ABSTRACT
In the present study, 30 lactic acid bacteria (LAB) strains were isolated from Anbaris, a traditional fermented dairy product manufactured in rural villages of Lebanon from goat milk with a distinct sharp taste, and identified as members of the Lactobacillus genus. Among these strains, twelve showed potential probiotic characteristics with acid and bile tolerance, angiotensin-converting enzyme (ACE) inhibitory activity, and antimicrobial capacity against Staphylococcus aureus, but not against E-coli. Pooled isolates have displayed a very high ACE inhibitory activity (>70%). These isolates also had a high tolerance to all pH values between 3.0 and 5.0, making Anbaris contain probiotic LAB that can cross saliva, upper and lower stomach, and the intestines. The isolates were also characterized using pulse-field gel electrophoresis (PFGE). The obtained results are the first to be collected on Anbaris. This increases the marketability of this product as a source of probiotics, nutritional, economical, and empowering women cooperatives in rural areas.
Introduction
Fermentation is one of the traditional processes used to improve the shelf-life and safety of foods while providing food security for people in certain regions, enabling them to survive severe winters or drought periods.[Citation1–4] Lactic acid bacteria (LAB) is widely used to produce fermented dairy products.[Citation5–7] Traditional dairy products, such as Labneh Anbaris, are naturally fermented in rural Lebanon.
Labneh Anbaris is a unique artisanal type of strained yogurt produced using traditional methods. The manufacturing process of this type of dairy product usually starts with pouring fresh goat milk into an earthenware vessel and adding coarse salt (5% m/v). This is followed by continuous whey draining while refilling the container with fresh milk every 5–7 days for two. The final product, a highly acidic (pH = 3.14) cheese-like texture, is shaped into balls and stored in well-sealed glass jars with or without vegetable or olive oil.[Citation7,Citation8] Few studies investigated this traditional product.[Citation7,Citation9] These studies have explored the presence of mesophilic and thermophilic LAB of different genera contributing to Anbaris’ high acidity[Citation9] and evaluated the production methods and physicochemical properties across various regions in Lebanon.[Citation7]
Probiotics are live microorganisms that provide health benefits to the host organisms.[Citation10–12] Lactic acid bacteria (LAB) are Gram-positive and are naturally present in the gastrointestinal tract of humans and ruminants.[Citation13] These LAB play an important role in keeping the balance of microbial flora.[Citation14–16] Probiotic bacteria are effective against various enteropathogenic microorganisms.[Citation17] Probiotics from the genus Lactobacillus are known for their beneficial properties in preventing highly infectious diarrhea in the pediatric group.[Citation18] LAB, isolated from traditional dairy products, have been identified as perfect substitutes for the lost microbiota in the human body, acting as probiotics and exhibiting several health benefits such as improved digestibility, improved lactose utilization, aggressive action toward pathogenic bacteria, and anti-carcinogenic effects, among others. The antibacterial properties of probiotic strains have also been investigated in foods for shelf life extension.[Citation19]
Furthermore, a study reported that four Lactobacillus strains from the cheese were effective against enteropathogenic bacteria.[Citation20] These strains showed as well a high tolerance for bile salts and organic acid. In addition, another study identified Lactobacillus plantarum KCC-24, which was isolated and characterized from Italian rye-grass forage, as an excellent probiotic candidate for developing quality food for ruminant animals and humans.[Citation21]
Analyzing such dominant microorganisms in traditional cheeses and yogurt has been the trend to find new genetically stable strains that hold probiotic characteristics.[Citation22] The present study aimed to characterize the various LAB strains isolated from the traditional Labneh Anbaris produced in rural areas of Lebanon. Most importantly, this work also aimed to identify potential probiotic properties of these LAB strains to use them for the local production of functional foods.
Materials and methods
Samples
Labneh Anbaris is freshly produced during the summer season when goat milk is available in the rural villages and then stored to be consumed during the other seasons. Labneh Anbaris was prepared between July and August. Two different batches were purchased from two local villages, Erseil and Kweikh, Eastern Lebanon. Samples were then transferred aseptically at 4°C to the laboratory for further analysis. Each batch was sub-divided into two batches so that one was stored at 4°C and the other at 25°C to imitate the common storage conditions.
Microbiological analyses
Isolation of lactic acid bacteria: Lactic acid bacteria colonies were isolated using the selective plating technique that follows the homogenization and serial dilution method.[Citation23] In the procedure, 90 ml of sterile buffered peptone water (CM 0009-Oxoid-Cambridge, U.K.) was added to 10 g of each Anbaris sample and homogenized in a stomacher circulator 400 (Seward Ltd., London, UK) for 2 minutes at a speed of 250 rpm to obtain a 1:10 dilution. Serial dilutions were prepared, and dilutions 10−1 and 10−3 were spread plated (0.1 ml) in duplicates onto the agar plates designated for the isolation of the various Lactic acid bacteria strains: a) MRS agar pH 5.7 (CM 0361-Oxoid-Cambridge, U.K.) for presumptive Lactobacilli, b) M17 agar pH 7.2 (CM 0785-Oxoid- Cambridge, UK) for presumptive lactococci. All plates were then incubated at 30°C and 45°C for 72 h under aerobic and anaerobic conditions. From each M17 and MRS plate incubated at 30°C and 45°C, 30 to 50 colonies of presumptive LAB, corresponding to the highest dilution at which growth occurred, were extracted and streaked onto separate Plate Count Agar (64475- Biorad- C.A., USA) plates and incubated for another 24 hours. Three to five isolated colonies were then subcultured onto 5 ml M17 and MRS broth (Oxoid- CM 0817, Oxoid-CM 0359, respectively). After 24 hours of incubation, 1 ml of the obtained turbid broths was transferred to sterile microtubes and frozen with 1 ml of 15% glycerol at −20°C.
Cell morphology of the isolated strains was determined using light microscopy Lx500 (Labomed, NY, USA). All 158 isolates were subjected to Gram staining, catalase, and oxidase tests.[Citation24–26] According to the morphology of the bacterial cells (Gram-positive, catalase-negative, oxidase-negative, rods, or cocci), 64 of the 81 isolates were obtained from Kweikh, and 49 of the 77 isolates obtained from Erseil were selected for further analyses.
Isolates with similar biochemical and morphological characteristics from each batch (Kweikh or Erseil) were pooled to facilitate the analysis procedure. This resulted in 12 pooled isolates extracted from the Erseil batch labeled from E01 to E12 and 18 pooled isolates extracted from the Kweikh batch labeled from K01 to K18.
Phenotypic characterization: All phenotypic tests were conducted based on previous studies[Citation27–30]] Isolates were tested for temperature tolerance (at 15, 30, and 45°C), salt tolerance (at 4.0, 6.5, and 8.0%), carbohydrate utilization, CO2 production from glucose, and L-arginine hydrolysis, black zone formation (only for cocci), and citrate utilization.
Acid tolerance: A test for acid tolerance was conducted as described in a previous study.[Citation22] Tryptone broth tubes were adjusted to different pHs of 3.0, 3.5, 4.0, 4.5, and 5.0 using 0.1 N HCl. Three to five colonies of the active LAB cultures (37°C for 24 hours) were inoculated into the pH-adjusted broth media. Inoculated tubes were later incubated at 37°C for 72 hours, after which they were periodically checked for bacterial growth indicated by turbidity or for no growth indicated by clarity in the broth.
Bile tolerance: This test was performed as described in a previous study.[Citation31] Three to five colonies of the active LAB cultures were inoculated into both MRS broth and MRS broth containing 0.3% Oxbile (70168-Fluka- Sigma-Aldrich, St. Louis, MO). The rapid growth of isolated strains in a broth medium with and without added bile was deliberated. Bacterial growth was monitored hourly for 8 hours by measuring the absorbance at 620 nm using a spectrophotometer (U-1800 Spectrophotometer, Hitachi Co. Ltd. Japan) with and without bile. Absorbance values vs. incubation time were later plotted in graphs, and a comparison of the cultures was based on the times required for each to increase the A620 nm by 0.3 units.
Antibacterial activity test (Well Diffusion Assay): The antibacterial activity experiment was performed using the well diffusion assay method described in a previous study.[Citation32] Standard curves (CFU vs. Optical Density) were extrapolated to allow standardization of the lactic acid bacterial inocula and indicator strains (S. aureus and Escherichia coli). In the procedure, 0.1 ml of BHI broth containing 105 cells/ml of indicator strains, Staphylococcus aureus, and E. coli were spread onto BHI plates (0.7% agar, w/v). Wells were made in the lawn of the soft agar. Fifty μl aliquots of 24 hr grown cultures (108 CFU/ml) were poured into the wells where a crystal of pronase E (P 6911- Sigma-Aldrich, St. Louis, MO) was placed close to the edge of the well containing the bacteriocin sample to confirm the proteinaceous nature of bacteriocin. Plates were incubated at 37°C for 24 hours. Bacteriocin production was signified by the emergence of a clear inhibition zone around the well.
ACE inhibitory activity: This experiment was performed according to the method explained in a previous study.[Citation22] 1% (v/v) of 24-hour active LAB cultures were inoculated into 10 ml of 10% reconstituted skimmed milk media and incubated at 37°C for 72 hours. Later, fermented skimmed milk was adjusted to pH 4.6 (using 1 N NaOH or 1 N HCl) and centrifuged at 12,000 × g for 20 min at 4°C to obtain the whey supernatant. The pH of the supernatant was adjusted to 8.3 with 5 N NaOH and further centrifuged at 12,000 × g for 20 min in a bench centrifuge (the whey supernatant was used in the assay of ACE inhibitory activity). 60 μl of each sample was mixed with 200 μl of Hip-His-Leu solution {50 mM Hip-His-Leu (H 1635- Sigma-Aldrich, St. Louis, MO) dissolved in 100 mM Na-borate buffer (pH 8.3) and 300 mM NaCl} and 40 μl of 25 mM ACE {angiotensin converting enzyme (A 6778- Sigma-Aldrich, St. Louis, MO) dissolved in cold deionized water}. After incubation at 37°C for 1 hour, 250 μl of 1 N HCl was added to stop the reaction, and 1.7 ml of ethyl acetate was added to extract the hippuric acid liberated by ACE. The mix was centrifuged at 5,000 × g for 20 min, and 0.5 ml of the upper layer was transferred into a test tube where the ethyl acetate was evaporated at room temperature for 20 min in a rotary vacuum evaporator. The hippuric acid was diluted in 1.0 ml sterile distilled water, and the absorbance was measured at 228 nm using U.V. spectrophotometrically (U-1800 Spectrophotometer, Hitachi Co. Ltd. Japan). The percentage of ACE inhibitory activity was calculated by the following formula[Citation22]
ACE inhibitory activity (%) = [(B-A)/(B-C)] ×100
where A is the optical density in the presence of both ACE and ACE inhibitory compounds, B is the optical density without ACE inhibitory compounds, C is the optical density without ACE and ACE compounds
Results and discussion
Biochemical and phenotypic characteristics of isolated LAB
The biochemical and phenotypic characteristics of the 30 pooled isolates investigated by Erseil and Kweikh are displayed in . Among the 12 pooled isolates extracted from the Erseil batch of Anbaris, only two cocci isolates (E08 and E10) out of the five cocci (E06, E07, E08, E10, and E11) showed high tolerance to temperatures of 30 and 45°C, sustainability under harsh saline conditions of 4.0, 6.5, and 8.0%, based on the formation of a black zone upon incubation on bile esculin agar. Hence, this concludes that those pooled strains may belong to the Enterococcus genus. This genus is considered one of the few coccus groups with high tolerance to harsh conditions, similar to species belonging to the Lactobacillus genus.[Citation32,Citation33]
Table 1. Physiological, phenotypic and probiotic characteristics of lactic acid bacteria isolated from traditionally fermented Labneh Anbaris (number of strains positive for each character)
On the other hand, from the other 7 rod-shaped pooled isolates from this same batch (E01, E02, E03, E04, E05, E09, and E12) presumably of the Lactobacillus genus, only E02 and E05 showed high tolerance to temperature (15, 30, 45°C) and salt (4.0, 6.5, 8.0%) proving to be of a strong nature. Results also show that pooled isolate E12 seems to be heterofermentative, proved by its ability to produce CO2 as a by-product of fermentation recognized by the presence of a bubble in the Durham tube. Although it was salt-tolerant (4.0, 6.5, 8%) and arginine utilizing, it was unable to survive the high temperature of 45°C proving its non-thermophilic nature.
The majority of the pooled isolates from the Kweikh batch were morphologically characterized as rod-shaped cells presumably belonging to the Lactobacillus genus (K02, K03, K04, K05, K10, K12, K13, K14, K16, and K17) and heterofermentative, producing CO2 as a by-product of the fermentation. Amongst these isolates, only K03 and K04 showed the strongest tolerance nature surviving the different temperatures and salt concentrations. K03 utilized arginine as an energy source producing NH3 and the by-products ornithine, CO2, and ATP.[Citation34] This isolate also utilized citrate (key intermediate in Kreb’s cycle) as a carbon source, converting it into oxaloacetate, which is usually later metabolized by oxaloacetate decarboxylase to produce the aroma compound diacetyl, pyruvate, and other metabolic products.[Citation35]
Acid and bile tolerance
According to the literature, the two major conditions for a probiotic strain are tolerance to high acidity and high concentration of bile components, the former being dominant in the stomach and the latter being majorly present in the intestine.[Citation36,Citation37] Conforming to both conditions allows a probiotic strain ingested via food to survive the transit through the upper gastrointestinal tract, cross all barriers, and remain in a viable physiological state to benefit the host.[Citation22,Citation37]
This study showed that only pooled isolates E01, E02, E07, E12, and K01 had a high tolerance to all pH values between 3.0 and 5.0. This result is highly significant as it proved that Anbaris from both villages do contain LAB of probiotic nature that can cross the human digestive tract, including the saliva (pH 6.5–7.5), upper stomach (pH 4.0–6.5), lower stomach (pH 1.5–4.0) and the intestines (pH 4.0–7.0).[Citation38] Hence, this gives the product an added health value. This will allow the strains to survive, grow and perform all required beneficial effects in the intestinal tract simulating the originally present lactic acid microbiota responsible for the body’s immune system.[Citation39]
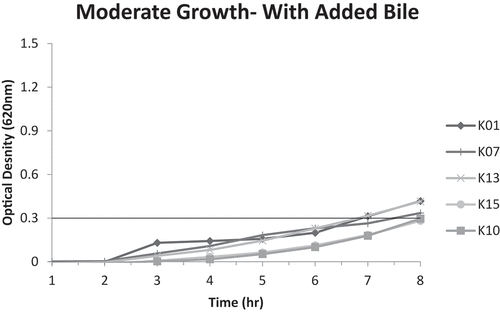
As for bile tolerance, results were divided based on four criteria of growth rates: no growth, slow (taking > 8 hours to reach A620 nm of 0.3 units), moderate (reaching an A620 nm of 0.3 units within 6–8 hours), and fast (reaching an A620 nm of 0.3 units within 4–6 hours). display the moderate and fast growth rates of Erseil and Kweikh isolates with and without added bile.
Figure 1. A. Moderate growth graph (optical density at 620 nm vs. time) of isolates E01, E04, E05 and E10 without added bile; reaching A620nm = 0.3 unit within 6–8 hours.B. Fast growth graph (optical density at 620 nm vs. time) of isolates E02, E03, E08, E09, and E11 without added bile; reaching A620nm = 0.3 unit within 4–6 hours.
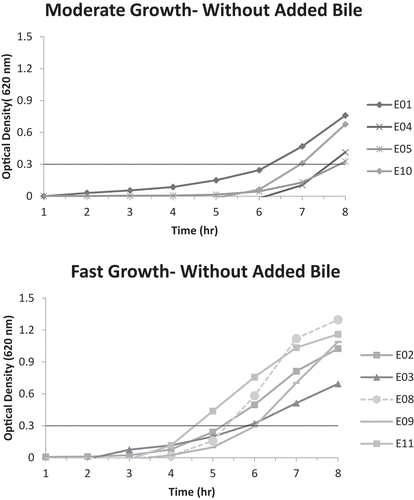
Figure 2. A. Moderate growth graph (optical density at 620 nm vs. time) of isolates E03 and E09 with added bile; reaching A620nm = 0.3 unit within 6–8 hours.B. Fast growth graph (optical density at 620 nm vs. time) of isolates E04 and E06 with added bile; reaching A620nm = 0.3 unit within 4–6 hours.
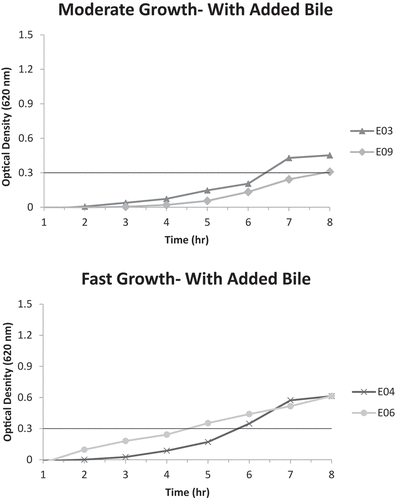
Figure 3. A. Moderate growth graph (optical density at 620 nm vs. time) of isolates K03, K04, K05, K06, K13, K15, and K16 without added bile; reaching A620nm = 0.3 unit within 6–8 hours .B. Fast growth graph (optical density at 620 nm vs. time) of isolates K08, K11, and K18 without added bile; reaching A620nm = 0.3 unit within 4–6 hours.
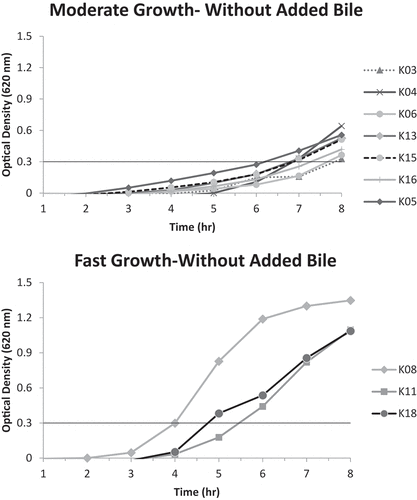
Figure 4. Moderate growth graph (optical density at 620 nm vs. time) of isolates K01, K07, K10, K13 and K15 with added bile; reaching A620nm = 0.3 unit within 6–8 hours.
It is noticeable that many of the isolates originating from the Erseil batch have displayed high tolerance to bile, with pooled isolates E04 and E06 having the best bile tolerance ability to reach an A620 nm of 0.3 units within 4–6 hours, showing a fast growth pace. However, none of the pooled isolates originating from the Kweikh batch were able to grow at a fast rate (4–6 hours to reach A620 nm of 0.3 units) in the presence of bile, but pooled isolates K13 and K15, showed very good bile tolerance ability and K01, K07 and K10, a moderate bile tolerance ability.
Antibacterial activity
The antibacterial activity test is another necessary probiotic selection test performed to establish the inhibition capabilities of the isolated strains against pathogenic organisms. Staphylococcus aureus (Gram-positive) and E. coli (Gram-negative) were used as indicators for the antibacterial abilities of the isolated strains. Pooled isolates E01, E09, and K03 showed a strong inhibition against S. aureus, as indicated by the clear inhibition zones. None of the pooled isolates had an effect against E. coli.
This result was particularly significant because probiotic isolates can provide a two-fold benefit. If added to food products, they may extend the shelf life by inhibiting major pathogenic and spoilage microorganisms while enriching the food with added health benefits known about probiotics. Furthermore, at the human level, those LAB strains, through their secretions, play a major role in inhibiting invasive pathogenic bacteria.[Citation40] The major antimicrobial metabolites produced by the Gram-positive LAB are bacteriocins, which attack closely related Gram-positive bacteria (excluding LAB, which display immunity to them) such as Staphylococcus, Bacillus, Listeria, Clostridium, and others.[Citation40] These proteinaceous agents act by “forming membrane channels or pores that destroy sensitive cells’ energy potential, leading to their instant death.[Citation41] This ability to suppress the growth of harmful bacteria is vital since such bacteria can invade the system via food, air, water, or any other source, causing harmful effects on the human body.[Citation22]
However, these bacteriocins are not frequently active against Gram-negative bacteria that are characterized to have an outer membrane that protects the cytoplasmic membrane from the action of antimicrobial compounds.[Citation42,Citation43] This membrane acts as a permeability barrier for the cell preventing the entry of antibiotics, detergents, and dyes.[Citation42] The Proteobacteria family is among these Gram-negative bacteria, including E. coli, Salmonella, Shigella, and other Enterobacteriaceae[Citation44] hence explaining the lack of activity against E. coli in this present study.
ACE inhibitory activity
Another basic prerequisite test performed on the LAB isolates to prove their probiotic nature is the angiotensin-converting enzyme (ACE) inhibitory activity test. According to the obtained results, pooled isolates E01, E02, E03, E04, E07, E08 as well as K11 have displayed a very high ACE inhibitory activity (>70%), reaching an approximate 99.90% inhibition in isolates E07, E08, a 93.18% inhibition in isolate E03, and 80.68% in isolate E01.
According to a study, Angiotensin II is a hormone recognized as the major cause of high blood pressure and elevated heart activity in pumping blood to the main arteries leading to its weakening.[Citation45] This hormone is one of the major causes of hypertension medications, heart failure, diabetes, and inflammatory responses to arterial injury.[Citation46] Pharmacological agents that function by either inhibiting the synthesis of Angiotensin II or blocking its action were utilized to cure hypertension.[Citation46] However, scientists have shifted toward the more natural sources of inhibition, discovering that fermented milk is active in lowering blood pressure.[Citation22] The probiotic LAB present in the milk exerts an ACE inhibitory effect to treat hypertension and congestive heart failure.[Citation22,Citation46] Having an ACE inhibitory activity allows the probiotic strains to prevent constriction of the vessels, lower blood pressure, and maintain normal activity of the heart.[Citation45]
Preliminary probiotic selection
The isolation and characterization of microorganisms from traditional dairy products have always been the means to find new, genetically stable strains that hold probiotic characteristics. Such studies have been implemented in several countries, including the Balkan region,[Citation32] Spain,[Citation47] Greece,[Citation48,Citation49] Bulgaria,[Citation33] and Bangladesh.[Citation22] Depending on the production technology and the ecological characteristics of the areas in which production and fermentation occur, different microbiota can be identified.[Citation50,Citation51] In this study, tests performed on Labneh Anbaris originating from rural Lebanon indicated the occurrence of probiotic strains in this artisanal, traditional product. Obtained results proved that isolates E01 (homofermentative, acid-tolerant, moderate bile tolerant, antimicrobials against S. aureus and 80.68% of ACE inhibitory activity), E03 (homofermentative, acid-tolerant, bile tolerant, 93.80% ACE inhibitory activity), and K03 (heterofermentative, temperature tolerant (15, 30, 45°C), salt-tolerant (4.0, 6.5, 8.0%), acid-tolerant (3.5–5.0) with antimicrobial abilities against S. aureus) are the most suitable candidates to be recognized as probiotic strains, although K03 has low bile tolerance. Its tolerance to the other harsh conditions and its Gram-positive bacterial inhibitory nature makes it an interesting strain to be further investigated, assessing the array of its potential uses.
The previous promising and interesting results necessitate further investigation into isolated strains, especially genetic identification. Hence, the significant outcomes of our study open the doors to the various positive roles that identified probiotic strains might play in food production, mainly dairy, health benefits, and economic edge in the local and regional markets. For instance, isolated stains with a fermentative nature and health-promoting characteristics can replace the currently used dairy starters. This substitution can aid in producing fermented dairy products labeled as functional foods.[Citation52]
Furthermore, the isolation of these strains from the artisanal dairy products (e.g., Labneh Anbaris) and their characterization as probiotics can allow their incorporation as live beneficial microbial supplements in dairy (ice creams, yogurts) and nondairy products (energy bars, cereals, infant formula) exhibiting health-promoting effects. These live active cultures may be a safe, natural approach to improving public health conditions. The emergence of new public health threats (such as new foodborne pathogens, increased resistance to antibiotics, and new incurable diseases) suggests the important role of these probiotics as preventive and curing agents for many types of diseases. This identification of probiotic strains and their use in functional foods has been proven to prevent and treat several health conditions, including acid reflux, eczema, autism, adrenal fatigue, food allergies, lactose intolerance, respiratory and vaginal infections, among others.[Citation53]
This study is the first to address the probiotic properties of Labneh Anbaris. These findings will enhance the productivity and marketability of this traditional product by highlighting its health-promoting characteristics. Focusing on having good taste attributes is major since different regions have different taste preferences, and Anbaris has a strong acidic flavor that does not appeal to all consumers. Much like the strategy used for the promotion of yogurt, the focus should be on the possibility of offering this product with a variety of textures and flavors (depending on the fermentation time and storage conditions).[Citation54] For instance, the addition of thyme to the final product can reduce the strong acidic taste and add to the acceptability of this product.
Most importantly, the standardization of the production process of Anbaris is vital for the consistency of the final product. Maintaining the traditional cottage scent of production, goat milk fermentation should be standardized under controllable, precise conditions that permit the production of a safe, fermented final product with a high nutritive value.[Citation54] Offering this product at an acceptable price/value ratio and accentuating its positive attributes and health-promoting, immunity-boosting benefits is the key to increasing awareness and ensuring a successful marketing strategy.[Citation54] Later, this product can be subjected to modification strategies, offering Anbaris with less fat, less cholesterol, fewer calories, fortified with vitamins, calcium, and fibers.
Having certain economic, health, and development advantages over some other dairy products can increase Anbaris production and aid in the development of the rural areas where the product is being produced. Because gender inequality still exists in many rural regions, women cooperative unions aiding in the economic independence of rural women are being formed. This could also empower women and increase their contributions to their family income.
Conclusion
Traditionally, fermented dairy products have been the center of research for many years as reservoirs of naturally present LAB with probiotic characteristics. These well-characterized strains can replace the currently used dairy starters, producing fermented, functional dairy products. Expanding this research to the traditional dairy products of rural Lebanon was the aim of this study. Labneh Anbaris’ rich chemical composition, high acidity, health-promoting factors as well as long shelf life allowed it to be an excellent candidate for a probiotic search. The local microbiota of this Anbaris was investigated from the probiotic point of view to be used as live microbial supplements or as new starter cultures to produce functional foods. Results allowed the classification of pooled isolates E01, E03, and K03, belonging to the Lactobacillus genus, as potential probiotic strains. Such results allow the categorization of Labneh Anbaris as a functional dairy product that can improve digestibility and nutritional value and confer several health benefits (antagonistic effect against enteric pathogens, anti-carcinogenic effects, improved immunity etc.). These health-promoting strains can also be used as dietary adjuncts to many types of functional dairy and nondairy products, boosting their beneficial health effects.
This study, however, needs further expanded research aimed at the genetic identification (genus/species level) of the different species involved. The conduction of a PCR (Polymerase Chain Reaction) for this identification is recommended to understand the nature of the species, relate them to the current results, and characterize the new probiotic strains identified. Furthermore, to assure the probiotic nature of the isolates identified, studies should be conducted on the antimicrobial metabolites (diacetyl and bacteriocins) produced by the strains proving their health-promoting immunity-boosting nature. Today, the increasing demand for safer foods with fewer chemical additives has elevated the interest in finding good alternatives to antibiotics, to which several pathogenic strains have gained resistance. Bacteriocins may present a good alternative to antibiotics and may be the gateway to producing healthier, safer foods. Future studies about the different types of Bacteriocins produced from LAB, their potential contributions to the field, and their technical applications in food need to be done.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Hassan, H. F.; Haddad, R.; Saidy, L.; Hosri, C.; Asmar, S.; Serhan, M. Tracking of Enrofloxacin Antibiotic in the Making of Common Middle Eastern Cheeses. Appl. Food Res. 2021a, 1(1), 100004. DOI: 10.1016/j.afres.2021.100004.
- Hassan, H. F.; Saidy, L.; Haddad, R.; Hosri, C.; Asmar, S.; Jammoul, A.; Jammoul, R.; Hassan, H.; Serhan, M. Investigation of the Effects of Some Processing Conditions on the Fate of Oxytetracycline and Tylosin Antibiotics in the Making of Commonly Consumed Cheeses from the East Mediterranean. Vet. World. 2021b, 14(6), 1644–1649. DOI: 10.14202/vetworld.2021.1644-1649.
- Swain, M.; Anandharaj, M.; Ray, R.; Rani, R. Fermented Fruits and Vegetables of Asia: A Potential Source of Probiotics. Biotechnol. Res. Int. 2014, 2014, 1–19. DOI: 10.1155/2014/250424.
- Kabrite, S.; Bou-Mitri, C.; Fares, J.; Hassan, H.; Boumosleh, J. Identification and Dietary Exposure Assessment of Tetracycline and Penicillin Residues in Fluid Milk, Yogurt, and Labneh: A cross-sectional Study in Lebanon. Vet. World. 2019, 12(4), 527–534. DOI: 10.14202/vetworld.2019.527-534.
- Caplice, E.; Fitzgerald, G. F.; Fitzgerald, G. F. Food Fermentations: Role of Microorganisms in Food Production and Preservation. Rev. Int. J. Food Microbiol. 1999, 50(1–2), 131–149. DOI: 10.1016/S0168-1605(99)00082-3.
- Serhan, M.; Mattar, J. Characterization of Four Lebanese Artisanal Goat Milk Cheeses: Darfiyeh, Aricheh, Shankleesh and Serdale by physico-chemical, Microbiological and Sensory Analyses. J. Food Agric. Environ. 2013, 11(3–4), 97–101.
- Dimassi, O.; Iskandarani, Y.; Afram, M.; Akiki, R.; Rached, P. Production and Physicochemical Properties of Labneh Anbaris, a Traditional Fermented Cheese like Product, in Lebanon. Int. J. Environ. Agric. 2020, 5(3), 509–516.
- Barakat, D.; The Effect of Heat Treatment and Storage Temperature on The Microbiological and Chemical Qualities of Traditional Labneh Anbaris. MSc thesis, American University of Beirut, Lebanon. 2009.
- Abu Ghyda, T.; Fahs, D.; Thevenod, E., and Klingemann, A. Article on Inventory Products Potentially Eligible for PDOs and PGIs in Lebanon. Retrieved from. Accessed 15 December 2021, 2007. http://www.economy.gov.lb/NR/rdonlyres/06141E19-A303-47FE-A01444BA70482760/0/ArticleOnInventories03042007TAG.pdf
- Aarti, C.; Khusro, A.; Arasu, M. V.; Agastian, P.; Al-Dhabi, N. A. Biological Potency and Characterization of Antibacterial Substances Produced by Lactobacillus Pentosus Isolated from Hentak, a Fermented Fish Product of North-East India. Springerplus. 2016, 5(2), 1743. DOI: 10.1186/s40064-016-3452-2.
- Al-Dhabi, N. A.; Arasu, M. V.; Vijayaraghavan, P.; Esmail, G. A.; Duraipandiyan, V.; Kim, Y. O.; Kim, H.; Kim, H. J. Probiotic and Antioxidant Potential of Lactobacillus reuterilr12 and Lactobacillus lactisll 10 Isolated from Pineapple Puree and Quality Analysis of pineapple-flavored Goat Milk Yoghurt during Storage. Microorganisms. 2020, 8(10), 1–15. DOI: 10.3390/microorganisms8101461.
- Nagpal, R.; Kumar, A.; Kumar, M.; Behare, P. V.; Jain, S., and Yadav, H. Probiotics, Their Health Benefits and Applications for Developing Healthier Foods: A Review. FEMS Microbiol. Lett. 2012, 334(1), 1–15. DOI: 10.1111/j.1574–6968.2012.02593.x.
- Aarti, C.; Khusro, A.; Varghese, R.; Arasu, M. V.; Agastian, P.; Al-Dhabi, N. A.; Ilavenil, S.; Choi, K. C. In Vitro Investigation on Probiotic, anti-Candida, and Antibiofilm Properties of Lactobacillus Pentosus Strain LAP1. Arch. Oral Biol. 2018, 89, 99–106. DOI: 10.1016/j.archoralbio.2018.02.014.
- Aarti, C.; Khusro, A.; Varghese, R.; Arasu, M. V.; Agastian, P.; Al-Dhabi, N. A.; Ilavenil, S.; Choi, K. C. In Vitro Studies on Probiotic and Antioxidant Properties of Lactobacillus Brevis Strain LAP2 Isolated from Hentak, a Fermented Fish Product of North-East India. LWT - Food Sci. Technol. 2017, 86, 438–446. DOI: 10.1016/j.lwt.2017.07.055.
- Arasu, M. V.; Al-Dhabi, N. A.; Ilavenil, S.; Choi, K. C.; Srigopalram, S. In Vitro Importance of Probiotic Lactobacillus Plantarum Related to Medical Field. Saudi J. Biol. Sci. 2016, 23(1), S6–S10. DOI: 10.1016/j.sjbs.2015.09.022.
- George Kerry, R.; Patra, J. K.; Gouda, S.; Park, Y.; Shin, H. S.; Das, G. Benefaction of Probiotics for Human Health: A Review. J. Food Drug Anal. 2018, 26(3), 927–939. DOI: 10.1016/j.jfda.2018.01.002.
- Fijan, S.; Šulc, D.; Steyer, A. Study of the in Vitro Antagonistic Activity of Various single-strain and multi-strain Probiotics against Escherichia Coli. Int. J. Environ. Res. Public Health. 2018, 15(7). DOI: 10.3390/ijerph15071539.
- Delcaru, C.; Alexandru, I.; Podgoreanu, P.; Cristea, V. C.; Bleotu, C.; Chifiriuc, M. C.; Bezirtzoglou, E.; Lazar, V. Antagonistic Activities of Some Bifidobacterium Sp. Strains Isolated from Resident Infant Gastrointestinal Microbiota on Gram-negative Enteric Pathogens. Anaerobe. 2016, 39, 39–44. DOI: 10.1016/j.anaerobe.2016.02.010.
- Agriopoulou, S.; Stamatelopoulou, E.; Sachadyn-Król, M.; Varzakas, T. Lactic Acid Bacteria as Antibacterial Agents to Extend the Shelf Life of Fresh and Minimally Processed Fruits and Vegetables: Quality and Safety Aspects. Microorganisms. 2020, 8(6), 952. DOI: 10.3390/microorganisms8060952.
- Zhang, C.; Jiang, J.; Tian, F.; Zhao, J.; Zhang, H.; Zhai, Q.; Chen, W. Meta-analysis of Randomized Controlled Trials of the Effects of Probiotics on Functional Constipation in Adults. Clin. Nutr. 2020, 39(10), 2960–2969. DOI: 10.1016/j.clnu.2020.01.005.
- Vijayakumara, M.; Ilavenil, S.; Hye Kim, D.; Arasu, M.; Priya, K.; Choon Choi, K. In-vitro Assessment of the Probiotic Potential of Lactobacillus Plantarum KCC-24 Isolated from Italian rye-grass (Lolium Multiflorum) Forage. Anaerobe. 2015, 32, 90–97. DOI: 10.1016/j.anaerobe.2015.01.003.
- Rashid, H.; Togo, K.; Ueda, M.; Miyamoto, T. Probiotic Characteristics of Lactic Acid Bacteria Isolated from Traditional Fermented Milk ‘Dahi’ in Bangladesh. Pak. J. Nutr. 2007, 6(6), 647–652. DOI: 10.3923/pjn.2007.647.652.
- Mannu, L.; Riu, G.; Comunian, R.; Frozzi, M. C.; Scintu, M. F. A Preliminary Study of Lactic Acid Bacteria in Whey Starter Culture and Industrial Pecorino Sardo Ewe’s Milk Cheese: PCR Identification and Evaluation during Ripening. Int. Dairy J. 2002, 12(1), 17–26. DOI: 10.1016/S0958-6946(01)00163-7.
- Gerhardt, P.; Murray, R. G. E.; Wood, W. A.; Kreig, N. R. Catalase Activity Methods for General and Molecular Bacteriology; American Society for Microbiology: Washington, DC, 1994; pp 614.
- Harley, J. P.; Prescott, L. M. Catalase Activity. In Laboratory Exercises in Microbiology; Brown Publishers: Dubuque, Iowa, 1993a; pp 76–77.
- Harley, J. P.; Prescott, L. M. O. T. Laboratory Exercises in Microbiology; Brown Publishers: Dubuque, Iowa, 1993b; pp 105–107.
- Kandler, O.; Weiss, N. Genus Lactobacillus Beijerinck 1901. In Bergey’s Manual of Systematic Bacteriology; Sneath, P. H. A., Mair, N. S., Sharpe, M. E., Holt, J. G., Eds.; Williams and Wilkins: Baltimore, 1986; Vol. 2, pp 1209–1243.
- Mundt, J. O. Lactic Acid Streptococci. In Bergey’s Manual of Systematic Bacteriology; Sneath, P. H. A., Mair, N. S., Sharpe, M. E., Holt, J. G., Eds.; Williams and Wilkins: Baltimore, 1986a; Vol. 2, pp 1064–1071.
- Mundt, J. O. Enterococci. In Bergey’s Manual of Systematic Bacteriology; Butler, J. P., Ed.; Williams and Wilkins: Baltimore, 1986b; pp 1063–1065.
- Prescot, L. M.; Harley, J. P.; Klein, D. A. Microbiology, 3rd ed.; Wm. C. Brown Publishers: London, 1996.
- Walker, D. R.; Gilliland, S. E. Relationship among Bile Tolerance, Bile Salt Deconjugation and Assimilation of Cholesterol by Lactobacillus Acidophilus. J. Dairy Sci. 1993, 76(4), 956–961. DOI: 10.3168/jds.S0022-0302(93)77422-6.
- Nikolic, M.; Terzic-Vidojevic, A.; Jovcic, B.; Begovic, J.; Golic, N.; Topisirovic, L. Characterization of Lactic Acid Bacteria Isolated from Bukuljac, a Homemade Goat’s Milk Cheese. Int. J. Food Microbiol. 2008, 122(1–2), 162–170. DOI: 10.1016/j.ijfoodmicro.2007.11.075.
- Tserovska, L.; Stefanova, S.; Yordanova, T. Identification of Lactic Acid Bacteria Isolated from Katyk, Goat’s Milk and Cheese. J. Cult. Col. 2002, 3, 48–52.
- Fugelsang, K. C.; Edwards, C. G. Wine Microbiology: Practical Applications and Procedures; Chapman and Hall: New York, NY, 2007.
- Quintans, N. G.; Blancato, V.; Repizo, G.; Magni, C.; López, P. Citrate Metabolism and Aroma Compound Production in Lactic Acid Bacteria. Molecular Aspects of Lactic Acid Bacteria for Traditional and New Applications. 2008, 3, 65–88.
- Bacha, K.; Mehari, T.; Ashenafi, M.; Michael, K. W.; Lindstrom, D. In-vitro Probiotic Potential of Lactic Acid Bacteria Isolated from ‘Wakalim,’ A Traditional Ethiopian Fermented Beef Sausage. Ethiop. J. Health Sci. 2009, 19(1). DOI: 10.4314/ejhs.v19i2.69419.
- Hyronimus, B.; Le Marrec, C.; Hadj, S. A.; Deschamps, A. Acid and Bile Tolerance of Spore-Forming Lactic Acid Bacteria. Int. J. Food Microbiol. 2000, 61(2–3), 193–197. DOI: 10.1016/S0168-1605(00)00366-4.
- Fallingborg, J. Intraluminal pH of the Human Gastrointestinal Tract. Dan. Med. Bull. 1999, 46(3), 183–196.
- Usman, H. A. B. T.; Hosono, A. Bile Tolerance, Taurocholate Deconjugation, and Binding of Cholesterol by Lactobacillus Gasseri Strains. J. Dairy Sci. 1999, 82(2), 243–248. DOI: 10.3168/jds.S0022-0302(99)75229-X.
- De Vuyst, L. L. F.; Leroy, F. Bacteriocins from Lactic Acid Bacteria: Production, Purification, and Food Applications. J. Mol. Microbiol. Biotechnol. 2007, 13(4), 194–199. DOI: 10.1159/000104752.
- Oscariz, J. C.; Pisabarro, A. G. Classification and Mode of Action of Membrane-Active Bacteriocins Produced by Gram-Positive Bacteria. Int. J. Microbiol. 2001, 4(1), 13–19. DOI: 10.1007/s101230100003.
- Parada, J. L.; Caron, R. C.; Bianchi, A.; Medeiros, P.; Soccol, C. R. Bacteriocins from Lactic Acid Bacteria: Purification, Properties and Use as Biopreservatives. Braz. Arch. Biol. Technol. 2007, 50(3), 521–542. DOI: 10.1590/S1516-89132007000300018.
- Ammor, E.; Tauveron, G.; Dufour, E.; Chevallier, I. Antibacterial Activity of Lactic Acid Bacteria against Spoilage and Pathogenic Bacteria Isolated from the Same Meat Small-Scale Facility 1-Screening and Characterization of the Antibacterial Compounds. Food Control. 2006, 17(6), 454–461. DOI: 10.1016/j.foodcont.2005.02.006.
- Frank, J. F. Enteropathogenic Escherichia Coli. Food Technol. 1988, 42(4), 192–193.
- Sweitzer, N. K. What Is an Angiotensin Converting Enzyme Inhibitor? Circulation. 2003, 108(3), 16–18. DOI: 10.1161/01.CIR.0000075957.16003.07.
- Taubman, M. B. Angiotensin II: A Vasoactive Hormone with Ever-Increasing Biological Roles. Circ. Res. 2003, 92(1), 9–11. DOI: 10.1161/01.RES.0000052920.70316.AE.
- Sanchez, I.; Sesena, S.; Poveda, J. M.; Cabezas, L.; Palop, L. Phenotypic and Genotypic Characterization of Lactobacilli Isolated from Spanish Goat Cheeses. Int. J. Food Microbiol. 2005, 102(3), 355–362. DOI: 10.1016/j.ijfoodmicro.2004.11.041.
- Litopoulou, T. E.; Tzanetakis, N. Microbiological Study of White-Brined Cheese Made from Raw Goat Milk. Food Microbiol. 1992, 9(1), 13–19. DOI: 10.1016/0740-0020(92)80057-B.
- Xanthopoulos, V.; Polychroniadou, A.; Litopoulou-Tzanetaki, E.; Tzanetakis, N. Characteristics of Anevato Cheese Made from Raw or Heat-treated Goat Milk Inoculated with a Lactic Starter. LWT - Food Sci. Technol. 2000, 33(7), 483–488. DOI: 10.1006/fstl.2000.0699.
- Terzic-Vidojevic, A.; Nikolic, M.; Veljovic, K.; Tolinacki, M.; Busarcevic, M.; Topisirovic, L. Analysis of the Lactic Acid Bacteria Microflora in Traditional Caucasus Cow’s Milk Cheeses. Arch. Biol. Sci. 2009a, 61(3), 395–406. DOI: 10.2298/ABS0903395T.
- Terzic-Vidojevic, A.; Lozo, J.; Topisirovic, L. Dominant Lactic Acid Bacteria in Artisanal Pirot Cheeses of Different Ripening Period. Genetika. 2009b, 41(3), 339–352. DOI: 10.2298/GENSR0903341T.
- Fernández, E.; Alegría, A.; Delgado, S.; Martín, M. C.; Mayo, B. Comparative Phenotypic and Molecular Genetic Profiling of Wild Lactococcus Lactis Subsp. Lactis Strains of the L. Lactis Subsp. Lactis and L. Lactis Subsp. Cremoris Genotypes, Isolated from Starter-Free Cheeses Made of Raw Milk. Appl. Environ. Microbiol. 2011, 77(15), 5324–5335. DOI: 10.1128/AEM.02991-10.
- Herich, R.; Levkut, M. Lactic Acid Bacteria, Probiotics and Immune System. Vet. Med. Czech. 2002, 47(6), 169–180. DOI: 10.17221/5821-VETMED.
- Khurana, H. K.; Kanawjia, S. K. Recent Trends in Development of Fermented Milks. Curr. Nutr. Food Sci. 2007, 3(1), 91–108. DOI: 10.2174/1573401310703010091.
