ABSTRACT
Glucose and lipid metabolism disorders (GLMD) are typically characterized by the metabolic disturbance of glucose and lipid. Clinically, GLMD includes diabetes, hyperlipidemia, obesity and nonalcoholic fatty liver disease. Studies found that Lycium barbarum polysaccharides (LBP) have activities of hypoglycemic, lowering blood lipids, anti-inflammatory, antioxidant and antitumor. Among them, the effects of LBP on improving insulin sensitivity, regulating blood lipids and reducing blood glucose have attracted much attention. This paper reviews the beneficial effects of LBP on controlling glucose and lipid metabolism diseases, so as to provide evidence for the potential use of LBP as a healthy food material for the control of metabolic diseases.
Introduction
Glucose and lipid metabolism disorders (GLMD) are a series of clinical manifestations such as diabetes, hyperlipidemia, obesity, and nonalcoholic fatty liver disease. It is easy to induce high blood pressure, atherosclerosis and other cardiovascular and cerebrovascular diseases.[Citation1] High-fat diet (HFD), lack of exercise and other factors have caused the incidence of glucose and lipid metabolism diseases rapidly, which seriously endangers human health.[Citation2] At present, there is no suitable drug to treat glucose and lipid metabolism diseases. Chemical synthesis drugs only intervene in single indicators such as blood sugar or lipids, but can’t solve the problem of “energy accumulation” in patients with glucose and lipid metabolism diseases as a whole.[Citation3] In recent years, traditional Chinese medicine has attracted much attention in GLMD. Many plant polysaccharides can use as a prebiotic for preventing metabolic syndrome by modulating the gut microbiota.[Citation4] Flaxseed polysaccharides effectively reduced the serum fasting glucose, total triglyceride and total cholesterol levels.[Citation5] Ginseng polysaccharide, lentinan, and fucoidan regulate cholesterol and triglyceride metabolism via ATGL-PPAR-α/PGC-1α, SREBP-1c-ACC/FAS or other pathways,[Citation6] which have great potential to develop as functional food or drugs for GLMD.
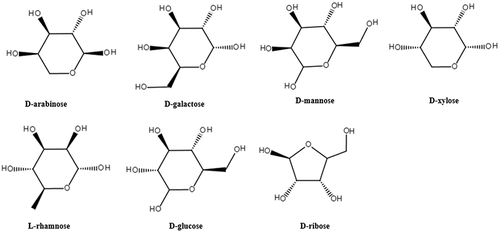
Lycium barbarum is the mature fruit of Ningxia Lycium barbarum. Lycium barbarum polysaccharide (LBP), as the main active component of Lycium barbarum, is mainly composed of D-arabinose, D-galactose, D-mannose, D-xylose, L-rhamnose, D-glucose, D-ribose and other monosaccharides and various uronic acids.[Citation7] LBP is a water-soluble polyglycoprotein complex with a molecular weight range of 10–2300 kDa.[Citation8] The molecular structure of LBP contains amino or imino groups and consists mainly of α-D-pyranose, with O-links between the sugar chain and the peptide chain. The main extraction methods of LBP include hot water extraction, alkali extraction, zymolytic extraction, microwave-assisted extraction and ultrasonic extraction, etc. The monosaccharide composition of LBP obtained by different extraction methods is slightly different (), which has different effects on the quality of LBP.[Citation9,Citation10]
Many research reported that LBP exhibited good pharmacological activities, such as anti-tumor,[Citation11] anti-aging,[Citation12] improving immunity, modulating gut microbiota, treating cardiovascular diseases,[Citation13] etc. Noteworthy, LBP has obvious therapeutic effects on GLMD, including hyperglycemia, hyperlipidemia, obesity and related complications.[Citation14] LBP protected ethanol-induced liver cell injury, which is beneficial to the application of LBP in functional food.[Citation15] Clinical studies reported taking LBP (150 mg, 2 daily) for 3 months significantly reduces the blood glucose level of type 2 diabetes patients.[Citation16] This article intends to summarize the role and mechanism of LBP in the prevention and treatment of glucose and lipid metabolism diseases, hoping to provide a theoretical basis for application of LBP as functional food.
Effect of LBP on diabetes and it’s complications
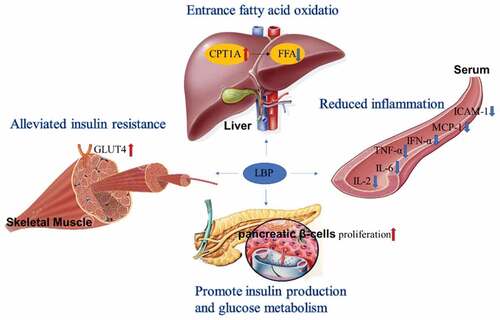
Diabetes mellitus (DM) is a chronic metabolic disease characterized by hyperglycemia. DM is prone to cause many complications, such as diabetic retinopathy, diabetic nephropathy and diabetic neuropathy.[Citation17] The pathological mechanism of DM is related to the decreased insulin sensitivity in metabolic organs (liver, skeletal muscle and adipose tissue), resulting in an increased glucose level of plasma. Hyperglycemia stimulates the secretion of insulin, which leads to hyperinsulinemia and even insulin resistance (IR).[Citation18] Inflammation also plays an important role in this process. The increase of T cells, macrophages and the inflammatory factors interleukin 6 (IL-6), interleukin 1β (IL-1β) or tumor necrosis factor α (TNF-α) promotes lipolysis and interferes with glucose consumption. Eventually lead to metabolic disorders.[Citation19] At present, the treatment strategies of DM include promoting insulin sensitivity, inhibiting glucose absorption and reducing inflammation.[Citation20]
It was reported that LBP dose-dependently (100, 250, and 500 mg/kg) reduced blood glucose levels in diabetic rats without cytotoxicity,[Citation21] and alleviated insulin resistance by promoting the translocation and activation of the glucose transporter GLUT4 in skeletal muscle.[Citation22] 30 and 60 mg/kg LBP administration reduced serum insulin, IL-6 and TNF-α levels in diabetic rats, indicating that LBP can improve insulin resistance and reduce inflammation levels.[Citation23] LBP promoted the proliferation of pancreatic β-cells, strengthen insulin secretion and glucose metabolism.[Citation24] 40 mg/kg LBP-4a served as a potential dietary therapeutic agent to improve hyperglycemia and hyperinsulinemia in type 2 diabetic rats by increasing the melatonin receptors (MT2) in epididymal adipose tissue.[Citation25] 150 mg/kg LBP-supplemented HFD for 6 weeks significantly alleviated glucose intolerance, dyslipidemia, and liver disease in gestational diabetic mice by regulating the microRNA expression profile of plasma exosomes.[Citation26] Clinical studies also show that 300 mg LBP daily for 3 consecutive months significantly reduced the blood glucose level and increased the insulin production of type 2 diabetes patients.[Citation16] These evidence all suggest LBP is a potential reagent for diabetes and related diseases ().
LBP also has a good effect on the complications of diabetes. Diabetic retinopathy is associated with suppression of Sirt1, Bcl-2 and activation of cell viability or apoptosis protein. Treating diabetic animals with LBP (25 or 50 mg/kg per day) hindered the development of cataract in lenses and improved retinal function by upregulating Sirt1, Bcl-2 and suppressing cell death related genes [Citation27] Diabetic nephropathy is another harmful complication of diabetes. LBP (100, 250, and 500 mg/kg) prevented renal damage of diabetic rats via NF-κB-mediated antioxidant and anti-inflammatory (IL-2, IL-6, TNF-α, IFN-α, MCP-1and ICAM-1) activities.[Citation21] After 12 weeks of LBP (500 mg/kg/d) administration, DM-induced structural damage to the nerve fiber myelination showed great improvement. These benefit effects are likely to be mediated through the induction of autophagy by inhibiting the activation of the mTOR/p70S6K pathways.[Citation28] LBP also treats male reproductive dysfunction caused by diabetes. 40 mg/kg LBP significantly down-regulated the expression of Beclin-1 and LC3I proteins in male diabetic mice, and up-regulated the expression of p-PI3K and p-Akt proteins. It improved testicular function and sperm activity in diabetic mice by inhibiting abnormal autophagy and apoptosis.[Citation29,Citation30]
Therapeutic effect and mechanism of LBP on hyperlipidemia
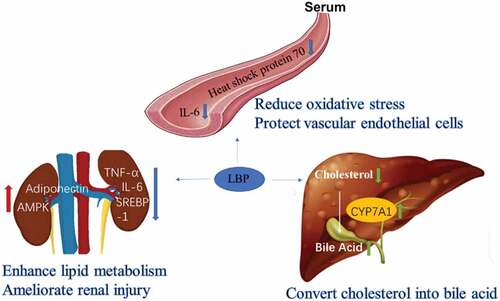
Hyperlipidemia, also known as “dyslipidemia,” refers to the total cholesterol (TC), triglycerides (TG) and low-density lipoprotein cholesterol (LDL-C) in blood is too high. Hyperlipidemia is a common clinical chronic metabolic disease caused by abnormal lipid metabolism or transport in the liver. It is also a high-risk factor that induces cardiovascular and cerebrovascular diseases such as atherosclerosis, coronary heart disease, and stroke.[Citation31] Long-term control of blood lipids can reduce the incidence and mortality of cardiovascular and cerebrovascular complications and the disability rate of diabetes.
A clinical double-blind randomized trial showed that taking dietary wolfberry water extract for 8 weeks had antioxidative and anti-inflammatory effects in overweight and hypercholesterolemic subjects by modulating inflammatory mRNA expression, and had therapeutic significance for patients with hypercholesterolemia and obesity.[Citation32] Hyperlipidemia rats were given 300 mg/kg LBPs daily for 12 weeks, and the levels of blood lipid, blood urea nitrogen, serum creatinine and urinary protein returned to normal, while SREBP-1, TNF-α and IL-6 in renal tissues were down-regulated, and adiponectin and AMPK were up-regulated.[Citation33] These results indicate that LBPs may mediate lipid metabolism, enhance anti-inflammatory responses, and ameliorate renal injury caused by lipid metabolism disorders in the hyperlipidemia rats. Giving 80 mg/kg/day LBP for 30 days to hyperlipidemic mice significantly reduced levels of liver malondialdehyde, serum heat shock protein 70 and IL-6, while increased liver cholesterol 7α-hydroxylase (CYP7A1) mRNA expression [Citation34] CYP7A1 is a rate-limiting enzyme involved in the conversion of cholesterol into bile salt. It is proved that LBP has a direct role in regulating the liver and blood vessel damage caused by blood cholesterol and hyperlipidemia ().
Therapeutic effect and mechanism of LBP on obesity
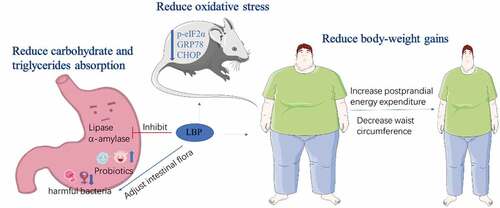
Obesity is caused by abnormal glucose and lipid metabolism, which can easily lead to diabetes, hyperlipidemia, hypertension, atherosclerosis and other cardiovascular diseases.[Citation35] Pancreatic lipase and alpha-amylase are two key enzymes in the process of carbohydrate and triglycerides metabolism. In clinically, the inhibitors of pancreatic lipase and alpha-amylase are often used for obesity.[Citation36] A lot of research has demonstrated that the gut microbiota plays an important role in regulating nutrient acquisition and body weight, thus serving as a key factor to control obesity and its associated disorders.[Citation37] In addition, increasing the basal metabolism and promoting white fat convert to brown is conducive to fat consumption,[Citation38] which is also an effective strategy for weight loss.
It is reported that LBP can significantly inhibit the activities of lipase and α-amylase to exert anti-obesity effect ().[Citation14] Obese mice treated with 40 mg/kg LBP increased insulin sensitivity and testosterone levels. The expressions of p-eIF2α, GRP78 and CHOP in the testis were down-regulated, indicating that LBP alleviated organ damage by reducing oxidative stress.[Citation39] Daily administration of 50 mg/kg LBP improved community diversity of intestinal flora and increased the short-chain fatty acids (SCFAs), which showed that LBP could be used as a prebiotic agent to control obesity.[Citation40] A clinical randomized trial investigated that LBP increased metabolic rate and reduced body-weight gains. The waist circumference after LBP intervention for 14 days reduced significantly.[Citation41]
Therapeutic effect and mechanism of LBP on nonalcoholic fatty liver disease
Nonalcoholic fatty liver disease (NAFLD) includes fatty liver, nonalcoholic steatohepatitis (NASH) and liver cirrhosis, which is characterized by a large number of diffuse fatty lesions in hepatocyte.[Citation42] NAFLD often interacts with other metabolic diseases such as diabetes, obesity and hyperlipidemia. The combination of these diseases is also called “metabolic syndrome “ (MS).[Citation43] Fatty acid oxidative stress, insulin resistance, inflammation and intestinal microbiota all play important role in the pathogenesis of NAFLD.[Citation44] Abnormal lipids metabolism leads to large accumulation of triglycerides and fatty acids in hepatocyte. This causes macrophages and pro-inflammatory factors infiltrate, further lead to insulin resistance (IR). Inflammatory and IR promote the development of NAFLD or MS.[Citation45] Therefore, correcting liver metabolic disorders and inflammation is an important way to treat NAFLD.
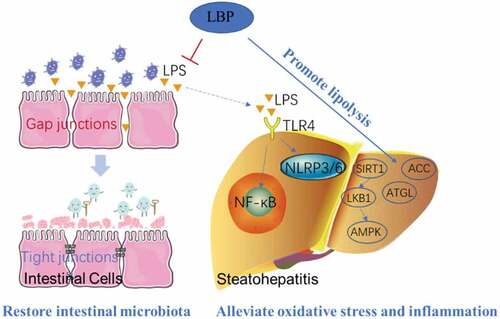
Many studies have reported that LBP has a significant effect on NAFLD (). 1 mg/kg LBP was given daily for 6 weeks alleviated liver injury in NASH mice. The mechanism involved inhibiting inflammasome NLRP3/6 and nuclear transcription factor NF-κB activity, improving liver oxidative stress, inflammation and apoptosis.[Citation46] LBP increased acetyl-CoA carboxylase (ACC) phosphorylation and adipose triglyceride lipase (ATGL) protein expression and decreased fatty acid synthase by activating the SIRT1/LKB1/AMPK pathway in vitro and in vivo. The activation of SIRT1 by LBP may be a strategy for the prevention of NAFLD.[Citation47] LBP restored the diversity of the intestinal microbiota and tight junctions of intestinal cells. High-fat diet with 50 mg/kg LBP supplement increased the Bacteroides phylum, zonula occludens-1 and occludin, reduced Proteobacteria and Firmicutes. At the same time, LBP down-regulated intestinal-derived lipopolysaccharide (LPS), liver LPS-binding protein, inflammatory factors via LPS/TLR4/NF-κB signaling pathway.[Citation48]
Conclusion
The balance of glucose and lipid metabolism is the basis for maintaining life activities. Natural healthy food for the prevention of glucose and lipid metabolism diseases is a current trend. Studies have found that LBP has significant therapeutic effects on glucose and lipid metabolism diseases, and its mechanism of action may be related to LBP’s ability to increase insulin sensitivity, improve insulin resistance, regulate the activity of glucose and lipid metabolism enzymes, improve antioxidant capacity and improve anti-inflammatory effects, etc. It is suggested that LBP as edible Chinese medicine has a good application prospect in the prevention and treatment of glucose and lipid metabolism diseases, and can developed as a healthy food for disease control.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Lin, Y.; Zhang, Z.; Wang, S.; Cai, J.; Guo, J. Hypothalamus-pituitary-adrenal Axis in Glucolipid Metabolic Disorders. Rev. Endocr. Metab. Disord. 2020, 21(4), 421–429. DOI: 10.1007/s11154-020-09586-1.
- Zhang, Q.; Liu, C.; Wang, Y.; Gong, J.; Wang, G.; Ge, W.; Kan, H. Associations of Long-term Exposure to Ambient Nitrogen Dioxide with Indicators of Diabetes and Dyslipidemia in China: A Nationwide Analysis. Chemosphere. 2021, 269, 128724. DOI: 10.1016/j.chemosphere.2020.128724.
- Agudelo, L. Z.; Ferreira, D. M.; Cervenka, I.; Bryzgalova, G.; Dadvar, S.; Jannig, P. R.; Ruas, J. L. Kynurenic Acid and Gpr35 Regulate Adipose Tissue Energy Homeostasis and Inflammation. Cell Metab. 2018, 27(2), 378–392. DOI: 10.1016/j.cmet.2018.01.004.
- Wang, X.; Wang, X.; Jiang, H.; Cai, C.; Li, G.; Hao, J.; Yu, G. Marine Polysaccharides Attenuate Metabolic Syndrome by Fermentation Products and Altering Gut Microbiota: An Overview. Carbohydr. Polym. 2018, 195, 601–612. DOI: 10.1016/j.carbpol.2018.05.003.
- Yang, C.; Xu, Z.; Deng, Q.; Huang, Q.; Wang, X.; Huang, F. Beneficial Effects of Flaxseed Polysaccharides on Metabolic Syndrome via Gut Microbiota in High-fat Diet Fed Mice. Food Res. Int. 2020, 131, 108994. DOI: 10.1016/j.foodres.2020.108994.
- Wu, Q.; Wang, Q.; Fu, J.; Ren, R. Polysaccharides Derived from Natural Sources Regulate Triglyceride and Cholesterol Metabolism: A Review of the Mechanisms. Food Funct. 2019, 10(5), 2330–2339. DOI: 10.1039/C8FO02375A.
- Ni, J.; Au, M.; Kong, H.; Wang, X.; Wen, C. Lycium Barbarum Polysaccharides in Ageing and Its Potential Use for Prevention and Treatment of Osteoarthritis: A Systematic Review. BMC Complement. Med. Therap. 2021, 21(1), 1–16. DOI: 10.1186/s12906-021-03385-0.
- Tian, X.; Liang, T.; Liu, Y.; Ding, G.; Zhang, F.; Ma, Z. Extraction, Structural Characterization, and Biological Functions of Lycium Barbarum Polysaccharides: A Review. Biomolecules. 2019, 9(9), 389. DOI: 10.3390/biom9090389.
- Zhou, S.; Rahman, A.; Li, J.; Wei, C.; Chen, J.; Linhardt, R. J.; Chen, S. Extraction Methods Affect the Structure of Goji (Lycium Barbarum) Polysaccharides. Molecules. 2020, 25(4), 936. DOI: 10.3390/molecules25040936.
- Hao, W.; Wang, S. F.; Zhao, J.; Li, S. P. Effects of Extraction Methods on Immunology Activity and Chemical Profiles of Lycium Barbarum Polysaccharides. J. Pharm. Biomed. Anal. 2020, 185, 113219. DOI: 10.1016/j.jpba.2020.113219.
- Chen, J. R.; Li, E. Q.; Dai, C. Q.; Yu, B.; Wu, X. L.; Huang, C. R.; Chen, X. Y. The Inducible Effect of LBP on Maturation of Dendritic Cells and the Related Immune Signaling Pathways in Hepatocellular Carcinoma (HCC). Curr. Drug Delivery. 2012, 9(4), 414–420. DOI: 10.2174/156720112801323107.
- Tang, R.; Chen, X.; Dang, T.; Deng, Y.; Zou, Z.; Liu, Q.; Wang, Z. Lycium Barbarum Polysaccharides Extend the Mean Lifespan of Drosophila Melanogaster. Food Funct. 2019, 10(7), 4231–4241. DOI: 10.1039/C8FO01751D.
- Zhang, Z.; Liu, H.; Yu, B.; Tao, H.; Li, J.; Wu, Z.; Cui, B. Lycium Barbarum Polysaccharide Attenuates Myocardial Injury in High-fat Diet-fed Mice through Manipulating the Gut Microbiome and Fecal Metabolome. Food Res. Int. 2020, 138, 109778. DOI: 10.1016/j.foodres.2020.109778.
- Kwok, S. S.; Bu, Y.; Lo, A. C. Y.; Chan, T. C. Y.; So, K. F.; Lai, J. S. M. A Systematic Review of Potential Therapeutic Use of Lycium Barbarum Polysaccharides in Disease. Biomed Res. Int. 2019, 2019, 1–18. DOI: 10.1155/2019/4615745.
- Wei, J.; Zhang, L.; Liu, J.; Pei, D.; Wang, N.; Wang, H.; Liu, Y. Protective Effect of Lycium Barbarum Polysaccharide on Ethanol‐induced Injury in Human Hepatocyte and Its Mechanism. J. Food Biochem. 2020, 44(10), e13412. DOI: 10.1111/jfbc.13412.
- Cai, H.; Liu, F.; Zuo, P.; Huang, G.; Song, Z.; Wang, T.; Sun, G. Practical Application of Antidiabetic Efficacy of Lycium Barbarum Polysaccharide in Patients with Type 2 Diabetes. Med. Chem. 2015, 11(4), 383–390. DOI: 10.2174/1573406410666141110153858.
- Sabanayagam, C.; Banu, R.; Chee, M. L.; Lee, R.; Wang, Y. X.; Tan, G.; Wong, T. Y. Incidence and Progression of Diabetic Retinopathy: A Systematic Review. Lancet Diabetes Endocrinol. 2019, 7(2), 140–149. DOI: 10.1016/S2213-8587(18)30128-1.
- Guilherme, A.; Henriques, F.; Bedard, A. H.; Czech, M. P. Molecular Pathways Linking Adipose Innervation to Insulin Action in Obesity and Diabetes Mellitus. Nat. Rev. Endocrinol. 2019, 15(4), 207–225. DOI: 10.1038/s41574-019-0165-y.
- Saxton, S. N.; Clark, B. J.; Withers, S. B.; Eringa, E. C.; Heagerty, A. M. Mechanistic Links between Obesity, Diabetes, and Blood Pressure: Role of Perivascular Adipose Tissue. Physiol. Rev. 2019, 99(4), 1701–1763. DOI: 10.1152/physrev.00034.2018.
- Samms, R. J.; Christe, M. E.; Collins, K. A.; Pirro, V.; Droz, B. A.; Holland, A. K.; Roell, W. C. GIPR Agonism Mediates Weight-independent Insulin Sensitization by Tirzepatide in Obese Mice. J. Clin. Invest. 2021, 131(12). DOI: 10.1172/JCI146353.
- Du, M.; Hu, X.; Kou, L.; Zhang, B.; Zhang, C. Lycium Barbarum Polysaccharide Mediated the Antidiabetic and Antinephritic Effects in Diet-streptozotocin-induced Diabetic Sprague Dawley Rats via Regulation of NF-κB. Biomed Res. Int. 2016, 2016, 3140290. DOI: 10.1155/2016/3140290.
- Zhao, R.; Qiu, B.; Li, Q.; Zhang, T.; Zhao, H.; Chen, Z.; Zheng, X. LBP-4a Improves Insulin Resistance via Translocation and Activation of GLUT4 in OLETF Rats. Food Funct. 2014, 5(4), 811–820. DOI: 10.1039/C3FO60602C.
- Liu, Q.; Han, Q.; Lu, M.; Wang, H.; Tang, F. Lycium Barbarum Polysaccharide Attenuates Cardiac Hypertrophy, Inhibits Calpain-1 Expression and Inhibits NF-κB Activation in Streptozotocin-induced Diabetic Rats. Exp. Ther. Med. 2019, 18(1), 509–516. DOI: 10.3892/etm.2019.7612.
- Zhu, J.; Liu, W.; Yu, J.; Zou, S.; Wang, J.; Yao, W.; Gao, X. Characterization and Hypoglycemic Effect of a Polysaccharide Extracted from the Fruit of Lycium Barbarum L. Carbohydr. Polym. 2013, 98(1), 8–16. DOI: 10.1016/j.carbpol.2013.04.057.
- Zhao, R.; Gao, X.; Zhang, T.; Li, X. Effects of Lycium Barbarum. Polysaccharide on Type 2 Diabetes Mellitus Rats by Regulating Biological Rhythms. Iran. J. Basic Med. Sci. 2016, 19(9), 1024–1030.
- Xiao, Y.; Chen, W.; Chen, R.; Luo, A.; Chen, D.; Liang, Q.; Tan, W. Exosomal MicroRNA Expression Profiling Analysis of the Effects of Lycium Barbarum Polysaccharide on Gestational Diabetes Mellitus Mice. Evid. Based Complement. Altern. Med. 2020, 2020, 2953520. DOI: 10.1155/2020/2953502.
- Yao, Q.; Zhou, Y.; Yang, Y.; Cai, L.; Xu, L.; Han, X.; Li, P. A. Activation of Sirtuin1 by Lyceum Barbarum Polysaccharides in Protection against Diabetic Cataract. J. Ethnopharmacol. 2020, 261, 113165. DOI: 10.1016/j.jep.2020.113165.
- Liu, S. Y.; Chen, L.; Li, X. C.; Hu, Q. K.; He, L. J. Lycium Barbarum Polysaccharide Protects Diabetic Peripheral Neuropathy by Enhancing Autophagy via mTOR/p70S6K Inhibition in Streptozotocin-induced Diabetic Rats. J. Chem. Neuroanat. 2018, 89, 37–42. DOI: 10.1016/j.jchemneu.2017.12.011.
- Shi, G. J.; Zheng, J.; Han, X. X.; Jiang, Y. P.; Li, Z. M.; Wu, J.; Yu, J. Q. Lycium Barbarum Polysaccharide Attenuates Diabetic Testicular Dysfunction via Inhibition of the PI3K/Akt Pathway-mediated Abnormal Autophagy in Male Mice. Cell Tissue Res. 2018, 374(3), 653–666. DOI: 10.1007/s00441-018-2891-1.
- Lei, X.; Huo, P.; Wang, Y.; Xie, Y.; Shi, Q.; Tu, H.; Zhang, S. Lycium Barbarum Polysaccharides Improve Testicular Spermatogenic Function in Streptozotocin-induced Diabetic Rats. Front. Endocrinol. 2020, 11, 164. DOI: 10.3389/fendo.2020.00164.
- Baumer, Y.; McCurdy, S.; Weatherby, T. M.; Mehta, N. N.; Halbherr, S.; Halbherr, P.; Boisvert, W. A. Hyperlipidemia-induced Cholesterol Crystal Production by Endothelial Cells Promotes Atherogenesis. Nat. Commun. 2017, 8(1), 1–14. DOI: 10.1038/s41467-017-01186-z.
- Lee, Y. J.; Ahn, Y.; Kwon, O.; Lee, M. Y.; Lee, C. H.; Lee, S.; Kim, J. Y. Dietary Wolfberry Extract Modifies Oxidative Stress by Controlling the Expression of Inflammatory mRNAs in Overweight and Hypercholesterolemic Subjects: A Randomized, Double-blind, Placebo-controlled Trial. J. Agric. Food Chem. 2017, 65(2), 309–316. DOI: 10.1021/acs.jafc.6b04701.
- Liao, J.; Liu, B.; Zhong, W.; Wang, G. D.; Xu, Y. L.; Chen, X. Protective Effect of Lycium Barbarum Polysaccharides against High-fat Diet-induced Renal Injury and Lipid Deposition in Rat Kidneys. J. Biol. Regul. Homeost. Agents. 2019, 33(1), 7–17.
- Zhu, X.; Hu, S.; Zhu, L.; Ding, J.; Zhou, Y.; Li, G. Effects of Lycium Barbarum Polysaccharides on Oxidative Stress in Hyperlipidemic Mice following Chronic Composite Psychological Stress Intervention. Mol. Med. Rep. May 2015, 11(5): 3445–3450.
- Maury, E.; Navez, B.; Brichard, S. M. Circadian Clock Dysfunction in Human Omental Fat Links Obesity to Metabolic Inflammation. Nat. Commun. 2021, 12(1), 1–17. DOI: 10.1038/s41467-021-22571-9.
- Rajan, L.; Palaniswamy, D.; Mohankumar, S. K. Targeting Obesity with Plant-derived Pancreatic Lipase Inhibitors: A Comprehensive Review. Pharmacol. Res. 2020, 155, 104681. DOI: 10.1016/j.phrs.2020.104681.
- Yang, M.; Yin, Y.; Wang, F.; Zhang, H.; Ma, X.; Yin, Y.; Chen, J. Supplementation with Lycium Barbarum Polysaccharides Reduce Obesity in High-Fat Diet-Fed Mice by Modulation of Gut Microbiota. Front. Microbiol. 2021, 12, 719967. DOI: 10.3389/fmicb.2021.719967.
- van den Berg, S. M.; van Dam, A. D.; Rensen, P. C.; de Winther, M. P.; Lutgens, E. Immune Modulation of Brown (Ing) Adipose Tissue in Obesity. Endocrine Rev. 2017, 38(1), 46–68. DOI: 10.1210/er.2016-1066.
- Yang, F. L.; Wei, Y. X.; Liao, B. Y.; Wei, G. J.; Qin, H. M.; Pang, X. X.; Wang, J. L. Effects of Lycium Barbarum Polysaccharide on Endoplasmic Reticulum Stress and Oxidative Stress in Obese Mice. Front. Pharmacol. 2020, 11, 742. DOI: 10.3389/fphar.2020.00742.
- Yang, Y.; Chang, Y.; Wu, Y.; Liu, H.; Liu, Q.; Kang, Z.; Duan, J. A Homogeneous Polysaccharide from Lycium Barbarum: Structural Characterizations, Anti-obesity Effects and Impacts on Gut Microbiota. Int. J. Biol. Macromol. 2021, 183, 2074–2087. DOI: 10.1016/j.ijbiomac.2021.05.209.
- Amagase, H.; Nance, D. M. Lycium Barbarum Increases Caloric Expenditure and Decreases Waist Circumference in Healthy Overweight Men and Women: Pilot Study. J. Am. Coll. Nutr. 2011, 30(5), 304–309. DOI: 10.1080/07315724.2011.10719973.
- Borrelli, A.; Bonelli, P.; Tuccillo, F. M.; Goldfine, I. D.; Evans, J. L.; Buonaguro, F. M.; Mancini, A. Role of Gut Microbiota and Oxidative Stress in the Progression of Non-alcoholic Fatty Liver Disease to Hepatocarcinoma: Current and Innovative Therapeutic Approaches. Redox Biol. 2018, 15, 467–479. DOI: 10.1016/j.redox.2018.01.009.
- de Gracia Hahn, D.; Duret, A.; Mann, J. P. An AGTR1 Variant Worsens Nonalcoholic Fatty Liver Disease and the Metabolic Syndrome. Off. J. Am. College Gastroenterol.| ACG. 2019, 114(4), 556–559. DOI: 10.14309/ajg.0000000000000193.
- Behary, J.; Amorim, N.; Jiang, X. T.; Raposo, A.; Gong, L.; McGovern, E.; Zekry, A. Gut Microbiota Impact on the Peripheral Immune Response in Non-alcoholic Fatty Liver Disease Related Hepatocellular Carcinoma. Nat. Commun. 2021, 12(1), 1–14. DOI: 10.1038/s41467-020-20422-7.
- Luo, P.; Qin, C.; Zhu, L.; Fang, C.; Zhang, Y.; Zhang, H.; Zhang, J. Ubiquitin‐specific Peptidase 10 (USP10) Inhibits Hepatic Steatosis, Insulin Resistance, and Inflammation through Sirt6. Hepatology. 2018, 68(5), 1786–1803. DOI: 10.1002/hep.30062.
- Xiao, J.; Wang, F.; Liong, E. C.; So, K. F.; Tipoe, G. L. Lycium Barbarum Polysaccharides Improve Hepatic Injury through NFkappa-B and NLRP3/6 Pathways in a Methionine Choline Deficient Diet Steatohepatitis Mouse Model. Int. J. Biol. Macromol. 2018, 120, 1480–1489. DOI: 10.1016/j.ijbiomac.2018.09.151.
- Jia, L.; Li, W.; Li, J.; Li, Y.; Song, H.; Luan, Y.; Yang, Y. Lycium Barbarum Polysaccharide Attenuates High-fat Diet-induced Hepatic Steatosis by Up-regulating SIRT1 Expression and Deacetylase Activity. Sci. Rep. 2016, 6(1), 1–11. DOI: 10.1038/srep36209.
- Gao, L. L.; Ma, J. M.; Fan, Y. N.; Zhang, Y. N.; Ge, R.; Tao, X. J.; Yang, J. J. Lycium Barbarum Polysaccharide Combined with Aerobic Exercise Ameliorated Nonalcoholic Fatty Liver Disease through Restoring Gut Microbiota, Intestinal Barrier and Inhibiting Hepatic Inflammation. Int. J. Biol. Macromol. 2021, 183, 1379–1392. DOI: 10.1016/j.ijbiomac.2021.05.066.