?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
There is higher energy in the protein content than in other weaning foods; sorghum and millets are utilized as weaning food in Ethiopia, India, Tanzania, Uganda, and several other African countries. Traditional beverages and giant beers made from sorghum seeds have more nutrients than beers made using malt. The chemical composition and mycoflora of dried sorghum bicolor seeds were studied for five-month of storage, and the mycoflora were isolated using different methods. The proximate and mineral analyses were also carried out to show the effect of the hold on the chemical composition of the seeds. During this five-month storage, the fungi isolated were Aspergillus fumigatus, Aspergillus Niger, Botrydioplodia sp., Fusarium sp., Mucor sp., Penicillium sp., and Rhizopus sp. The fungi count of the Sorghum bicolor seeds increased as the month of storage increased. The chemical composition of the Sorghum bicolor seeds decreased as the month of storage increased. Generally, the chemical composition of the freshly prepared seeds were more than those of the stored ones. Ash (2.63 g/100 g sample), fat (3.26 g/100 g sample), Fiber (2.96 g/100 g sample), and the carbohydrate (64.16 g/100 g sample) of the freshly prepared sun-dried Sorghum bicolor seeds were higher than the Ash (2.54 g/100 g sample), fat (2.83 g/100 g sample), and carbohydrate (63.36 g/100 g sample) after 20 weeks of storage. There is a general decrease in the mineral content of the Sorghum bicolor seeds as the month of storage increased.
Introduction
Sorghum bicolor is a cane-like grass, up to 6 m tall with a sizable-branched cluster of grains. The individual grains are small and about 3–4 mm in diameter. They vary in color from pale yellow through reddish brown to dark brown depending on the cultivar.[Citation1] The common names are called as sorghum (United States, Australia), durra (Africa), jowar (India), and banchatan (Ethiopia).[Citation2] Sorghum is a genus of numerous species of grasses. Some are raised for grains, fodder plants, or cultivated as part of the pasture. The species are natives to tropical and subtropical regions of all continents and Oceania, and Australasia. Sorghum bicolor grains are stored for the following reason: (i) For retaining a high proportion of viable seeds for planting at the next harvest, (ii) For future use by the consumers and processor, and (iii) For reselling by commercial traders on home market but occasionally for export.[Citation3,Citation4]
In some countries in West Africa, Sorghum bicolor grains are mixed with wood ash and stored in a clay pot. In Nigeria, Sorghum bicolor is stored as not threshed head in a solid-walled container called a rumbu.[Citation5] For short-term storage, bundles of sorghum heads are arranged in layers in the rumbu. For long-term storage of three to six years, the heads are laid out individually rather than in bundles. Some farmers spread gwanderdeji (Anonasenagalensis) on the bottom of the rumbu and between each year of the grains. When a rumbu is whole, the mouth is sealed with clay. In Uganda, Sorghum bicolor is threshed and stored in gunny sacks.[Citation6,Citation7] Grain deterioration in storage is caused mainly through[Citation7]: (i) Big-deterioration, (ii) Insect and pests, (iii) Molds and fungi.
Biodeterioration is due to the activity of enzymes present in the seeds. The extent of deterioration depends upon the level of enzyme activity, which in turn is determined by moisture and temperature.[Citation8] Fungal attack in storage generally occurs where dying has been inadequate, or a large number of insects is presented, thereby resulting in weight loss, which enhances big-deterioration, resulting in an enhanced loss of nutrients and contamination with anti-nutritional factors.[Citation4,Citation8] This research compares the chemical position and mycoflora of the freshly sundried and stored Sorghum bicolor color seeds.
Materials and methods
Collection of samples
The seeds of Sorghum bicolor were purchased at Oja-Ale market in Idanre, Ondo State, Nigeria. The black shell of the sources was picked, and the seeds were sun-dried for one week. The samples were stored for six months in an insect-free container, labeled, and kept in the laboratory at a temperature of 26 °C. The relative humidity of the storage area is 45% RH. One gram of the sample was taken monthly for analysis. The subsequent microbiological investigations were carried out.
Direct planting method
From the stored sun-dried Sorghum bicolor seeds, 15 seeds were examined randomly for external moldiness. They were surface sterilized with ethanol and later washed with sterile distilled water. Using sterile dissecting forceps, the surface of the stored sundried seeds was scrapped and was aseptically placed on a potato dextrose agar plate. It was incubated at room temperature for 3–4 days. The fungi cultures were further sub-cultured until pure colonies were obtained by successive hyphae tip transfer. The cultures were examined under the microscope to determine the type of fungi present.
Dilution plate method
About one gram of the stored sun-dried Sorghum bicolor seeds was surface sterilized with ethanol and was grinded. This was followed by the addition of 10 ml of sterile distilled water. The sample was shaken thoroughly, and 1 ml of the suspension was pipetted into a clean test tube containing 9 ml of pure distilled water and mixed. These were repeated until dilutions 10−1 to 10−6 were obtained. One milliliter from each of the dilutions 10−5 to 10−6 was added to molten potato dextrose agar in sterilized two Petri dishes for each dilution. The plates were allowed to solidify and then incubated at room temperature for 3–4 days; the fungal colonies were counted every 24 hours until they merged.
Washing method
This method was carried out by washing one gram of each in 10 ml of sterile distilled water in a beaker, and this was shaken thoroughly; drops of suspension of the contaminated water were introduced into Petri dishes containing potato dextrose agar. This was evenly spread on the agar surface; the plates were incubated at room temperature for 3–4 days and were observed for visible fungal growth.
Identification of the fungi isolates
The cultural and morphological features identified by the isolated fungi were examined under bright daylight for the color of the culture, and further examination was carried out using the following methods.
Needle mount preparation method
The needle-mounted preparation method described as a method where a fragment of the sporing surface of the initial culture was taken midway or between the center and edge of the colony. This was teased out on a sterile glass slide using a botany needle. The fragments were stained, adding a drop of lactophenol blue. A coverslip was placed on it and was observed under the x10 and x40 objective lens of the microscope.
Slide culture technique
This method is used when 1 cm of potato dextrose agar was cut from a plate approximately 2 mm deep and placed on a sterile glass slide. The fungus was macerated into four vertical drops of a sterile needle. A sterilized coverslip was placed on it so that it overlapped the medium on all sides, and the preparation was placed on proper support in a Petri dish containing blotting paper soaked in 20% glycerol in water. The preparation was suitably covered and examined under the low-power objective microscope. The proximate and mineral analyses were carried out.
Moisture content
The moisture content was determined using the oven drying method. Clean and dry Petri dishes were weighed using meter balance, and their respective and eights were recorded. About 5 g of each sample was transferred into the containers and weighed. The Petri dishes containing the samples were moved into the oven maintained at 105°C and dried for about 3 (three) hours. They were then transferred into the desiccator to cool and then weighed. The weighing process was repeated at intervals until constant weighted were obtained. The loss in weight during in percentage was taken to the percentage moisture content.
Ash
About 1 g of finely ground samples were weighed into a clean, dried, pre-weighed available with lid. The crucibles were then transferred to the muddle furnace set at 550°C (lid removed). Ashing was continued until a light gray, or white ash was obtained. The crucibles were cooled in a desiccator and weighed. A high ash figure suggests the presence of impurities.
Fat
About 5 g each sample was weighed and wrapped into a filter paper and placed in an extraction thimble. The thimble was considered before the sample, the thimble with sample was inserted into the soxhlet apparatus. Extraction under reflux was carried out with petroleum ether (40–60°C) boiling range for 5 h. At the end of the extraction, the thimble was dried in an oven for about 30 minutes at 100°C for the evaporation of the solvent, and the thimble was allowed to cool in a desiccator and later weighted.
Crude protein
About 1 g of each of the samples was weighed into the micro Kjeldahl digestion flask, one tablet of selenium catalyst and 15 ml of concentrated H2SO4 were added. The mixture was digested on an electrothermal heater until a clear solution was obtained. The flask was diluted to cool, after which the solution was diluted with distilled water to 50 ml, 5 ml of this was transferred into distillation apparatus, 5 ml of 2% boric acid was pipetted into a 100 ml conical flask (the receiver flask), and four drops of screened methyl red indicator were added. 50% NaOH was continually added to the digested sample until the solution turned cloudy, which indicated that the solution had become alkaline. The distillation was carried into the acid solution in the receiver flask with the delivery tube below the acid level. As distillation was going on, the pink color solution of the receiver flank turned blue, indicating ammonia. Distillation was continued until the content of the round bottom flask was about 50 ml, after which the delivery of the condenser was rinsed with distilled water. The resulting solution in the conical flask was then titrated with 0.1 M HCL.
Crude fiber
About 2.0 g of each sample was weighed into one-liter conical flasks, and 200 ml of boiling 1.25 g/100 H2SO4 was added and simmered gently for 30 minutes. The mixture was filtered through a muslin cloth and rinsed well with hot distilled water. The sample was scrapped back with a spatula, and 200 ml of boiling 1.25 g/100 NaOH was added and allowed of boiling 1.25 g/100 was added and allowed to boil gently for 30 min. It was filtered through muslin cloth, and the residue was washed thoroughly with hot distilled water and then rinsed once with 10 g/100 HCl and further rinsed twice with industrial methylated spirit and rinsed to drain dry. Then, the residue was scrapped into a crucible, dried in the oven at 105 ℃, cooled in a desiccator, and weighed. The residue was ashed at 50 ℃ for 90 minutes in a muffle furnace, cooked in a desiccator, and weighed again.
Carbohydrate
The carbohydrate is termed carbohydrate by the difference in which the total crude protein, water, fat, ash, and natural fiber is subtracted from 100. The nitrogen-free extractive (N.F.E), which is also referred to as soluble carbohydrate, is obtained by the difference.
Minerals
The mineral content of the samples was analyzed from the solution obtained by first dry ashing. The minerals were analyzed in an Atomic Absorption spectrophotometer (AAS). Some minerals analyzed include Ca, Fe, k, Na, Mg, Mn, Cu, and Zn. The standards for each metal were prepared. The instrument was switched on, and the lamp for each metal was fixed. All the metals analyzed, used to allow cathode lamps and air acetylene flame. The standard of each metal was aspirated into the light and the sample and their perspective concentration in mg/L.
Results and discussion
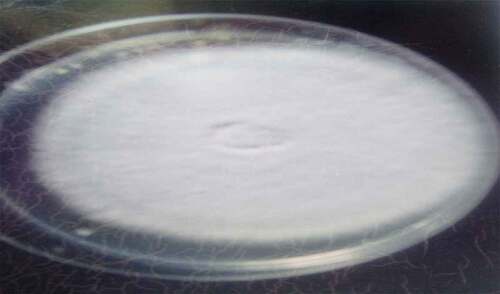
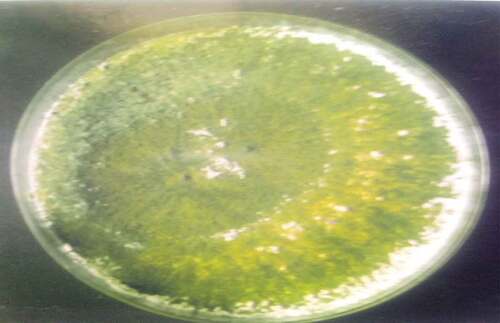
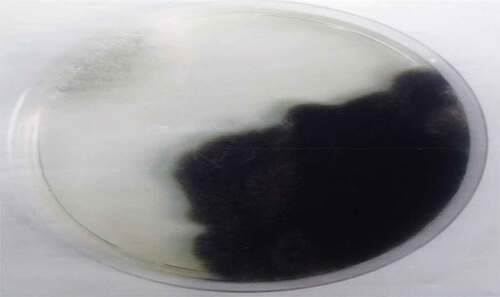
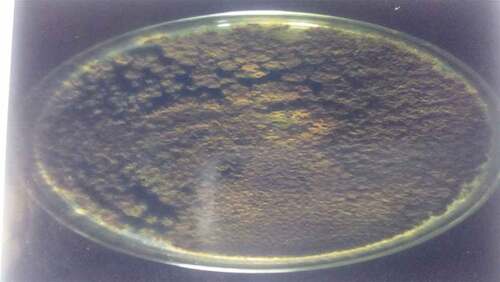
are the pictures of the seven fungi were isolated from the fresh and stored Sorghum bicolor seeds using various methods based on the cultural and morphological characteristics (). It was observed that the fungal counts increased with the storage time, and the fungi isolated from the freshly prepared sample were fewer than those stored. Sorghum grains are often contaminated by molds, they are ideal substrates for mold growth when poorly dried and stored,[Citation9] and is a serious biotic constraint in sorghum production areas. Many of these fungi are facultative parasites or saprophytic fungi, as they contribute to pre- and post-harvest deterioration of grains.[Citation10] The isolates vary in their cultural and morphological characteristics as in Rhizopus sp., which was quickly recognized as dirty white mycelium with the sporangia been seen by the naked eye as black dots, Aspergillus fumigatus which color is golden yellow, and Penicillium sp., established the characteristic green diamond color, sparkling as it matures.
Table 1. Cultural and morphological characteristics of fungi isolates
The fungi isolated from the fresh and stored sun-dried Sorghum bicolor seeds using the direct plating method were Aspergillium Niger, Aspergillus fumigatus, Botriodioplodia sp., Fusarium sp., Mucor sp., Penicillium sp., and Rhizopus sp. The fungi isolated from the fresh and stored sun-dried Sorghum bicolor seeds using the washing method were Aspergillus Niger, Fusarium sp., and Rhizopus sp. The fungi isolated from the new and stored sundried Sorghum bicolor seeds using the dilution plate method were A. Niger, Fusarium sp., Aspergillus fumigtus, Botriodioplodia sp., Penicillium sp., Rhizopus sp., and Mucor sp., all shown in .
Table 2. Fungi isolated from stored sundried Sorghum bicolor seeds using a direct plating method
Three common mold fungi were isolated in sorghum grains, which were collected from farmers’ stores and also from freshly harvested grains are , usually, Aspergillus species, Fusarium species, and Penicillium sp. Aspergillus species was predominant in sorghum from farmers’ stores, while Fusarium species and Penicillium sp. was predominant in freshly harvested grains.Citation[11] The isolation of fungal species belonging to the genera Aspergillus, Fusarium, and Penicilium species was in conformity with the findings that reported the isolation of the same fungal species.[Citation11,Citation12]
The predominance of Aspergillus species from farmers’ storage facilities observed in this study conforms to reports elsewhere.[Citation12,Citation13] It also concurs with the findings of stored rice grains in Nigeria.Citation[14] Aspergillus species dominates on cereals in the tropics. It grows at high moisture content and more rapidly than the Fusarium and Penicillium species as these latter two will take a longer time to sporulate. Aspergillus species infection in sorghum grains is a serious problem in a crop grown under rain fed conditions. Aspergillus species infection of the crop occurs in the field before harvest.Citation[15] Fusarium species are consistently associated with infection at early grain development stages across the agro-ecological zones.Citation[16] The fungus is a natural contaminant in cereals and other agricultural commodities. This makes Fusarium species an economically important genus of fungi as it causes diseases on a wide variety of plants at different developmental stages and also in plant products.
A widespread distribution of Fusarium species is attributed to the ability of the fungi to grow on a wide range of substrates and their efficient mechanism of spore dispersal.Citation[17] Fungal pathogens such as Helminthosorium sp., Aspergillus sp., Fusarium sp., Rhizoctonia sp., Fusarium sp., Penicillium sp., and Curvularia sp. The frequency of occurrence of the pathogens was higher in unwashed sorghum seeds than in those washed with distilled water. Therefore, of all the pathogens identified, Aspergillus sp. was the most abundant; occurring in all the washed and unwashed seed samples in a study of Seed borne pathogens on farmer-saved sorghum seeds.Citation[18]
The result of proximate analysis of the freshly prepared dried Sorghum bicolor seeds. It can be deduced from that the proximate analysis of the fresh and stored dried Sorghum bicolor seeds contained ash (2.63 g/100 g sample), fat (3.26 g/100 g sample), fiber (2.96 g/100 g sample), and the carbohydrate (64.16 g/100 g sample) of the freshly prepared sun-dried Sorghum bicolor seeds were higher than the ash (2.54 g/100 g sample), fat (2.98 g/100 g sample), fiber (2.83 g/100 g sample), and carbohydrate (63.36 g/100 g sample) of the stored ones, which shows that the ash, fat, fiber, and carbohydrate content decreases as the month of storage increases. This was due to the degradation activities of the fungi in the seeds feeding on the nutrients present in the Sorghum bicolor seeds.
Table 3. Proximate analysis of freshly prepared sundried and stored Sorghum bicolor seeds
The moisture content (11.94 g/100 g sample) and crude protein (15.08 g/100 g sample) of freshly prepared dried sorghum bicolor seeds were lower than the moisture content (12.41 g/100 g sample) and crude protein (15.88 g/100 g sample) of the stored ones, which shows that the moisture content and crude protein increases as the month of storage increases. The moisture content increases because of the storage environment because the seeds are longer exposed to sunlight, and the crude protein content increases because of the degradative activities of the Sorghum bicolor seeds by the fungi. This finding agrees with the finding of the proximate analysis of selected Sorghum cultivars.Citation[19]
Mineral analysis of the fresh and stored dried sorghum bicolor seeds dried sorghum seeds in mg/100 g of the sample are shown in . The various minerals were sodium (14.87 g), potassium (11.66 g), magnesium (11.76 g), zinc (23.32 g), iron (0.49 g), manganese (0.01 g), calcium (24.78 g), phosphorus (75.43 g) in the freshly prepared sample. At the same time, that of the stored ones was found to be Na(16.04 g), K(11.56 g), Mg(10.02 g), Zn(21.01 g), Fe(0.48 g), Mn(0.19 g), Ca(25.01 g), and P(69.52 g). It could be deduced from the table that potassium and calcium increase as the month of storage increases. Lead and copper were not detected both in the freshly prepared and in stored samples, and this result correlates with the findings on the mineral composition of sorghum varieties.Citation[20]
Table 4. Result of mineral analysis of sun-dried Sorghum bicolor seeds during storage (mg/100 g)
This study indicated that Aspergillus Niger, Aspergillus fumigatus, Botryodiplodia sp., Fusarium sp., Mucro sp., Penicillium sp., and Rhizopus were isolated from sun-dried Sorghum bicolor seeds during storage. Most of these fungi are known to be a surface contaminant of most stored grains, which reduces germination. Fusarium sp., was the most inhibitive, followed by Aspergillus, Penicillium, and others.Citation[21]
There was a reduction in the size and weight of sorghum grain artificially infected with Penicillium sp. The storage fungi (primarily Aspergillus sp., and Penicillium sp.) usually will damage sorghum seed under moisture conditions lower than those in which field, fungi attack seed before maturity (grain mold) and after maturity (grain weathering fungi).Citation[22]It was reported the most common fungi associated with grain weathering in species of Fusarium.Citation[23] The cumulative effect of damage of these organisms isolated from sundried Sorghum bicolor seeds can be loss of seed viability, retarded germination, low seed seedling vigor, and sometimes blighting of the seeds.
Similarly, some reported isolated fungi involved in the biodegradation of slices of white yam (Discorearotundata) and water yam (Discoreaalata) are stored under different conditions.Citation[24] They also recognized that mold might develop in the sorghum inflorescence at any stage, from young inflorescence to the mature head, provided climatic conditions are suitably moist. The second central area of concern when considering the nutritional value of moldy sorghum grains is the possibility that the fungi will produce metabolic-toxic chemicals known as mycotoxins. Also, aflatoxins are potent hepatic carcinogens produced by Aspergillus sp., as reported that moldy sorghum ear heads were contaminated with aflatoxin B and E in India, Uganda, and the United States of America.[Citation16,Citation25,Citation26] Aflatoxin is hepatoxic, carcinogenic, mutagenic, and teratogenic and the proper drying and storage would significantly prevent the contamination of food grain.
The ash, fat fiber, and the carbohydrate of the freshly prepared sun-dried Sorghum bicolor seeds were higher than that of storage grains, which showed that the ash, fat, fiber, and carbohydrate content decreased as the month of storage increased. This is due to the degradation activities of the fungi in the sorghum seeds, sorghum grain deterioration results from physical, physiological, and chemical changes.Citation[26] During the storage period, there was also an increase in the moisture content of the stored Sorghum bicolor seeds as the month of storage increases from 11.94% of freshly prepared grains to 12.41% after 20 weeks of storage.
This is similar to the work on the stored grain of legumes in which the moisture content was found to increase as the month of storage increased.Citation[16] During the six-month analysis, there was a general decrease in the mineral content of the stored Sorghum bicolor seed. Potassium and phosphorus content that decreased as the storage weeks increased may be attributed to fungal degradation of the seed using the mineral for its metabolism.Citation[27]
Conclusion
This study showed the microbiological and chemical reactions of different fungi found in the sun-dried Sorghum bicolor seeds during storage and their effect on their chemical composition and food and functional value. The fungi count of the Sorghum bicolor seeds increased as the month of storage increased. The result of proximate analysis of the freshly prepared dried Sorghum bicolor seeds. It can be deduced which shows the proximate analysis of the fresh and stored dried Sorghum bicolor seeds that the ash (2.63 g/100 g sample), fat (3.26 g/100 g sample), fiber (2.96 g/100 g sample), and carbohydrate (64.16 g/100 g sample) of the freshly prepared sun-dried Sorghum bicolor seeds were higher than the ash (2.54 g/100 g sample), fat (2.98 g/100 g sample), fiber (2.83 g/100 g sample), and carbohydrate (63.36 g/100 g sample) of the stored ones, which shows that ash, fat, fiber, and carbohydrate content decreases as the month of storage increases. This is due to the degradation activities of the fungi in the seeds feeding on the nutrients present in the Sorghum bicolor seeds. The chemical composition of the Sorghum bicolor seeds decreased as the month of storage increased, and the chemical composition of the freshly prepared seeds was more than those of the stored ones. Some of these fungi isolated were also capable of eliciting toxic responses when ingested, and most of the mineral content of freshly prepared seeds were more than the stored ones.
Author contributions
All the authors contribute equally and jointly in writing, revising, and approval of the final version.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The raw data supporting the conclusion of this article will be made available by the authors upon request, without any reservation.
References
- Kakuru, K. M.; Bagamba, F.; Okori, P. Consumer Preferences for Quality Characteristics of Sorghum Grain in Eastern Uganda: A Choice Experiment Approach. Afr Crop Sci J. 2021, 29(4), 497–512. DOI: 10.4314/acsj.v29i4.6.
- Adugna, A.; Endashaw, B.; Zemede, A.; Teshome, A. Patterns of Morphological Variation of Sorghum (Sorghum Bicolor (L.) Moench) Landraces in Qualitative Characters in North Shewa and Sout Welo, Ehtiopia. Hereditas. 2002, 137(3), 161–172. DOI: 10.1034/j.1601-5223.2002.01604.x.
- Martin, J. H.; Waldren, R. P.; L, D. Stamp, Principles of Field Crop Production; Pearson prentice Hall Publishers: USA, 2002; pp. 8.
- Carlos, A. P.; Lima, R. B.; Paul, D.; Benedito, C. Mycoflora and Aflatoxigenic Species in Derivatives of Milled Rice Science. Technol Aliment. 2000, 20(1), 1–8.
- Amura, N. A. Fungi Associated with Yam Chips in Storage and the Effect on the Chip Nutrient Composition. Moor J. Agri. Res. 2001, 2, 35–39.
- Fagboun, E. D; Anibijuwon, I. I;Egbebi, O; Lawal, O.Fungi Associated with Spoilage of Dried Cocoa Beans during Storage in Ekiti State of Nigeria. J. Microbiol Biotechnol Food Sci. 2011, 1(2), 204-214
- Lawal, O. U. Effect of Storage on the Nutrient Composition and the Mycobiota of Sundried Water Melon Seeds (Citrullus Lanatus. J Microbiol Biotechnol Food Sci. 2011, 1(3), 267–276.
- A.O.A.C. Official Method of Analysis, 14th ed.; Association of an official analytical chemist: Washington D.C., 2005; pp. 23–28.
- Martin, J. H.; Waldren, R. P.; Stamp, D. L. Principles of Field Crop Production; Pearson prentice Hall Publishers: USA, 2006; pp 8.
- Leslie, J. F.; Summerell, B. A. The Fusarium, Laboratory Manual, 1st ed.; Blackwell Publishing Professional: USA, 2006; pp 274.
- Kange, A. M.; Cheruiyot, E. K.; Ogendo, J. O., and Arama, P. Effect of Sorghum (Sorghum Bicolor L. Moench) Grain Conditions on Occurrence of mycotoxin-producing Fungi. Agric Food Secur. 2015, 4, 15. DOI: 10.1186/s40066-015-0034-4.
- Monica, M. S.; Simas, M. B.; Botura, B. C.; Sabino, M.; Mallmann, C. A.; Bitencourt, T. C.; Batatinha, M. J. Determination of Fungal Microbiota and Mycotoxins in Brewer’s Grain Used in Dairy Cattle Feeding in the State of Bahia, Brazil. Food Control. 2007, 18, 404–408. DOI: 10.1016/j.foodcont.2005.11.007.
- Gerbaldo, G. A.; Pereyra, C. M.; Cavaglieri, L. R.; Ruiz, F.; Pascual, L.; Dalcero, A. M.; Barberis, I. L. Surveillance of Aflatoxin and Microbiota Related to Brewer’s Grain Destined for Swine Feed in Argentina. Veterinary Medicine International 2011, 2011, 1–7. Accessed 20 Aug 2013. DOI: 10.4061/2011/912480.
- Bankole, S. A.; Somorin, Y. M. Mycoflora of Stored “Ofada” and “Abakiliki” Rice in Lagos and Ogun States. Afr. J. Microbiol. Res. 2010, 4, 1724–1726.
- Klich, M. A. Aspergillus Species: The Major Producer of Aflatoxin. Mol. Plant Pathol. 2007, 8(6), 713–722. DOI: 10.1111/j.1364-3703.2007.00436.x.
- Prom, L. K; Waniska, R. D; Abdourahmane, I. K; Rooney, R. D. Response of eight sorghum cultivars innoculated with Fusarium thapsinum, Culvunaria Lunata and a mixture of two fungi.(2003). Crop Prot.; DOI: 10.1016/S0261-2194(02)00246-6.
- Summerell, B. A.; Salleh, B.; Leslie, J. F. A Utilitarian Approach to Fusarium Identification. Plant Dis. 2003, 87(87), 117–128. DOI: 10.1094/PDIS.2003.87.2.117.
- Abdulsalaam, S.; Shenge, K. C. Seed Borne Pathogens on farmer-saved Sorghum (Sorghum Bicolor L.) Seeds. J. Stored Prod. Postharvest. Res. 2011, 2(2), 24–28.
- Jimoh, W. L. O.; Abdullahi, M. S. Proximate Analysis of Selected Sorghum Cultivars. Bayero J Pure Appl Sci. 2017, 10(1), 285–288. DOI: 10.4314/bajopas.v10i1.43.
- Dicko, M. H.; Gruppen, H.; Traore, A. S.; Van Berkel, W. J. H.; A.g.l, V. Evaluation of the Effect of Germination on Phenolic Compounds and Antioxidation Activities in Sorghum Varieties. J. Agric. Food Chem. 2005, 53, 2581–2588. DOI: 10.1021/jf0501847.
- Dwivedi, S. K. In Vitro Efficacy of Some Fungal Antagonists against Fusarium Solani and Fusarium Oxysporum F. Sp. Lycopersici Causing Brinjal and Tomato Wilt. J Biol Pharm Res. 2013, 4(1), 46–52.
- Yanjarappa, S. M.; Sharmila, D. R.; Ramanayaka, J. G. Survey of Postharvest Fungi Associated with Sorghum Grains Produced in Karnataka (India). J. Plant Protect. Res. 2010, 50(3), 335–339.
- Mohamed-Nor, N. M. I.; Salleh, B.; Leslie, J. F. Fusarium Species from Sorghum in Thailand. Plant Pathol. J. 2019, 35(4), 301–312. DOI: 10.5423/PPJ.OA.03.2019.0049.
- Okaigbo, R. N.; Nwakammah, P. T. Biodegradation of White Yam (Discoreaalata L) Sun-dried under Different Conditions. KMITL Sci. Tech. J. 2005, 5(3) 513.
- Magan, N.; Sanchis, V.; Aldred, D. Role of Spoilage Fungi in Seed Deterioration, Chapter 28. In Fungal Biotechnology in Agricultural, Food and Environmental Application; Aurora, D. K., Ed.; Marcell Dekker: New York, 2004; pp 311–323.
- Brennan, J. M.; Fagan, B.; Van, M. A.; Cooke, B. M.; Doohan, F. M. Studies on in Vitro Growth and Pathogenicity of Fusarium Fungi. Eur. J. Pl. Path. 2003, 109, 577–587. DOI: 10.1023/A:1024712415326.
- Campos, F. R.; Bressan, J.; Jassinski, V. C. G.; Zuccolotto, T.; Silva, L. E.; Cerqueira, L. B. Baccharis (Asteracecae): Chemical Constituents and Biological Activities’. Chem. Biodiversity. 2016, 13, 1–17. DOI: 10.1002/cbdv.201400363.