?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
The phenolic extracts of walnut cultivars with a red pellicle (HWE), a purple pellicle (ZWE) and a yellow pellicle (WWE) were compared. The total contents of phenolics, flavonoids and condensed tannins, as well as in vitro antioxidant activities (ABTS and DPPH) of the phenolic extracts were evaluated. The results showed that 25 phenolic compounds were identified in three colored walnuts. The HWE contained the highest contents of total phenolics and condensed tannins, while the ZWE contained the highest content of flavonoids. The DPPH and ABTS assays indicated that the phenolic extracts of colored-walnut pellicles showed a higher free radical scavenging ability than butylated hydroxytoluene (BHT) at the same concentration. Phenolic extracts of colored walnuts were also evaluated as natural antioxidants in walnut oil, substituting for BHT. The efficacy of phenolic extracts in walnut oil preservation was evaluated using accelerated storage (60 °C) for 12 days. After accelerated storage, walnut oil enriched with the phenolic extracts kept better qualities, with significantly lower peroxides, p-anisidine, and TOTOX values. The volatiles were measured and identified using the headspace solid phase micro-extraction gas chromatography to further assess oil quality. Aldehydes such as hexanal and 2-heptenal were identified as representative oxidative volatiles. Hexanal and 2-heptenal content indicated that adding phenolic extracts could prevent flavor deterioration of the oil. This study suggested that phenolic extracts of walnut pellicles could offer an effective alternative to synthetic antioxidants for walnut oil preservation.
Introduction
Walnuts, a nutritious tree nut known for its high oil and protein content, is produced worldwide. China is now the major walnut producer with 56% (>2,520,000 tonnes) of global walnut production occurring in China during 2019 (FAOSTAT, http://faostat.fao.org/faostat/). Walnut oil is rich in polyunsaturated fatty acids, including linoleic acid (C18:2; 47.4%) and α-linolenic acid (C18:3; 15.8%), which can prevent the occurrence of coronary heart disease [Citation1]. Due to the high concentrations of polyunsaturated fatty acids, walnut oil is susceptible to oxidation, resulting in off-flavors, toxic chemicals, and decreased nutritional values and qualities [Citation2]. A few methods have been proposed to retard oil oxidation, such as isolating the oil from oxygen, inactivating the oxidation related enzymes, adding chelating agents, using appropriate packaging and storing at lower temperatures [Citation3]. In addition to these methods, specific antioxidants are also known to protect the oil against oxidation.
Commercial synthetic antioxidants such as butylated hydroxyanisole, butylated hydroxytoluene (BHT), propyl gallate, and tert-butyl hydroquinone have been used as antioxidants to slow the oxidation process and extend the shelf life of the oil. However, synthetic antioxidants have regulatory limits in several nations. Previous research found that these synthetic antioxidants might be unsafe for humans due to their potential carcinogenic effects, particularly at high concentrations or long-term consumption [Citation4,Citation5]. As a result, natural antioxidants are receiving more attention. The effects of natural antioxidants during storage of vegetable oils have been reported. Mangosteen peel extracts could inhibit the primary and secondary oxidation of sunflower oil for 24 days during accelerated oxidation [Citation6]. A rosemary extract was also used to increase the oxidative stability of vegetable oil [Citation7]. Other natural phenolic extracts from seaweed,[Citation5] Sorbus aucuparia (L.) and Malus baccata (L.),[Citation8] peanut skins,[Citation9] cocoa bean husks,[Citation10] vine tea (Ampelopsis grossedentata),[Citation11] and Camellia oleifera seed cake[Citation12] were also used as antioxidants in the preservation of oils.
Walnuts have been reported to have the most abundant amount of phenolic compounds among tree nuts. Among the walnut fruit’s parts, the pellicle is one of the richest sources of phenolic chemicals, despite its relatively small mass[Citation13]. The polyphenol compounds in walnut pellicles include phenolic acids, hydrolysable tannins and a small number of flavonoids [Citation13–15]. Medic et al. found 19 ellagitannins, 12 ellagic acid derivatives, 4 anthocyanins, and 5 additional phenols in the pellicles of six different walnut cultivars [Citation16]. Previous research also found 120 compounds in walnuts using liquid chromatography combined with electrospray ionization hybrid linear trap quadrupole-Orbitrap mass spectrometry (LC-LTQ-Orbitrap), the majority of which were ellagitannins, and ellagic acid and its derivatives [Citation17]. The health benefits of walnut polyphenols include lessening arteriosclerosis, hypercholesterolemia, hypertriglyceridemia, cardiovascular disease, and diabetes mellitus [Citation14, Citation17–19]. Changes in eating habits caused consumers to demand shelled walnuts for convenience, health, and nutrition [Citation20]. The preliminary processing of walnuts in China is usually divided into the following procedures: green husk removal, drying, size classification, shell-breaking, shell-kernel separation, and pellicle removal [Citation21]. The high content of tannins contained in walnut pellicles could affect the taste and color of walnut products like walnut milk and beverage. The walnut pellicle is usually removed [Citation22]. However, the walnut pellicle is usually removed and discarded as waste [Citation23]. Colored walnuts, such as red and purple walnuts, are valuable walnut germplasm resources. Thus, the purpose of this study was to characterize the phenolic extracts of three different colored walnut pellicles including total phenolic content (TPC), flavonoids (FL), and condensed tannins (CT). ABTS and DPPH free radical scavenging activity were used to measure in vitro antioxidant activity. Besides, the effectiveness of colored-walnut pellicle extracts in retarding the oxidation of walnut oil with accelerated conditions compared to BHT was studied using peroxide, p-anisidine and TOTOX values, and volatile oxidation compounds.
Materials and methods
Materials and reagents
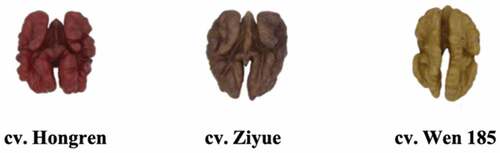
Purple walnuts (cv. Ziyue), red walnuts (cv. Hongren) and the yellow walnuts (cv. Wen 185) were used as raw materials. Two kg of Ziyue were harvested in Huidong (Liangshan, Sichuan, China) and three kg of Hongren in Luonan (Shangluo, Shanxi, China) in September 2021. The Wen 185 were obtained from Aksu, Xinjiang, China in 2021. The harvested walnuts were obtained by mechanical dehulling and drying at 40 °C to a constant weight. Walnuts were maintained at the local room temperature, mailed to the laboratory and stored at 4 °C for a maximum of 3 month.
Gallic acid, 1,1-diphenyl-2-picrylhydrazyl (DPPH), 2,2ʹazino-bis-(3-ethylbenzthiazoline-6-sulfonic acid) (ABTS) and BHT were purchased from Sigma-Aldrich (St Louis, MO, USA). All other reagents and solvents used were analytical grade and purchased from Macklin Biochemistry Co., Ltd. (Shanghai, China). Authentic standards of hexanal, 2-heptenal, 2-octenal, 1-octen-3-ol, 2,4-heptadienal and 2,4-decadienal were obtained from Sigma-Aldrich (St. Louis, MO, USA).
Preparation of walnut pellicles’ phenolic extracts
The pellicles of walnuts were carefully stripped manually and ground to powder using liquid nitrogen and freeze-dried using a freeze-drier (FD-1A-50, Hefan Instrument Co., Ltd., Shanghai, China). Phenolic extracts were obtained using the method described by Zhang et al. with minor modifications [Citation13]. About 2.0 g of pellicles were extracted with 400 mL 70% ethanol with shaking at 55 °C for 30 min. The extracts were centrifuged at 7104 x g (8000 rpm) at 4 °C for 10 min (Sigma, Bremen, Germany). This procedure was repeated twice. The supernatants were combined and the solvent removed at 40 °C using a Buchi Rotavapor (R-100, Buchi, Flawil, Switzerland) to remove the solvent. The extracts were lyophilized, powdered, and stored at – 20 °C in the darkness until further analysis.
Extraction of walnut oil
Walnut oils were obtained using a hydraulic oil press (Hengxiang Machinery Co., Ltd., Dezhou, Shandong, China) at 40 MPa. After the extraction was completed, the walnut oils were packed in 100 mL amber brown glass bottles with a screw top and stored at −20 °C for a maximum of 2 wk.
Determination of phenolic compounds
Identities of phenolic compounds were determined using a Waters UPLC system coupled with a Waters Xevo TQ/MS (Waters Corporation, Milford, MA, USA) and a ZORBAX Eclipse Plus C18 column (3.0 × 150 mm, 1.8 µm, Agilent, Santa Clara, CA, USA). The mobile phase included solutions A (0.1%, v/v, formic acid in H2O) and solution B (acetonitrile) with the following gradient: 0–1 min, 5%; 1–8 min, 5–30%; 8–12 min, 30–40%; 12–16 min, 40–95%; 16–17 min, 95–100%; 17–21 min, 100%; and then decreased to 5% for 3 min. The flow rate was 1 mL/min with an injection volume of 1 µL. The column temperature was set as 40 °C. The mass analysis was performed by ESI source in positive ion mode with an m/z scanning range of 80–800. Nitrogen was used as the desolvation gas, with a flow of 800 L/h and a temperature of 400 °C. The ion source was set at 150 °C, cone voltage of 30 V and collision voltage of 20 V. Compounds were identified and quantified by comparing their retention time and accurate MS and MS/MS spectral data with commercial standards or information previously reported in reference [Citation17]. The content of phenolic compounds was expressed µg/g dry weight (DW) of the pellicle.
Determination of TPC
The TPC of the walnut pellicles’ phenolic extracts was determined using the Folin-Ciocalteu method [Citation8]. The phenolic extracts were diluted in the 95% ethanol solution at a concentration of 50 µg/mL and then diluted to various concentrations ranging from 5 to 25 µg/mL. Then the reaction mixture contained 100 µL of diluted pellicle extracts, 500 µL of Folin-Ciocalteu reagent and 2.5 mL of 20% sodium carbonate. The mixture was then diluted to a final volume of 10 mL with deionized water (Canrex Analytical In-strument Co., Ltd., Shanghai, China). Samples were stored in the dark at room temperature (25 ± 1 °C) for 1 h. The absorbance was determined using a spectrophotometer (TU-1901, Beijing Pu Analysis General Instrument Co., Ltd., Beijing, China) at 765 nm. Results were expressed as mg of gallic acid equivalents (GAE)/g pellicle DW (dry weight).
Determination of total FL
Total FL were determined according to the method of Zhang et al. [Citation13]. Briefly, 2 mL of extract (1000 µg/mL in 60% ethanol) was mixed with 0.7 mL sodium nitrite (5%, w/v) and 0.7 mL aluminum nitrate (10%, w/v). After shaking, the mixture was allowed to stand for 6 min. Then, 6 mL of sodium hydroxide (5%, w/v) was added, the mixture was further diluted with 60% ethanol to 25 mL. After 30 min at room temperature, the absorbance was measured at 510 nm. Total FL were expressed as mg of Rutin /g pellicle DW (dry weight).
Determination of CT
The CT was measured using the method of Zhang et al. [Citation13]. Briefly, 0.5 mL of extracts (250 µg/mL in methanol) was mixed with 2.5 mL of vanillin reagent (Macklin Biochemical Co., Ltd., Shanghai, China) with 4% concentrated hydrochloric acid. After gentle mixing, the mixture was incubated in the dark at room temperature for 20 min. The absorbance was measured at 510 nm. CT were expressed as mg of CatE/g pellicle DW (dry weight).
Antioxidant activities
The antioxidant activities were determined using an ABTS radical decolorization assay and a DPPH assay, which were reported as stable assays and widely used to determine the antioxidant activity of plant materials [Citation24]. The absorbance was determined at 734 and 517 nm, respectively. BHT was used as the positive control and Trolox was used for the calibration curve. Results were expressed as the mg of Trolox E/100 g pellicle DW.
Phenolic extract enrichment of walnut oil
Lecithin (Sigma-Aldrich) was used as an emulsifier for the phenolic enrichment of walnut oil [Citation25 Briefly, 0.3% of lecithin (w/v) was dissolved in walnut oil, and then a solution of ethanol:water (50:50, v/v) containing walnut pellicle’s phenolic extract was drop-wise added to the walnut oil. The mixture was then ultrasonicated (Nanjing Safer Biotech Co., Ltd., Nanjing, Jiangsu, China) at 20 kHz using a probe for 15 min to incorporate the emulsified phenols at 1000 ppm GAE. BHT was added at 200 ppm with ultrasonication. Walnut oil without any antioxidants was used as a control.
Evaluation of walnut oil oxidation
Oil samples were stored at 60 °C for 12 days. At the end of days 3, 6, 9 and 12, ~20 g of oil samples was transferred to a screw-cap glass vial and immediately placed at −20 °C for a maximum of 3 days. Peroxide (PV)[Citation26] and p-anisidine (p-AV)[Citation26] values were determined using the standard AOCS methods. Total oxidation (TOTOX) value was calculated using the following equation[Citation6]:
Determination of volatile compounds
Volatile compounds were determined by headspace solid phase micro-extraction (HS-SPME) combined with gas chromatography-mass spectrometry (GC-MS), using the method already established by our research group [Citation2]. Two g of oil were weighed into 20 mL headspace vials with a silicone septa (Thermo Fisher Scientific Company, Waltham, MA, USA). Vials were incubated at 50 °C for 10 min. After equilibration, a 50/30 µm divinylbenzene/carboxen/polydimethylsiloxane (DVB/CAR/PDMS) fiber (Supelco, Bellefonte, PA, USA) was used to extract the volatiles at 50 °C for 30 min. The fiber was then immediately transferred to the GC’s injection port (7890A-5975C, Agilent Technologies Inc., Santa Clara, CA, USA) and desorbed at 250°C for 5 min.
Volatiles were separated using a polar HP-INNOWAX column (30 m × 0.25 mm i.d., 0.25 μm film thickness, Agilent) connected to the GC. The column temperature was programmed as follows: 40 °C for 3 min, increasing at 4 °C/min to 220 °C, and then held for 5 min. Purified helium gas was used as the carrier gas at 1 mL/min. The mass spectrometer was operated in the electron impact mode at 70 eV with a scan range of 33–650 amu. The ion source and the transfer line temperature were 230 and 280 °C, respectively.
Identification of volatiles was carried out by matching their retention times and mass spectra with authentic standards or tentatively matched with the mass spectrum with those in the US National Institutes of Standards and Technology (NIST) search library version 2.0 (https://www.nist.gov/). The identified volatile compounds were quantified using the total ion current (TIC) peak area, i.e., as a percentage of the area of the total peak areas assuming an identical linear response for each compound.
Statistical analyses
All the analyses were carried out in triplicate and the results were expressed as mean ± standard deviation. Statistical analysis was done using one-way analysis of variance (ANOVA) with the Statistical Program for the Social Sciences software (SPSS Inc., Chicago, IL, USA). Significant differences using Duncan’s multiple range test were accepted when P < .05.
Results and discussion
Phenolic compounds in colored walnuts
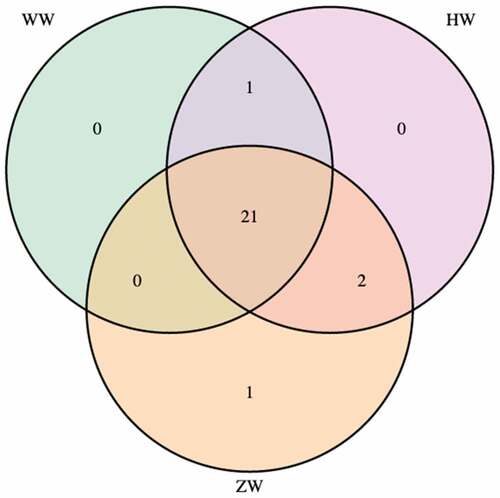
As shown in , phenolic compounds were identified by matching and analyzing the mass-to-charge ratio and the retention time of peaks in the mass spectrum, including phenolic acids, flavonoids, anthocyanins, and other polyphenols. Among them, 25 compounds were found in all colored walnut (, ), including vanillic acid, syringic acid, p-hydroxybenzoic acid, coumaric acid, and other 21 phenolic compounds in . Particularly, kaempferol were only identified in ZW. Meanwhile, two anthocyanins, such as cyanidin-3-arabinoside and cyanidin-pentoside isomer, were unique to HW and ZW. The phenolic compounds identified in our study was in consistent with the previous research, which reported the phenolic compounds in walnut pellicle and husk[23]. Persic et al. [15]reported that five anthocyanins were found in the red walnut pellicle, including four derivatives of cyanidin and one derivative of delphinidin. The total content of anthocyanins (delphinidin hexoside, cyanidin glucoside, cyanidin galactoside, cyanidin arabinoside and cyanidin xyloside) reached 3167 µg/kg fresh weight of pellicle when the red walnut was fully ripening[15]. Phenolic compounds could correspond to the antioxidant activity of walnut[40]. A previous study reported that the colored barley contains a large number of phenolic compounds with antioxidant capacity. However, the concentration of phenolic compounds is not the only factor that affects the antioxidant activity[29].
Table 1. The content of phenolic compounds in colored walnut.
TPC, total FL and CT content of phenolic extracts
shows the colors of the three different walnut cultivars. The TPC, total FL, and CT of their phenolic extracts are shown in . The HWE had the highest total phenolic content, whereas the WWE had the lowest TPC (P< .05). The TPC of colored walnut pellicles ranged from 227.1 ± 1.7 to 574.5 ± 5.6 mg GAE/g DW. Previously study found that the phenolic concentration was 370 ± 140 mg of GAE/g [Citation27]. The different concentrations between our study and the previous report could correspond to the different walnut cultivars. The total flavonoids of the HWE and ZWE were significantly higher (P < .05) than those in WWE. However, HWE contained the most condensed tannins and ZWE the lowest (P< .05).
Table 2. The total contents of phenolics (TPC), flavonoids (FL) and condensed tannins (CT) of different colored walnuts’ pellicles.
Antioxidant activities of phenolic extracts
The antioxidant activities of colored-walnut pellicle extracts (HWE, ZWE and WWE) at all tested concentrations were evaluated using the most commonly used antioxidant indicators: DPPH and ABTS. DPPH is a free radical that could accept the electrons and hydrogen radicals from donor compounds, while ABTS assay could determine the antioxidant activity through the reaction of antioxidant agent (potassium permanganate or potassium persulfate) and ABTS salt [Citation24,Citation28]. As shown in , the results indicated that the antioxidant activity of DPPH increased proportionally with the extracts concentration from 5 to 25 µg/mL. Initially, at a lower concentration of 5 µg/mL, the highest value of DPPH scavenging activity was observed in ZWE, whereas the lowest scavenging activity was found in BHT (P< .05). Moreover, the same trend of antioxidant activity was recorded for all the extracted samples at the concentration of 15 to 25 µg/mL (P < .05). The ZWE showed the highest antioxidant activity, followed by HWE, WWE, and BHT at a 25 µg/mL concentration. The results indicated that the purple walnut pellicle extract was the most effective DPPH radical scavenger than HWE and WWE. On the other hand, the colored-walnut pellicle extracts also exhibited proportional increase in antioxidant activity with a concentration of extracts (10–80 µg/mL) using ABTS method. The ABTS antioxidant efficacy of BHT was observed to be lower than that of phenolic extracts (ZWE, WWE and HWE) and showed a significant difference (P< .05) at all concentrations (10–80 µg/mL). The ZWE demonstrated higher antioxidant activity than WWE and HWE at the concentration of 80 µg/mL.
Figure 3. The in vitro antioxidant activities of colored-walnut pellicle extracts in different concentrations, (A) DPPH radical scavenging ability; (B) ABTS radical scavenging activity. The phenolic extracts of red walnut (cv. Hongren), purple walnut (cv. Ziyue) and yellow walnut (cv. Wen 185) pellicles are abbreviated as HWE, ZWE and WWE, respectively. Different letters represent a significant difference (P < .05) between the different samples at the same concentration.
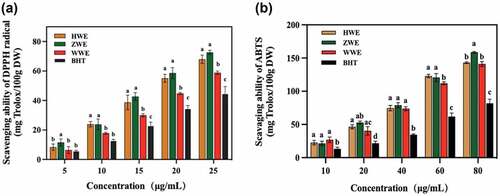
Other antioxidant studies of naturally colored plants showed that their extracts had greater antioxidant activity in vitro. Previously research studied oats’ antioxidant activity of oats with black, red, and yellow and found that the phenolic extracts of black oats showed the highest antioxidant activity than that of yellow and red oat[Citation29.] Ge et al.[Citation30] compared the antioxidant activity of phenolic compounds of naked barely with white, yellow, black, and blue colors. The results indicated that the naked black barley had higher DPPH and hydroxyl radical scavenging ability than the naked blue barley[Citation30]. The various composition and content of phenolic compounds could be responsible for the different antioxidant properties of colored naked barleys. Ge et al.[Citation30] also reported that the color barley contained many phenolic compounds with antioxidant activity. The protocatechuic acid, glycitein, and protocatechuic acid found in the color barley showed a high correlation coefficient against the oxidation. However, these compounds were not highly contained in the color barley[Citation30].
PV, p-AV and TOTOX analysis of walnut oils
The PV, p-AV, and TOTOX values of oils incubated at 60 °C for 12 days are shown in . Walnut oil without any antioxidants was most susceptible to oxidation. Walnut oil containing BHT or phenolic extracts had a decreased oxidation rate. WWE had a stronger antioxidant capacity than either HWE or ZWE.
Figure 4. Peroxide value (A), p-AV value (B) and TOTOX value (C) of walnut oil supplemented with phenolic extracts and BHT with accelerated storage at 60 °C for 12 days. The phenolic extracts of red walnut (cv. Hongren), purple walnut (cv. Ziyue) and yellow walnut (cv. Wen 185) pellicles are abbreviated as HWE, ZWE and WWE, respectively.
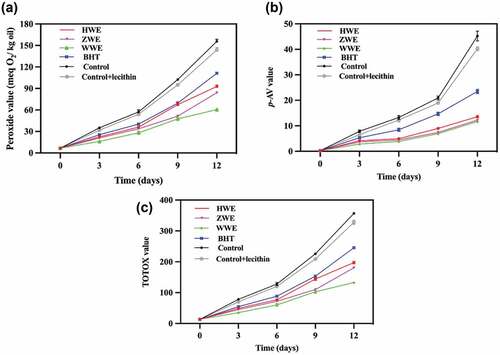
PV > 9 meqO2/kg suggests oil oxidation[Citation31]. As shown in , the same increasing trend was observed for all the oil samples during accelerated oxidation. Walnut oil without an addition had the highest PV. After 12 days of storage, the peroxide value was in the range of 60.6–92.7 meqO2/kg for phenolic extract samples, while it was 111.2 meqO2/kg for BHT samples. During the accelerated storage, all the samples showed a proportional increase in peroxide value. Peroxide value was almost 2 times higher in oil sample containing BHT than that of oil samples with WWE after 12 days of storage (P< .05). The results showed that phenolic extracts effectively protected walnut oil against oxidation.
The p-AV has been used to evaluate the secondary oxidation products of unsaturated fatty acids. ) shows that the p-AV increased with accelerated oxidation. Walnut oils stabilized with the phenolic extracts had lower p-AV than the BHT sample. A previous study suggested that p-AV <10 reflected better oil quality[Citation31]. The highest p-AV of 44.2 was found in the walnut oil without antioxidants after 12 days of storage, while the p-AV with HWE, ZWE, WWE, and BHT were 13.6, 12.2, 11.7, and 23.5, respectively. Our findings agree with those reported by Miraliakbari et al.,[Citation32] who indicated that the p-AV value of walnut oil under accelerated oxidation (60 °C) ranged from 8.6 at 3 days to 48.8 at 12 days. A significant difference (P < .05) was found between HWE and WWE during the accelerated storage of all the tested oil samples. The results of p-AV showed the following decreasing oxidative stability: WWE > ZWE > HWE > BHT. The phenolic extracts were again better than the BHT.
TOTOX values could be used to estimate the overall value of an antioxidant at both the early and late stages of oil oxidation. A lower value of TOTOX represents greater oxidative stability[Citation6]. As shown in ), the TOTOX values of all samples increased with increasing storage time. Walnut oil with phenolic extracts had lower TOTOX values than those with BHT. Consistent with the PV and p-AV results, oil stabilized with WWE showed greater oxidative stability, followed by ZWE and HWE.
Volatile compounds
Lipid oxidation is the main problem with oils as it changes an oil’s chemical, sensory, and nutritional properties. The characterization of volatile compounds can be related to oil quality and flavor deterioration [Citation33]. The results are shown in , which shows the total ion chromatograms of volatile compounds obtained from walnut oil with or without antioxidants. After storage, the oil samples enriched with phenolic extracts showed lower amounts of volatile oxidation compounds.
Figure 5. Total ion chromatograms of the volatiles formed with different oil samples with 12 days accelerated oxidation. Note: Control (walnut oil without antioxidant at 0 days), WOH (with HWE), WOZ (with ZWE), WOW (with WWE), WOB (with BHT), and WO (without antioxidant).
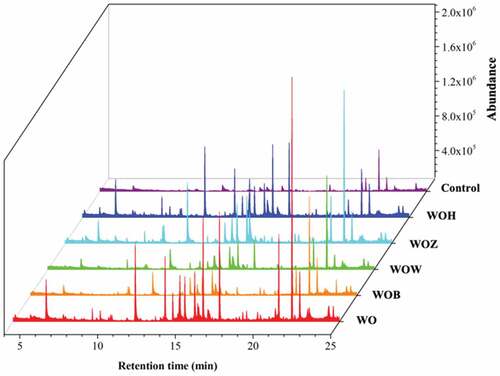
As shown in , 12 different volatile compounds were identified, including 7 aldehydes, 3 ketones, 1 alcohol, and 1 acid. The aroma descriptions of these volatiles were fatty, oily, green, rancid, fruit and nut. These compounds suggested oxidation, resulting in a loss of flavor and nutritional value that would be unacceptable to consumers and have economic consequences for oil manufacturers[Citation34]. shows a heatmap describing the changes in oil volatiles’ concentrations. After 12 days of storage at 60 °C, the highest concentrations of volatiles were found in the walnut oil without antioxidants. On the other hand, fewer volatiles were found in the oils containing phenolic extracts and BHT.
Figure 6. A heatmap of the volatile compounds in the oil samples with 12 days accelerated oxidation. Low to high rankings were determined from the peak areas. The color in green means a lower content of the compound indicate at right, the yellow means a medium content, and red means higher. Note: Control (walnut oil without antioxidant at 0 days), WOH (with HWE), WOZ (with ZWE), WOW (with WWE), WOB (with BHT), and WO (without antioxidant).
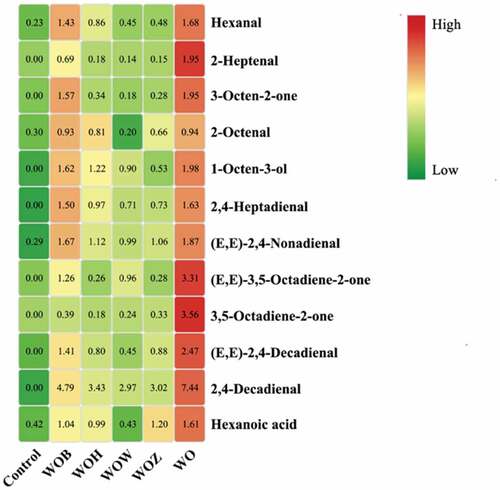
Aldehydes are the most prominent volatiles generated during lipid oxidation,[Citation2] as confirmed by this study. Hexanal, mainly produced from linoleic acid oxidation through 13-LOOH, has been an important indicator of bitter and rancid flavors[Citation2,Citation35]. Hexanal is also an important marker for detecting the degree of oxidation in nuts, such as walnut kernels, roasted almonds, and olive oil[Citation16,Citation36,Citation37]. In this study, after 12 days at 60 °C, walnut oil without antioxidants had the highest content of hexanal (1.68 x 106 e.c.) compared to BHT (1.43 x 106 e.c.), and with HWE (0.86 x 106 e.c.), ZWE (0.48 x 106 e.c.) and WWE (0.45 x 106 e.c.). Grilo and Wang also showed that after 28 wk of storage, hexanal concentration in ‘Chandler’ and ‘Howard’ walnut cultivars increased[Citation38]. A previous study reported that 2-heptanal could also be used as a marker for oxidized walnut oil[Citation2]. Walnut oil without antioxidants had the greatest 2-heptanal content (1.95 x 106 e.c.) after accelerated oxidation, whereas the other oil samples had lower levels (BHT, HWE, ZWE and WWE had 0.69, 0.18, 0.15 and 0.14 × 106 e.c., respectively). Previous studies also found that 2-heptenal was the most abundant volatile component in the oxidized walnut oil, followed by hexanal, 1-octen-3-ol, hexanoic acid, and 2,4-heptadienal[Citation2]. Most of these volatiles are generated mainly from linoleic acid and linolenic acid oxidation, which is consistent with walnut oil being mainly composed of linoleic acid (57 ~ 68%) and linolenic acid (7 ~ 9%)[Citation39,Citation40,Citation41,Citation42,Citation43,Citation44,Citation45,Citation46]. Thus, walnut oil without antioxidants had higher contents of hexanal and 2-heptanal, while walnut oil with antioxidants showed lower contents.
Other volatiles found such as 1-octen-3-ol and hexanoic acid, contribute to fruit, rancid, cheese, and fat flavors. This two volatiles could also potentially serve as markers to identify oxidation levels of walnuts[Citation38]. The highest contents of 1-octen-3-ol and hexanoic acid were found in the control, indicating that walnut oil without antioxidants showed more off-flavors than other oil samples after accelerated storage.
Conclusion
HWE and ZWE showed higher TPC, FL and in vitro antioxidant activities than the WWE. However, the efficacy of phenolic extracts in retarding walnut oil oxidation showed the opposite trend. All the phenolic extracts added to walnut oil significantly reduced the PV, p-AV and TOTOX values during accelerated storage, with WWE performing the best, indicating that the content of phenolic compounds may not be proportional to the performance of an antioxidant activity in walnut oil. The contents of 2-heptenal and hexanal, the markers of walnut oil quality, were lower in the phenolic extracts stabilized oil after accelerated storage. Therefore, this study suggested that walnut pellicle could be a safe and natural potential source of antioxidants for food stabilization, especially for unsaturated vegetable oils.
CRediT authorship contribution statement
Feng Jin: Conceptualization, writing, original draft preparation. Yaping Wang: Methodology. Ruimin Huang: Data curation. Baoxin Li: Software. Ye Zhou: Supervision, project administration, funding acquisition. Dong Pei: Resources, project administration, funding acquisition.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Grosso, A. L.; Asensio, C. M.; Nepote, V.; Grosso, N. R. Antioxidant Activity Displayed by Phenolic Compounds Obtained from Walnut Oil Cake Used for Walnut Oil Preservation. J. Am. Oil Chem. Soc. 2018, 95(11), 1409–1419. DOI: 10.1002/aocs.12145.
- Zhou, Y.; Fan, W.; Chu, F.; Wang, C.; Pei, D. Identification of Volatile Oxidation Compounds as Potential Markers of Walnut Oil Quality. J. Food Sci. 2018, 83, 2745–2752. DOI: 10.1111/1750-3841.14342.
- Mohammadi, A.; Jafari, S. M.; Esfanjani, A. F.; Akhavan, S. Application of nano-encapsulated Olive Leaf Extract in Controlling the Oxidative Stability of Soybean Oil. Food Chem. 2016, 190, 513–519. DOI: 10.1016/j.foodchem.2015.05.115.
- Mohdaly, A. A. A.; Sarhan, M. A.; Mahmoud, A.; Ramadan, M. F.; Smetanska, I. Antioxidant Efficacy of Potato Peels and Sugar Beet Pulp Extracts in Vegetable Oils Protection. Food Chem. 2010, 123, 1019–1026. DOI: 10.1016/j.foodchem.2010.05.054.
- Agregán, R.; Munekata, P. E.; Domínguez, R.; Carballo, J.; Franco, D.; Lorenzo, J. M. Proximate Composition, Phenolic Content and in Vitro Antioxidant Activity of Aqueous Extracts of the Seaweeds Ascophyllum Nodosum, Bifurcaria Bifurcata and Fucus Vesiculosus. Effect of Addition of the Extracts on the Oxidative Stability of Canola Oil under Accelerated Storage Conditions. Food Res. Int. 2017, 99, 986–994. DOI: 10.1016/j.foodres.2016.11.009.
- Chong, Y. M.; Chang, S. K.; Sia, W. C. M.; Yim, H. S. Antioxidant Efficacy of Mangosteen (Garcinia Mangostana Linn.) Peel Extracts in Sunflower Oil during Accelerated Storage. Food Biosci. 2015, 12, 18–25. DOI: 10.1016/j.fbio.2015.07.002.
- Yang, Y.; Song, X.; Sui, X.; Qi, B.; Wang, Z.; Li, Y.; Jiang, L. Rosemary Extract Can Be Used as a Synthetic Antioxidant to Improve Vegetable Oil Oxidative Stability. Ind. Crop Prod. 2016, 80, 141–147. DOI: 10.1016/j.indcrop.2015.11.044.
- Aladedunye, F.; Matthäus, B. Phenolic Extracts from Sorbus Aucuparia (L.) and Malus Baccata (L.) Berries: Antioxidant Activity and Performance in Rapeseed Oil during Frying and Storage. Food Chem. 2014, 159, 273–281. DOI: 10.1016/j.foodchem.2014.02.139.
- Franco, D.; Rodríguez-Amado, I.; Agregán, R.; Munekata, P. E. S.; Vázquez, J. A.; Barba, F. J.; Lorenzo, J. M. Optimization of Antioxidants Extraction from Peanut Skin to Prevent Oxidative Processes during Soybean Oil Storage. LWT- Food Sci. Technol. 2018, 88, 1–8. DOI: 10.1016/j.lwt.2017.09.027.
- Hernández-Hernández, C.; Morales-Sillero, A.; Fernández-Prior, M. A.; Fernández-Bolaños, J.; Aguilera-Herrera, M. P.; Rodríguez-Gutiérrez, G. Extra Virgin Olive Oil Jam Enriched with Cocoa Bean Husk Extract Rich in Theobromine and Phenols. LWT - Food Sci. Technol. 2019, 111, 278–283. DOI: 10.1016/j.lwt.2019.05.027.
- Jia, C.; Li, J.; Zhang, M.; Ma, W.; Zhao, S.; Liu, R.; Rong, J.; Li, X. Antioxidant Properties of the Extracts of Vine Tea (Ampelopsis Grossedentata) with the Different Color Characteristics and Inhibition of Rapeseed and Sunflower Oil Oxidation. LWT - Food Sci. Technol. 2021, 136, 110292. DOI: 10.1016/j.lwt.2020.110292.
- Wu, G.; Han, S.; Li, X.; Karrar, E.; Xu, L.; Jin, Q.; Zhang, H.; Wang, X. Effect of the Phenolic Extract of Camellia Oleifera Seed Cake on the Oxidation Process of Soybean Oil by 1H Nuclear Magnetic Resonance during Frying. LWT - Food Sci. Technol. 2021, 150, 111900. DOI: 10.1016/j.lwt.2021.111900.
- Zhang, Y. G.; Kan, H.; Chen, S. X.; Thakur, K.; Wang, S.; Zhang, J. G.; Shang, Y. F.; Wei, Z. J. Comparison of Phenolic Compounds Extracted from Diaphragma Juglandis Fructus, Walnut Pellicle, and Flowers of Juglans Regia Using Methanol, Ultrasonic Wave, and Enzyme assisted-extraction. Food Chem. 2020, 321, 126672. DOI: 10.1016/j.foodchem.2020.126672.
- Slatnar, A.; Mikulic-Petovsek, M.; Stampar, F.; Veberic, R.; Solar, A. Identification and Quantification of Phenolic Compounds in Kernels, Oil and Bagasse Pellets of Common Walnut (Juglans Regia L.). Food Res. Inter. 2015, 67, 255–263. DOI: 10.1016/j.foodres.2014.11.016.
- Persic, M.; Mikulic-Petkovsek, M.; Slatnar, A.; Solar, A.; Veberic, R. Changes in Phenolic Profiles of red-colored Pellicle Walnut and Hazelnut Kernel during Ripening. Food Chem. 2018, 252, 349–355. DOI: 10.1016/j.foodchem.2018.01.124.
- Medic, A.; Jakopic, J.; Hudina, M.; Solar, A.; Veberic, R. Identification and Quantification of the Major Phenolic Constituents in Juglans Regia L. Peeled Kernels and Pellicles, Using HPLC–MS/MS. Food Chem. 2021, 352, 1294. DOI: 10.1016/j.foodchem.2021.129404.
- Regueiro, J.; Sánchez-González, C.; Vallverdú-Queralt, A.; Simal-Gándara, J.; Lamuela-Raventós, R.; Izquierdo-Pulido, M. Comprehensive Identification of Walnut Polyphenols by Liquid Chromatography Coupled to Linear Ion trap–Orbitrap Mass Spectrometry. Food Chem. 2014, 152, 340–348. DOI: 10.1016/j.foodchem.2013.11.158.
- Pan, A.; Sun, Q.; Manson, J. E.; Willett, W. C.; Hu, F. B. Walnut Consumption Is Associated with Lower Risk of Type 2 Diabetes in Women. J. Nutr. 2013, 143, 512–518. DOI: 10.3945/jn.112.172171.
- Ni, Z. J.; Zhang, Y. G.; Chen, S. X.; Thakur, K.; Wang, S.; Zhang, J. G.; Shang, Y. F.; Wei, Z. J. Exploration of Walnut Components and Their Association with Health Effects. Crit. Rev. Food. Sci. Nutri. 2021, 1–17. DOI: 10.1080/10408398.2021.1881439.
- Ortiz, C. M.;.; Vicente, A. R.;.; Fields, R. P.;.; Grilo, F.;.; Labavitch, J. M.; Donis-Gonzalez, I.;.; Crisosto, C. H. Walnut (Juglans Regia L.) Kernel Postharvest Deterioration as Affected by Pellicle Integrity, Cultivar and Oxygen Concentration. Postharvest. Biol. 2019, 156, 110948. DOI: 10.1016/j.postharvbio.2019.110948.
- Liu, M.; Li, C.; Cao, C.; Wang, L.; Li, X.; Che, J.; Yang, H.; Zhang, X.; Zhao, H.; He, G., et al. Walnut Fruit Processing Equipment: Academic Insights and Perspectives. Food Eng. Rev. 2021, 13(4), 822–857. DOI: 10.1007/s12393-020-09273-6.
- Zhu, Y.; Song, H.; Zhang, X.; Chen, C.; Zhao, S.; Ge, F.; Liu, D. Recovery of Flavonoids from Walnuts de‐Pellicle Wastewater with Macroporous Resins and Evaluation of Antioxidant Activities in Vitro. J. Food Eng. 2017, 40(1), e12275.
- Sheng, F.; Hu, B. Y.; Jin, Q.; Wang, J. B.; W, C. Y.; L, Z. R. The analysis of Phenolic Compounds in walnut Husk and Pellicle by UPLC-Q-Orbitrap HRMS and HPLC. Molecules. 2021, 26, 3013. DOI: 10.3390/molecules26103013.
- Sridhar, K.; Charles, A. L. In Vitro Antioxidant Activity of Kyoho Grape Extracts in DPPH and ABTS Assays: Estimation Methods for EC50 Using Advanced Statistical Programs. Food Chem. 2019, 275, 41–49. DOI: 10.1016/j.foodchem.2018.09.040.
- Fregapane, G.; Guisantes-Batan, E.; Ojeda-Amador, R. M.; Salvador, M. D. Development of Functional Edible Oils Enriched with Pistachio and Walnut Phenolic Extracts. Food Chem. 2020, 310, 125917. DOI: 10.1016/j.foodchem.2019.125917.
- AOCS. Method Cd 8-53. In Official Methods and Recommended Practices of the American Oil Chemists’ Society, 5th ed.; AOCS Press: Champaign, Il, USA, 2009.
- Hayes, D.; Angove, M. J.; Tucci, J.; Dennis, C. Walnuts (Juglans Regia) Chemical Composition and Research in Human Health. Crit. Rev. Food Sci. Nutr. 2016, 56(8), 1231–1241. DOI: 10.1080/10408398.2012.760516.
- Chen, Z.; Bertin, R.; Froldi, G. EC50 Estimation of Antioxidant Activity in DPPH Assay Using Several Statistical Programs. Food Chem. 2013, 138, 414–420. DOI: 10.1016/j.foodchem.2012.11.001.
- Varga, M.; Jójárt, R.; Fónad, P.; Mihály, R.; Palágyi, A. Phenolic Composition and Antioxidant Activity of Colored Oats. Food Chem. 2018, 268, 153–161. DOI: 10.1016/j.foodchem.2018.06.035.
- Ge, X.; Jing, L.; Zhao, K.; Su, C.; Zhang, B.; Zhang, Q.; Han, L.; Yu, X.; Li, W. The Phenolic Compounds Profile, Quantitative analysis and Antioxidant Activity of Four Naked Barley Grains with Different Color. Food Chem. 2021, 335, 127655. DOI: 10.1016/j.foodchem.2020.127655.
- Drinić, Z.; Mudrić, J.; Zdunić, G.; Bigović, D.; Menković, N.; Šavikin, K. Effect of Pomegranate Peel Extract on the Oxidative Stability of Pomegranate Seed Oil. Food Chem. 2020, 333, 127501. DOI: 10.1016/j.foodchem.2020.127501.
- Miraliakbari, H.; Shahidi, F. Oxidative Stability of Tree Nut Oils. J. Agric. Food Chem. 2008, 56(12), 4751–4759. DOI: 10.1021/jf8000982.
- Ramos-Escudero, F.; Morales, M. T.; Escudero, M. R.; Muñoz, A. M.; Chavez, K. C.; Asuero, A. G. Assessment of Phenolic and Volatile Compounds of Commercial Sacha Inchi Oils and Sensory Evaluation. Food Chem. 2021, 140, 110022.
- Agregána, R.; Lorenzoa, J. M.; Munekatab, P. E. S.; Domingueza, D.; Carballoc, J.; Franco, D. Assessment of the Antioxidant Activity of Bifurcaria Bifurcata Aqueous Extract on Canola Oil. Effect of Extract Concentration on the Oxidation Stability and Volatile Compound Generation during Oil Storage. Food Res. Int. 2017, 99, 1095–1102. DOI: 10.1016/j.foodres.2016.10.029.
- Gallego, M. G.; Gordon, M. H.; Segovia, F. J.; Almajano, M. P. Caesalpinia Decapetala Extracts as Inhibitors of Lipid Oxidation in Beef. Molecules. 2015, 20(8), 13913–13926. DOI: 10.3390/molecules200813913.
- Clark, R. G.; Nursten, H. E. The Sensory Analysis and Identification of Volatiles from Walnut (Juglans Regia L.) Headspace. J. Sci. Food Agric. 1977, 28, 69–77. DOI: 10.1002/jsfa.2740280111.
- Yang, J.; Pan, Z.; Takeoka, G.; Mackey, B.; Bingol, G.; Brandl, M. T.; Garcin, K.; Mchugh, T. H.; Wang, H. Shelf-life of Infrared dry-roasted Almonds. Food Chem. 2013, 138, 671–678. DOI: 10.1016/j.foodchem.2012.09.142.
- Grilo, F. S.; Wang, S. C. Walnut (Juglans Regia L.) Volatile Compounds Indicate Kernel and Oil Oxidation. Foods. 2021, 10(2), 329. DOI: 10.3390/foods10020329.
- Gao, P.; Liu, R.; Jin, Q.; Wang, X. Comparative Study of Chemical Compositions and Antioxidant Capacities of Oils Obtained from Two Species of Walnut: Juglans Regia and Juglans Sigillata. Food Chem. 2019, 279, 279–287. DOI: 10.1016/j.foodchem.2018.12.016.
- Wu, S.; Shen, D.; Wang, R.; Li, Q.; Mo, R.; Zheng, Y.; Zhou, Y.; Liu, Y. Phenolic Profiles and Antioxidant Activities of Free, Esterified and Bound Phenolic Compounds in Walnut Kernel. Food Chem. 2021, 350, 129217. DOI: 10.1016/j.foodchem.2021.129217.
- Javed, H. U.; Wang, D.; Wu, G.; Kaleem, Q. M.; Duan, C.; Shi, Y. Post-storage Changes of Volatile Compounds in Air- and sun-dried Raisins with Different Packaging Materials Using HS-SPME with GC/MS. Food Res. Inter. 2019, 119, 23–33. DOI: 10.1016/j.foodres.2019.01.007.
- Matthäus, B.; Bonte, A.; Sinning, B.; Charrouf, Z. Aroma-relevant Volatile Compounds as Markers for the Sensory Quality of Argan Oil. Eur. J. Lipid Sci. Technol. 2019, 121(12), 1900279. DOI: 10.1002/ejlt.201900279.
- Wu, Y.; Duan, S.; Zhao, L.; Gao, Z.; Luo, M.; Song, S.; Xu, W.; Zhang, C.; Ma, C.; Wang, S. Aroma Characterization Based on Aromatic Series Analysis in Table Grapes. Sci. Rep. 2016, 6(1), 1–16. DOI: 10.1038/s41598-016-0001-8.
- Morales, M. T.; Rios, J. J.; Aparicio, R. Changes in the Volatile Composition of Virgin Olive Oil during Oxidation: Flavors and off-flavors. J. Agric. Food Chem. 1997, 45, 2666–2673.
- Hu, S.; Pan, B. S. Modification of Fish Oil Aroma Using a Macroalgal Lipoxygenase. J. Am. Oil Chem. Soc. 2000, 77(4), 343–348. DOI: 10.1007/s11746-000-0056-y.
- Flavornet and Human Odor Space. http://www.flavornet.org. Accessed December 20, 2021.