJV on the 13th day. The fermentation time of RJV was about 11 days, and BJV was about 15 days. The fermentation period of BJV was longer, but BJV produced more acid in the end.
Changing comparison of cAMP on BJV and RJV
) showed that the content of cAMP in RJV was 0.64 ± 0.02 μg/mL at the beginning of fermentation, and remained extremely low within 1–5 days, then increased to 1.97 ± 0.1 μg/mL in 5 ~ 11 days and maintained this content level until the end of fermentation. The cAMP in BJV was 7.62 ± 0.03 μg/mL at the beginning of fermentation and maintained this level for 1–9 days. After the ninth day, its content showed a slight downward trend, and dropped to 6.60 ± 0.04 μg/mL at the end of fermentation. On the whole, the cAMP content of BJV during the whole fermentation process was significantly higher than that of RJV. At the end of fermentation, the content in BJV was 3.5 times of that of RJV.
The reason may be that during the process of blackening, the long-term high temperature and high humidity environment changed the structure and composition of the jujube, reduced, or released the coupling effect of cAMP and other substances in the jujube, thereby increasing its extraction rate, as a result, its initial fermentation broth was higher than that of RJV.
At the end of BJV fermentation, the content of cAMP decreased gradually. It may be due to the lack of carbon sources, such as sugar and alcohol, Acetobacter aceti consumed other nutrients, cAMP was also used as a fermentation substrate to continue to provide Acetobacter aceti to maintain growth and metabolism, resulting in a decrease. And the increase in the content of RJV in the middle of fermentation may be caused by the contamination of yeast, Arthrobacter sp. and other that can produce cAMP in the fermentation broth.[Citation38,Citation39] The specific reasons need to be studied further.
Comparison of polysaccharide contents on BJV and RJV
Polysaccharide is an important active substance in jujube fruit. It has several health benefits, such as anti-oxidation, anti-tumor, liver protection, and lowering blood fat abilities. It is converted into acetic acid and other fermentation products as a substrate of acetic acid bacteria fermentation during the fermentation process.[Citation40] It can be seen from ) that the content of RJV in the initial fermentation broth was 75.72 ± 3.81 mg/mL. In addition, its content decreased significantly as the fermentation progressed, and its consumption rate gradually increased within 1–9 days, followed by a decrease in the rate to 21.95 ± 0.85 mg/mL at the end of fermentation. The polysaccharide content in the initial fermentation broth of BJV was 88.08 ± 4.05 mg/mL, which was higher than that of RJV. The consumption rate gradually increased within 1 ~ 13 days, and then decreased after 13 days with the final value of 21.52 ± 0.23 mg/mL. In general, the consumption trend of polysaccharides in RJV and BJV was roughly the same and they were negatively correlated with their total acid content. Their contents were similar at the end of fermentation. Both of the vinegars had a relatively high content of polysaccharides after fermentation, which helped maintaining the bioactivities of black jujube polysaccharides.
Comparison of 5-HMF contents on BJV and RJV
5-HMF has several adverse effects, such as causing cell damage, stimulating mucosal cells, and inducing cell mutations. However, some studies have also proved that it has certain antioxidant activity, inhibits tumor cells, and promotes internal circulation, which are beneficial to human body.[Citation41–44] This is a substance which food safety characteristic is controversial and may require removal from food.
It can be seen from ) that the 5-HMF content in RJV had been at a very low level during the fermentation process with a value of 0.002 ± 0.000 mg/mL throughout. The initial content of 5-HMF in BJV was 0.367 ± 0.002 mg/mL, which decreased rapidly with the fermentation process and dropped to 0.015 ± 0.02 mg/mL on the 11th day and then 0.010 ± 0.001 mg/mL in the end.
There was a significant difference in 5-HMF content in the initial fermentation broth of RJV and BJV. This was because a large amount of 5-HMF was produced by blackening process of jujube and the non-enzymatic browning of jujube during the blackening process mainly includes Maillard reaction, ascorbic acid oxidation decomposition, caramelization reaction, and polyphenol oxidation condensation, all of which produce 5-HMF.[Citation36]
In the research of biodegradation of furfural, strains, such as Saccharomyces cerevisiae H.[Citation45] and Corynebacterium glutamicum[Citation46] have a degrading effect on 5-HMF. Meyerozyma guilliermondii SC1103 can also reduce furan aldehyde to furan alcohol.[Citation47] During the fermentation of BJV, the content of 5-HMF was significantly reduced as well, indicated that the process can significantly reduce the furfural generated during blackening of jujube. This change reduced the harmful substances in vinegar and improved the quality and safety of black jujube products.
Antioxidant activity comparison on BJV and RJV
Fruit vinegar is rich in nutrients and has certain antioxidant activity.[Citation48] It can be seen from that using blackened and red jujube as raw materials, these two kinds of fermented vinegar had different antioxidant activities despite having same one-step fermentation method. The DPPH scavenging activities of BJV and RJV were 0.10 ± 0.01 mg Trolox/mL and 0.15 ± 0.02 mg Trolox/mL, respectively. The ABTS scavenging activity and FRAP reduction activity of BJV were 0.52 ± 0.03 mg Trolox/mL and 1.23 ± 0.02 mg Trolox/mL, respectively, which were significantly higher than those of RJV (ABTS 0.18 ± 0.01 mg Trolox/mL, FRAP 0.35 ± 0.01 mg Trolox/mL). Overall, BJV had stronger antioxidant activity than RJV (P < .05) in vitro.
Figure 4. DPPH, ABTS and FRAP reduction activity between BJV and RJV.
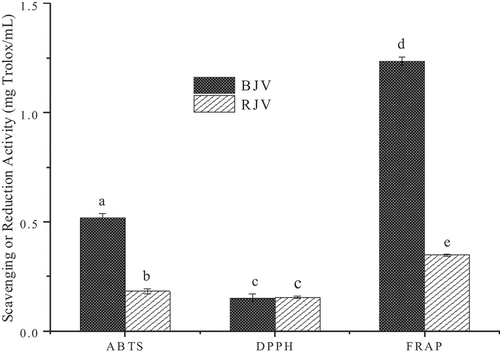
In general, the DPPH scavenging activity of fruit vinegar was positively correlated with the contents of polyphenols, flavonoids, and other substances.[Citation49,Citation50] However, the scavenging activity of BJV and RJV on DPPH was weak, which indicated that the contents of polyphenols and flavonoids were low in both jujube vinegar after fermentation. The reason of stronger scavenging activity of ABTS and the reducing activity of FRAP of BJV than those of RJV maybe due to the presence of substances like melanoidin[Citation51,Citation52] in BJV, which was produced during the blackening and fermentation processes of jujube, providing strong antioxidant activity. The specific active substance and its content need further study.
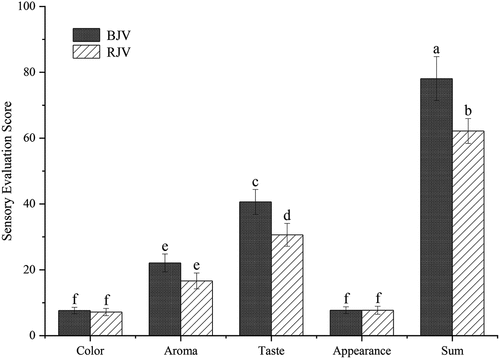
Sensory evaluation comparison on BJV and RJV
It can be seen from that the score of color and appearance of BJV and RJV are slightly different with clear and transparent colors. However, there were significant differences in aroma and taste. The aroma and taste scores of BJV are 22.1 and 40.6, respectively, possessing a typical vinegar and ester fragrance and a harmonious taste, providing a refreshing flavor to throat. The aroma and taste of RJV scored 16.6 and 30.6, respectively, showing characteristics of prominent sourness and thinner ester fragrance, slightly irritating to the throat. In summary, by comparing BJV with RJV, the color and appearance of the two can reach a better level, but BJV can be significantly better than RJV in terms of aroma and taste. The total scores of BJV and RJV were 78.1 points and 62.2 points, respectively, indicating that the overall sensory characteristics of BJV are better (P < .05).
Changing comparisons of volatile compounds on BJV and RJV
Sixteen kinds of volatile gas components were detected in the initial fermentation of the broth of BJV. As the fermentation process progressed, the types of volatile compounds continued to increase. At the 13th day, a maximum of 31 kinds of volatile compounds were detected. As the fermentation continued further, the total number of volatiles was reduced to 26 kinds in the end. On the other hand, there were 17 kinds in the initial fermentation broth of RJV, and the maximum of 24 kinds were detected on the 15th day, which maintained the amount to the end of fermentation. Therefore, the number of volatile compounds detected for BJV was more than that for RJV during the entire processing. It can be seen from that the composition and relative content of volatile components obtained in the fermentation process of BJV and RJV can be mainly acids, esters, alcohols, aldehydes, and ketones. However, the composition and relative content of the two kinds of vinegars are quite different. The relative content of acid substances in these two kinds of vinegar increased during the fermentation process, and the ester substances first increased and then decreased. Acids and esters are the main aroma components of vinegar. A small amount of ketones, aldehydes, and other compounds are also indispensable parts of the aroma of fruit vinegar. All detected volatile compounds and their relative contents of BJV and RJV were shown in the supplemental table S1 and S2, and the significance analysis of relative content of volatile components in BJV and RJV before, middle and late fermentation were shown in the supplemental table S3 and S4.
Figure 6. Changes of the proportion of volatile components in BJV (A), and RJV (B) during fermentation; Changes of relative contents of main acids in volatile components of BJV (C) and RJV (D); Changes of relative contents of main esters in volatile components of BJV (E) and RJV (F); Changes of relative contents of main alcohols and aldehydes in volatile components of BJV (H) and RJV (I).
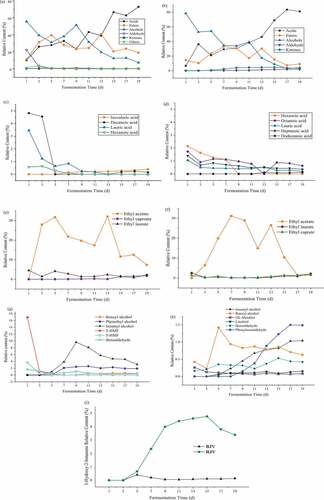
Acids and esters were the main components of the aroma of BJV and RJV. Appropriate volatile acids can make fruit vinegar fresh, elegant, and refreshing. Most esters have fruity and floral aromas and other aromatic odors, and esters are the main source of the soft aroma of fruit vinegar. In BJV, the relative content of acids increased from 10.3% at the beginning of fermentation to 72.9% during the fermentation process. Esters increased from 11.33% to 19.11%. In RJV, the relative content of acid increased from 6.82% to 81.15%, and the proportion of esters decreased from 14.54% to 9.28%. The results showed that BJV had less volatile acids and higher esters than RJV. These characteristics of BJV make it softer than jujube vinegar, and more esters make it less irritating in sensory evaluation.
The main acids and esters in volatile components of BJV and RJV were shown in . Except for acetic acid, which accounts for the most in BJV, the isovaleric acid began to appear on the seventh day of fermentation and finally accounted for 0.4%. The low concentration of isovaleric acid presented sweet fruit aroma-like blueberry. Decanoic acid that has an unpleasant fatty taste was rapidly reduced from 4.82% at the beginning of fermentation and disappeared on fifth day, but it appeared again at 15d and accounted for 0.16% of the total aroma components at the end. Both Dodecanoic acid and Hexanoic acid disappeared at the end of fermentation. In RJV, the Hexanoic acid decreased rapidly with the fermentation process from 2.14% in the beginning to 0 on the ninth day. Proportion of Octanoic acid, Dodecanoic acid, and Heptanoic acid decreased gradually with the fermentation process. In BJV, Ethyl acetate presents a clear and slightly fruity wine aroma, which content first increased and then decreased during fermentation, finally accounting for 7.35% at the end of the fermentation. Ethyl caproate has an oily, pineapple-like odor, which began to produce on seventh day and accounted for 2.25% at the end. In RJV, the relative content of Ethyl laurate first decreased and then increased, accounting for 2.16% at the end of fermentation, showing a mild leafy and petal-like aroma. Similarly, the relative content of Ethyl acetate first increased and then decreased, accounting for 1.99% at the end of fermentation.
showed the alcohols and aldehydes contents in BJV and RJV. Among the volatile alcohols, in addition to ethanol added as a fermentation substrate, BJV also contained relatively high content of isoamyl alcohol, benzyl alcohol, and phenethyl alcohol. Isoamyl alcohol and phenylethanol have special floral or fruity aromas, which enrich the flavor of fruit vinegar. Benzyl alcohol was detected from the 11th day of fermentation, the relative content remained at about 0.5%, showing an aromatic taste. In addition, benzyl alcohol was easily oxidized to benzaldehyde and benzyl ether, attributing to an almond aroma. The relative content of isoamyl alcohol and benzyl alcohol in RJV was high. Among them, these alcohols enriched the aroma components of fruit vinegar and enhanced the taste of fruit vinegar.
Among the volatile aldehydes, 3-HMF and 5-HMF in BJV occupied a relatively high proportion in the initial fermentation broth, and then declined rapidly to undetectable level as the fermentation process progressed. This phenomenon was similar to the change of 5-HMF in fermentation broth, which suggested that the fermentation process can reduce the content of 5-HMF.
Among volatile ketones, it can be seen from ) that the content of 3-Hydroxy-2-butanone in BJV had been at a low level and the proportion of it in the end of fermentation was 0.14%, whereas it increased first in RJV with reaching the highest concentration in 15 days and then decreased to 3.93% of the total volatile components at the end of fermentation.3-Hydroxy-2-butanone provides a pleasant creamy aroma at a lower concentration, but at higher concentration, it has a strong pungent smell. This could be one of the reasons for irritating aroma of RJV compared to BJV. In addition, the volatile components of fruit vinegar contained other ketones, al-kanes, phenols, and other substances, which interact with each other and jointly play a certain role in the flavor of fruit vinegar.
Conclusion
The optimal conditions for one-step fermentation of blackened jujube vinegar obtained by RSM were 15% of Acetobacter aceti (Huniang 1.01) inoculation, 30.44°C of fermentation temperature, and 6.45% vol of initial alcohol content. By comparing BJV and RJV fermented under the same conditions, BJV had a longer fermentation period and showed a delay at the beginning. Besides, 5-HMF, the potentially risky substance can be rapidly reduced during fermentation. BJV’s cAMP content in BJV was 3.5 times that of RJV, and showed higher antioxidant capacity in terms of ABTS and FRAP. In addition, the proportion of volatile acids in BJV was less and the esters proportion was about 2 times higher than that of RJV, indicating that BJV has a better aroma. In general, BJV showed better characteristics in the above-mentioned aspects, it’s a better new type of fruit vinegar. in addition, the change mechanism of bioactive substances such as cAMP and 5-HMF during the fermentation process and bioactive function of BJV need to be further studied.
Supplemental Material
Download ()Acknowledgments
Projects funded by the central government to guide local scientific and Technological Development (YDZX2021071), Shandong Province Key Research and Development Plan (the rural revitalization scientific and technological innovation improving action) (2021TZXD011), Dezhou health food industry innovation and entrepreneurship Community and Shandong Land Development Group Co. LTD Project “High efficient green production and high-value utilization of the whole industrial chain key technology integration and industrialization demonstration of red jujube.”
Supplemental data
Supplemental data for this article can be accessed on the publisher’s website.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Song, J.; Bi, J.; Chen, Q.; Wu, X.; Lyu, Y.; Meng, X. Assessment of Sugar Content, Fatty Acids, Free Amino Acids, and Volatile Profiles in Jujube Fruits at Different Ripening Stages. Food Chem. 2018, 270. DOI: 10.1016/j.foodchem.2018.07.102.
- Zhang, R.; Sun, X.; Zhang, K.; Zhang, Y.; Song, Y.; Wang, F. Fatty Acid Composition of 21 Cultivars of Chinese Jujube Fruits (Ziziphus Jujuba Mill.). J. Food Meas. Charact. 2021, 15(2), 1225–1240. DOI: 10.1007/s11694-020-00718-4.
- Gao, Q. H.; Wu, C. S.; Wang, M. The Jujube (Ziziphus Jujuba Mill.) Fruit: A Review of Current Knowledge of Fruit Composition and Health Benefits. J. Agric. Food Chem. 2013, 61(14), 3351–3363. DOI: 10.1021/jf4007032.
- Reche, J.; Hernández, F.; Almansa, M. S.; Carbonell-Barrachina, Á. A.; Legua, P., and Amorós, A. Effects of Organic and Conventional Farming on the Physicochemical and Functional Properties of Jujube Fruit. LWT Food Sci. Technol. 2019, 99, 438–444. DOI: 10.1016/j.lwt.2018.10.012.
- Wojdylo, A.; Figiel, A.; Legua, P.; Lech, K.; Carbonell-Barrachina, Á. A., and Hernández, F. Chemical Composition, Antioxidant Capacity, and Sensory Quality of Dried Jujube Fruits as Affected by Cultivar and Drying Method. Food Chem. 2016, 207, 170–179. DOI: 10.1016/j.foodchem.2016.03.099.
- K, R. A.; Naymul, K.; Islam, S. M. R.; Tao, B.;Yang, L.; Wei, C. Jujube Fruit: A Potential Nutritious Fruit for the Development of Functional Food Products. J. Funct. Foods. . 2020, 75, 104205.
- Reche, J.; Hernández, F.; Almansa, M. S.; Carbonell-Barrachina, Á. A.; Legua, P., and Amorós, A. Physicochemical and Nutritional Composition, Volatile Profile and Antioxidant Activity Differences in Spanish Jujube Fruits. LWT Food Sci. Technol. 2018, 98, 1–8. DOI: 10.1016/j.lwt.2018.08.023.
- Xia, T.; Zhang, B.; Duan, W. H.; Zhang, J.; Wang, M. Nutrients and Bioactive Components from Vinegar: A Fermented and Functional Food. J. Funct. Foods. 2020, 64. DOI: 10.1016/j.jff.2019.103681.
- Gao, L.; Gu, D., Sun, X.; Zhang, R. . Investigation of Processing Technology for Aged Black Jujube. Food Science and Nutrition Studies. 2019,3(4), 107, DOI: 10.22158/fsns.v3n4p107.
- Sun, X.; Gu, D.; Fu, Q.; Gao, L.; Shi, C., and Zhang, R. , et al. Content Variations in Compositions and Volatile Component in Jujube Fruits during the Blacking Process. Food Sci. Nutr. 2019, 7(4), 1387–1395.
- Hong, J.-Y.; Nam, H.-S.; Yoon, K.-Y.; Shin, S.-R. Physicochemical Properties and Nutritional Components of Fermented Black Jujube. Korean J. Food Preserv. 2012, 19(2), 243–248.
- Kharchoufi, S.; Gomez, J.; Lasanta, C.; Castro, R.; Sainz, F.; Hamdi, M. Benchmarking Laboratory-scale Pomegranate Vinegar against Commercial Wine Vinegars: Antioxidant Activity and Chemical Composition. J. Sci. Food Agric. 2018, 98(12), 4749–4758. DOI: 10.1002/jsfa.9011.
- Men, Y.; Zhu, P.; Zhu, Y. M.; Zheng, Y.; Yang, J. G.; Sun, Y. X. The Development of Low-calorie Sugar and Functional Jujube Food Using Biological Transformation and Fermentation Coupling Technology. Food Sci. Nutr. 2019, 7(4), 1302–1310. DOI: 10.1002/fsn3.963.
- Zhao, M. N.; Zhang, F.; Zhang, L.; Liu, B. J.; Meng, X. H. Mixed Fermentation of Jujube Juice (Ziziphus Jujuba MilL.) With L. Rhamnosus GG and L. Plantarum-1: Effects on the Quality and Stability. Int. J. Food Sci. Technol. 2019, 54(8), 2624–2631. DOI: 10.1111/ijfs.14174.
- Cao, M.; Niu, W. T.; Wang, S. H. Study on Flavor Substances of Jujube Fruit Vinegar. Basic Clin. Pharmacol. Toxicol. 2020, 126, 112.
- Zhao, L. W.; Liu, F. M.; Wu, L. M.; Xue, X. F.; Hou, F. Fate of Triadimefon and Its Metabolite Triadimenol in Jujube Samples during Jujube Wine and Vinegar Processing. Food Control 2017, 73, 468–473. DOI: 10.1016/j.foodcont.2016.08.039.
- Budak, N. H.; Aykin, E.; Seydim, A. C.; Greene, A. k.; Guzel-Seydim, Z. B. Functional Properties of Vinegar. J. Food Sci. 2014, 79(5), R757–R764. DOI: 10.1111/1750-3841.12434.
- Ali, Z.; Ma, H. L.; Rashid, M. T.; Wali, A.; Younas, S. Preliminary Study to Evaluate the Phytochemicals and Physiochemical Properties in Red and Black Date’s Vinegar. Food Sci. Nutr. 2019, 7(6), 1976–1985. DOI: 10.1002/fsn3.1009.
- Ali, Z.; Li, J. K.; Zhang, Y. H.; Naeem, N.; Younas, S.; Javeed, F. Dates (Phoenix Dactylifera) and Date Vinegar: Preventive Role against Various Diseases and Related in Vivo Mechanisms. Food Rev. Int. 2020. DOI: 10.1080/87559129.2020.1735411.
- Ali, Z.; Wang, Z. B.; Amir, R. M.; Younas, S.; Wali, A.; Adowa, N., et al. Potential Uses of Vinegar as a Medicine and Related in Vivo Mechanisms. Int. J. Vitam. Nutr. Res. 2016, 86(3–4), 140–151. DOI: 10.1024/0300-9831/a000440.
- Ali, Z. S.; Ma, H. L.; Wali, A.; Ishmael, A.; Sharif, M. N., et al. Daily Date Vinegar Consumption Improves Hyperlipidemia, Beta-carotenoid and Inflammatory Biomarkers in Mildly Hypercholesterolemic Adults. J. Herbal Med. 2019, 17–18. DOI: 10.1016/j.hermed.2019.100265.
- Zhai, H. Y.; Zhu, W. X.; Jin, J. L.; Liu, E. W. Research Progress in Liquid Fermented Jujube Products. (In Chinese). Farm Prod Process. 2015, 13, 56–59. DOI: 10.3969/j.1671-9646(X).07.017.
- Chen, Y.; Bai, Y.; Li, D. S.; Wang, C.; Xu, N.; Hu, Y. Improvement of the Flavor and Quality of Watermelon Vinegar by High Ethanol Fermentation Using Ethanol-Tolerant Acetic Acid Bacteria. Int. J. Food Eng. 2017, 13(4. DOI: 10.1515/ijfe-2016-0222.
- Dias, D. R.; Sliva, M. S.; de Souza, A. C.; Magalhaes-Guedes, K. T.; Ribeiro, F. S. D. Vinegar Production from Jabuticaba (Myrciaria Jaboticaba) Fruit Using Immobilized 2 Acetic Acid Bacteria. Food Technol. Biotechnol. 2016, 54(3), 351–359. DOI: 10.17113/ftb.54.03.16.4416.
- Luzon-Quintana, L. M.; Castro, R.; Duran-Guerrero, E. Biotechnological Processes in Fruit Vinegar Production. Foods. 2021, 10(5), 945. DOI: 10.3390/foods10050945.
- Zhang, W. Y.; Zhang, J. S.; Zhang, X. Study on Technological Conditions for One-step Fermentation of Hawthorn Fruit Vinegar. (In Chinese). China Condiment. 2003, 07, 23–25. DOI: 10.1111/j.1365-2621.2010.02413.x.
- Yang, Y. C.; Yang, X. Y.; Zhou, X. J.; Xue, G. X. Comparative of Quality Characteristics of Citrus Fruit Vinegar Prepared by Two Fermentation Methods. (In Chinese). China Brewing. 2020, 39(9), 152–156. DOI: 10.11882/j.0254-5071.2020.09.029.
- Kang, X. F.; Huang, Z. W.; Wu, G. F. Study on Technological Conditions for One-step Fermentation of Nanfeng Mandarin Vinegar. (In Chinese). China Condiment. 2015, 40(11), 59–63. DOI: 10.3969/j.100-9973.2015.11.015.
- Yuan, L.; Li, G.; Yan, N.; Wu, J.; Due, J. Optimization of Fermentation Conditions for Fermented Green Jujube Wine and Its Quality Analysis during Winemaking.J. Food Sci. Technol. 2021, 59, 288–299 , prepublish.
- Zhao, H.-X.; Zhang, H.-S.; Yang, S.-F. Phenolic Compounds and Its Antioxidant Activities in Ethanolic Extracts from Seven Cultivars of Chinese Jujube. Food Sci. Hum. Wellness. 2014, 3(3), 183–190. DOI: 10.1016/j.fshw.2014.12.005.
- Wang, B.; Huang, Q.; Venkitasamy, C.; Chai, H.; Gao, H.; Cheng, N.et al. Changes in Phenolic Compounds and Their Antioxidant Capacities in Jujube (Ziziphus Jujuba Miller) during Three Edible Maturity Stages. LWT. Food Sci. Technol. 2016, 66, 56–62. DOI: 10.1016/j.lwt.2015.10.005.
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M. ; Rice-Evans, C. Antioxidant Activity Applying an Improved ABTS Radical Cation Decolorization Assay. Free Radical Biol. Med. 1999, 26(9), 1231–1237. DOI: 10.1016/S0891-5849(98)00315-3.
- Feng, C.; Wang, B.; Zhao, A.; Wei, L.; Shao, Y., Wang, Y., et al. Quality Characteristics and Antioxidant Activities of Goat Milk Yogurt with Added Jujube Pulp. Food Chem. 2019, 277, 238–245. DOI: 10.1016/j.foodchem.2018.10.104.
- Tao, Y.; Sun, D.-W.; Górecki, A.; Błaszczak, W.; Lamparski, G., and Amarowicz, R., et al. A Preliminary Study about the Influence of High Hydrostatic Pressure Processing in Parallel with Oak Chip Maceration on the Physicochemical and Sensory Properties of A Young Red Wine. Food Chem. 2016, 194, 545–554. DOI: 10.1016/j.foodchem.2015.07.041.
- Butkhup, L.; Jeenphakdee, M.; Jorjong, S.; Samappito, S.; Samappito, W., and Chowtivannakul, S. HS-SPME-GC-MS Analysis of Volatile Aromatic Compounds in Alcohol Related Beverages Made with Mulberry Fruits. Food Sci. Biotechnol. 2011, 20(4), 1021–1032. DOI: 10.1007/s10068-011-0140-4.
- Sun, X.; Gu, D.; Fu, Q.; Gao, L.; Shi, C., and Zhang, R., et al. Content Variations in Compositions and Volatile Component in Jujube Fruits during the Blacking Process. Food Sci. Nutr. 2019, 7(4), 1387–1395. DOI: 10.1002/fsn3.973.
- Ra, C. H.; Jeong, G.-T.; Shin, M. K., and Kim, S.-K. Biotransformation of 5-hydroxymethylfurfural (HMF) by Scheffersomyces Stipitis during Ethanol Fermentation of Hydrolysate of the Seaweed Gelidium Amansii. Bioresour. Technol. 2013, 140, 421–425. DOI: 10.1016/j.biortech.2013.04.122.
- Chen, X.-C.; Bai, J.-X.; Cao, J.-M.; Li, Z.-J.; Xiong, J.; Zhang, L., and Hong, Y., et al. Medium Optimization for the Production of Cyclic Adenosine 3’,5’-monophosphate by Microbacterium Sp. No. 205 Using Response Surface Methodology. Bioresour. Technol. 2009, 100(2), 919–924. DOI: 10.1016/j.biortech.2008.07.062.
- Li, L.; Chen, X.; Ren, H.; Cao, J.; Xiong, J., and Bai, J. , et al. Dynamic Mathematical Models of Batch Experiments and Fed-batch Cultures for Cyclic Adenosine Monophosphate Production by Arthrobacter A302. World J. Microbiol. Biotechnol. 2011, 27(10), 2379–2385. DOI: 10.1007/s11274-011-0707-5.
- Wang, D.; Zhao, Y.; Jiao, Y.; Yu, L.; Yang, S.; Yang, X. Antioxidative and Hepatoprotective Effects of the Polysaccharides from Zizyphus Jujube Cv . Shaanbeitanzao. Carbohydrate Polymers. 2012, 88(4), 1453-1459.
- Surh, Y. J.; Tannenbaum, S. R. Activation of the Maillard Reaction Product 5-(hydroxymethyl)furfural to Strong Mutagens via Allylic Sulfonation and Chlorination. Chem. Res. Toxicol. 1994, 7(3), 313–318. DOI: 10.1021/tx00039a007.
- Hashim, M. N. M.; Abd-Talib, N.; Yaji, E. L. A.; Kelly, Y. T. L.; Razali, N., and Pa’ee, K. F. The Effect of Frying on Browning, Acrylamide and 5-hydroxymethylfurfural Formation on Malaysian Curry Puff Skin Treated with L-asparaginase. Food Sci. Biotechnol. 2021, 30(1), 149–158. DOI: 10.1007/s10068-020-00849-w.
- Kim, H. K.; Choi, Y.-W.; Lee, E. N.; Park, J. K.; Kim, S.-G., and Park, D.-J., et al. 5-Hydroxymethylfurfural from Black Garlic Extract Prevents TNF Alpha-induced Monocytic Cell Adhesion to HUVECs by Suppression of Vascular Cell Adhesion Molecule-1 Expression, Reactive Oxygen Species Generation and NF-kappa B Activation. Phytotherapy Res. 2011, 25(7), 965–974. DOI: 10.1002/ptr.3351.
- Zhao, L.; Su, J.; Li, L.; Chen, J.; Hu, S., and Zhang, X., et al. Mechanistic Elucidation of Apoptosis and Cell Cycle Arrest Induced by 5-hydroxymethylfurfural, the Important Role of ROS-mediated Signaling Pathways. Food Res. Int. 2014, 66, 186–196. DOI: 10.1016/j.foodres.2014.08.051.
- Jarboe, L. R. YqhD: A Broad-substrate Range Aldehyde Reductase with Various Applications in Production of Biorenewable Fuels and Chemicals. Appl. Microbiol. Biotechnol. 2011, 89(2), 249–257. DOI: 10.1007/s00253-010-2912-9.
- Tsuge, Y.; Kudou, M.; Kawaguchi, H.; Ishii, J.; Hasunuma, T., and Kondo, A. FudC, a Protein Primarily Responsible for Furfural Detoxification in Corynebacterium Glutamicum. Appl. Microbiol. Biotechnol. 2016, 100(6), 2685–2692. DOI: 10.1007/s00253-015-7115-y.
- Li, Y. M.; Zhang, X.-Y.; Li, N.; Xu, P.; Lou, W.-Y., and Zong, M.-H. Biocatalytic Reduction of HMF to 2,5-Bis(hydroxymethyl)furan by HMF-Tolerant Whole Cells. Chemsuschem.2017, 10(2), 372–378. DOI: 10.1002/cssc.201601426.
- Liu, Q.; Tang, G.-Y.; Zhao, C.-N.; Gan, R.-Y., and Li, H.-B. Antioxidant Activities, Phenolic Profiles, and Organic Acid Contents of Fruit Vinegars. Antioxidants.2019, 8(4), 12. DOI: 10.3390/antiox8040078.
- Kalemba-Drozdz, M.; Kwiecień, I.; Szewczyk, A.; Cierniak, A., and Grzywacz-Kisielewska, A. Fermented Vinegars from Apple Peels, Raspberries, Rosehips, Lavender, Mint, and Rose Petals: The Composition, Antioxidant Power, and Genoprotective Abilities in Comparison to Acetic Macerates, Decoctions, and Tinctures. Antioxidants.2020, 9(11), 18. DOI: 10.3390/antiox9111121.
- Sun, H. L.; Lu, Z. M.; Bao, R.; Zhao, N.; Bai, W. D. Change of Antioxidant Activity in Persimmon Vinegar during Brewing Process. (In Chinese). Food Sci. 2011, 32(19), 37–41. DOI: 10.1007/s10068-017-0099-x.
- Wu, J. F.; Jin, Y.; Zhang, M. Evaluation on the Physicochemical and Digestive Properties of Melanoidin from Black Garlic and Their Antioxidant Activities in Vitro. Food Chem. 2021, 340, 10. DOI: 10.1016/j.foodchem.2020.127934.
- Zhang, C.; Luo, H. T. Advancement of the Study on the Food Melanoidins. (In Chinese). China Food Additiv. 2005, 03, 11–13+29. DOI: 10.3390/foods8100516.