?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
Adlay is a cereal that has been used as food, but its use is still limited. Adlay consists of high starch content, but native adlay starch has a low water absorption capacity and other functional properties. The characteristics of adlay starch can be improved; some of them are modified into porous starch. This study aimed to define porous adlay starch granules and improve the physicochemical properties by synergic modification of microwave heat treatment (MHT) and ozonation. This study consisted of MHT, ozonation, and synergic modification of MHT-ozonation on adlay starch (native, ozonation 20 min, MHT 3 min, MHT 6 min, MHT 3 min followed by ozonation 20 min, and MHT 6 min followed by ozonation 20 min). The results showed that the best treatment was the synergic modification of 6 min of MHT followed by 20 min of ozonation. It showed the highest swelling volume of 22.43 ± 0.39 mL/g and highest water absorption capacity of 3.31 ± 0.34 g/g, the best starch stability with the lowest breakdown viscosity of 3779,33 ± 191,81 cP, and formed the most porous adlay starch. The dual modification effectively created porous starch without changing the new functional groups in starch.
Introduction
Adlay (Coix lachryma-jobi) is a cereal plant widely distributed in various countries. Adlay seeds have starch that reaches approximately 70% of their dry weight[Citation1]; this makes adlay starch be used as food. Adlay seeds are generally used as a base for adlay porridge and puffed products, whereas adlay flour can be used as a thickener for soups.[Citation2,Citation3] Even so, adlay as a food ingredient is still limited, especially in the form of starch. This limitation is due to the characteristics of native adlay flour and starch. Adlay starch has low water solubility and absorption capacity.[Citation4]
The limitations of adlay starch can be improved by changing the characteristics of native adlay starch, especially by making the adlay starch granules more porous with some modifications.[Citation4,Citation5] The pores of the starch granules can increase the solubility and water absorption of starch. Porous starch granules can also be used as absorbents, supporting the encapsulation of food compounds and slow-release agents.[Citation6] Porous starch can be prepared by partial hydrolysis using enzymes, but control of the process is quite difficult, or hydrolysis using an acid such as hydrochloric acid, but it is less safe and has the potential for chemical residues. Treatment that is considered adequate, safe, and easy is to use microwave heat treatment (MHT). MHT is a starch modification or treatment that utilizes microwave energy to be converted into heat energy. The heat energy will change the characteristics of starch.[Citation7] Factors affecting the modification are starch moisture content, modification time, and microwave power.[Citation8] MHT can improve functional properties such as swelling volume and water absorption capacity, as well as more stable pasting properties to heating.[Citation8,Citation9] The advantages of MHT are fast and selective heating, which tends to be cost-effective and environmentally friendly because they do not leave residues.[Citation10,Citation11]
MHT can be combined with other types of modifications to give a more significant effect on starch. Another modification that can be combined with MHT is ozonation. Ozonation is a modification that utilizes ozone compounds as oxidizing agents.[Citation12,Citation13] The difference between ozone and other oxidizing agents is that it quickly decomposes back into oxygen, so the process does not leave a chemical residue. Ozonation can improve functional properties such as swelling power and solubility; and increase the surface porosity of starch granules.[Citation13,Citation14] The advantages of ozonation as a starch modification are that the process is fast, strong and effective, and environmentally friendly.[Citation12,Citation15] MHT and ozonation can improve the characteristics of starch. However, research related to combining these two types of modification is still very limited. The use of MHT treatment and ozonation to make porous starch is also still limited. Therefore, this study aimed to determine the porous adlay starch granules and improve the functional and pasting properties of adlay starch with a combination of modified MHT followed by ozonation.
Materials and methods
Materials
Adlay seed (Coix lacryma-jobi L.) “Mayuen” variety which was obtained from Majalengka, West Java, Indonesia. Additional materials used are distilled water, NaOH, and ozone. The research method used was an experimental method using descriptive explanatory research analysis. At the same time, the software used for data analysis was PASW Statistics 18.
Adlay starch extraction
Adlay starch extraction refers to Capule and Trinidad[Citation4] with modifications. Adlay seeds that have been ground into flour were soaked in 0.3% NaOH for 24 h with a ratio of 1:4. The flour solution was then stirred and filtered using a filter cloth. The first filtering dregs were soaked again with 0.3% NaOH and filtered using a filter cloth. The dregs of the second filtering were soaked again with 0.3% NaOH and filtered again as in the previous step. The filtering results were deposited in a container for 24 h. The filtered starch was then washed 3 times to neutralize the remaining NaOH and remove the remaining impurities. The adlay starch was then dried in a cabinet oven at 50°C for 17 h and sieved on a 100 mesh sieve.
Modification of adlay starch using microwave heat treatment (MHT) and ozonation
The modification process of adlay starch MHT was carried out according to Román et al.[Citation16] Adlay starch was adjusted to the water content up to 30% with the addition of aquadest. The starch was then stored at 4°C for 24 h. Next, the starch was put into a microwave oven (Sanyo EM-S1073) with a low strength level (Low) for 3 min and 6 min, respectively. The modified starch was then dried at 50°C for 12 h. While the ozonation process of adlay starch was carried out, referring to Sandhu et al. (2012). The adlay starch was then put into the ozone tube. Ozonizer was conditioned until it reached a steady flowrate of 2 L/min. Ozone gas was then passed through a tube containing adlay starch for 20 min. The sample was stirred for 5 minutes after completion of the ozonation. The adlay starch resulting from the ozonation was then transferred to a closed metalized pouch and stored at 4°C. This treatment consisted of MHT, ozonation, and synergic modification of MHT-ozonation on adlay starch (native, ozonation 20 min, MHT 3 min, MHT 6 min, MHT 3 min followed by ozonation 20 min, and MHT 6 min followed by ozonation 20 min).
Analysis of swelling volume and solubility
The swelling volume test was carried out using the Collado and Corke[Citation17] method. Adlay starch weighing 0.35 g (db) was put into a centrifugation tube and was added by 12.5 mL of aquadest. The sample was then stirred using a vortex for 30 seconds. The sample was then heated at 80°C for 30 min. Then, the sample was cooled in cold water for 1 minute. Samples were centrifuged in a centrifuge at a speed of 3500 rpm for 30 min. The supernatant and gel were then separated. The supernatant was then transferred to a constant cup to dry in an oven at 110°C for 24 h. The dry supernatant was weighed. The swelling volume and solubility values were measured using the following formula.
Analysis of water absorption capacity (WAC)
The WAC of starch was tested according to Beuchat.[Citation18] The adlay starch was weighed 1 g (dry base) and then put into a centrifugation tube. 10 mL of aquadest was added to the sample’s centrifugation tube, then stirred using a vortex for 30 seconds. The sample was then allowed to stand at 25°C for 30 min. The sample was then centrifuged using a centrifuge for 30 min at 3500 rpm. The supernatant and gel were separated, and the supernatant’s weight was weighed. The value of WAC was measured by the following formula:
Analysis of color by chromameter
The adlay starch color test was carried out using the Chromameter Lab Hunter. The chromameter tool was turned on, and the EZMQ application on the computer desktop was opened. The adlay starch sample was put into a cleaned chromameter glass. The bottom surface of the chromameter glass was ensured to be fully covered with the sample without any gaps, and the placement of the sample in the glass was parallel. The EZMQ application was run for color testing. The test was carried out three times (triple). The data displayed were L*, a*, and b*.
Analysis of carbonyl and carboxyl content
The carbonyl content of adlay starch was analyzed according to the method of Smith,[Citation19] and the carboxyl content of adlay starch was analyzed according to the method of Chattopadhyay et al.[Citation20]
Determination of pasting properties
The Pasting properties of adlay starch were tested using a rapid visco analyzer (RVA-SM2). The adlay starch sample was weighed ± 3.5 g and put into a canister. Then, ± 25 mL of aquadest was added to the sample’s canister. The canister was then attached to the RVA apparatus for testing the pasting properties. Testing began by stirring the sample at a constant temperature of 50°C for 1 minute. The sample was heated from 50°C until it reached 95°C at a speed of 6°C/minute. Stirring the sample was held at 95°C for 5 min, then the sample was cooled to 50°C. The sample was held back at 50°C for 5 min before the test was completed.[Citation21]
Determination of starch granule morphology
Morphological analysis of adlay starch granules was conducted using a scanning electron Microscope (SEM JEOL-JSM6510). The adlay starch was placed in a sample holder with double-side isolation. Then, a gold layer was applied to the sample, and the sample was placed in the SEM. The morphology of adlay starch was analyzed with a magnification scale of 2000x and 7000x.
Determination of functional group
The analysis of the functional group of adlay starch was carried out using a combination of Fourier transform infrared spectroscopy (FTIR) instruments (Nicolet iS10 FTIR spectrometer) and attenuated total reactance (ATR). Adlay starch samples were used for about 1–2 mg. Pure KBr powder weighed 200 g and was added to the sample. Then, the sample was stirred and placed in the mold. Sampling was carried out for several min while being held. The samples were then analyzed in the FTIR instrument.
Results and discussion
Swelling volume and solubility
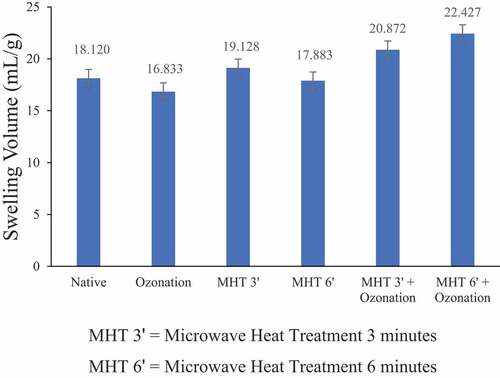
The swelling volume of adlay starch can be seen in . It was known that ozonation reduces the adlay starch swelling volume. The adlay starch with ozonation treatment had the lowest swelling volume value. The decrease in swelling volume can be caused by disintegration in the starch granules during ozonation.[Citation22] Oxidation is also claimed to penetrate starch granules deeply, especially in amorphous regions.[Citation23] Modifying MHT for 3 min and combining MHT-ozonation gave the opposite effect, where the swelling volume value of adlay starch increased after modification. Adlay starch with the highest swelling volume was found in adlay starch with MHT combination treatment for 6 min and followed by ozonation for 20 min. Under these conditions, the swelling volume increased 1.24 times compared to native starch. The increase in swelling volume can be caused by a synergistic combination of modification treatment, which affects changes in the amylopectin chain of starch compared to a single modification. The increase in swelling volume can also be caused by structural changes in the amylopectin chain and the expansion of amorphous regions in starch. According to Deka and Sit,[Citation9] the longer amylopectin chains increase the number of hydroxyl groups contained in starch granules, so the ability of starch to expand also increases. The increase in swelling volume occurred due to the high amylopectin content and the wider amorphous area, namely an increase in the porosity of starch granules which increases the surface area of starch granules. MHT and oxidation treatment can increase the surface porosity of starch granules.[Citation9,Citation13] The expansion of amorphous regions in starch can cause an increase in starch expansion.
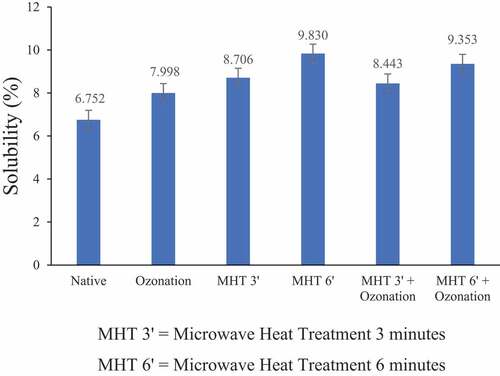
The adlay starch solubility can be seen in . Both ozonation and MHT increased the solubility of native adlay starch. The highest adlay starch solubility was shown in adlay starch with 6 min of MHT modification, which increased 1.46 times compared to native starch. The heating process could cause an increase in solubility after microwaves modified starch. The hydrogen bonds in the amylose and amylopectin branched chains were broken, making it easier for the hydrogen bonds of water to unite with the amylose hydroxyl groups of starch, which play a role in binding water molecules. Therefore, the solubility of starch also increases.[Citation24,Citation25] The high temperature caused the depolymerization of starch molecules, so amylose became simpler, shorter, and more water-soluble straight chains. The increase in the solubility of adlay starch after ozonation for 20 min was due to oxidation by ozone, causing the starch granule structure to weaken and causing depolymerization of starch molecules. The increasing pattern of swelling characteristic of ozone-treated starch has been associated with the introduction of a hydrophilic carboxyl group.[Citation26] Also, the negative charges of carboxyl groups repel each other, which causes an increase in the swelling of starch granules upon heating.[Citation27] Depolymerization weakens starch bonds and makes the water more easily absorbed so that starch solubility increases.[Citation28]
Water absorption capacity (WAC)
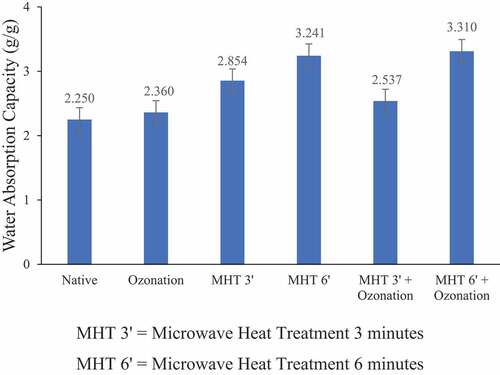
The WAC of adlay starch can be seen in . Both ozonation and MHT increased the water absorption capacity of adlay starch. Adlay starch with the highest water absorption capacity was adlay starch with MHT combination treatment for 6 min followed by ozonation for 20 min. Under these conditions, the WAC increased 1.47 times compared to native starch. The increase in water absorption capacity could be due to the addition of hydrophilic carboxyl groups by ozone so that the ability of starch to absorb water increases. The pores on the surface of the starch granules are also influential because they can facilitate the penetration of water into the granules, thereby increasing the water absorption capacity of starch.[Citation29]
Color chromaticity
The results of the adlay starch color can be seen in . L* represents the brightness level of starch, a* represents green for negative values and red for positive values, b* represents blue for negative values and yellow for positive values, and ΔE* represents the total color difference between the treatments sample and control (native starch). shows that native adlay starch has a brighter color than modified adlay starch. However, the total color change indicated by E* was very small between modified adlay starch and native starch (range 0.18–2.68), so the color differences were difficult to distinguish visually. In general, the ozonation process can lighten the color of starch, but in this study, ozonation did not give a significant difference to native adlay starch. This could be because the ozone is not strong enough to lighten the color of the adlay starch. MHT modification decreased the L* value of adlay starch, thus indicating a decrease in the brightness of adlay starch. This decrease could be due to the caramelization process in the starch. High temperatures can cause caramelization reactions of sugars in starch. The MHT adlay starch also showed an increase in the b* value, which indicated that the adlay starch became more yellow. An increase in the value of b* was due to the depolymerization of starch by microwave heating.
Table 1. Effect of MHT and ozonation on the color chromaticity of adlay starch.
Carbonyl and carboxyl content
Carbonyl content and carboxyl content of adlay starch and their modifications can be seen in . Ozonation and MHT treatments increased carbonyl and carboxyl content, with the highest increase occurring at the treatment of MHT 6 min followed by ozonation for 20 min at a flow rate of 2 L/min, where carbonyl content and carboxyl content increased 2.38 times and 1.93 times, respectively. Carbonyl groups are produced mainly in the early stages of starch oxidation, then further increase in carboxyl groups will result. The heat energy from the MHT treatment can break the C-C bond, especially between C2 and C3 on glucose molecules in starch polymers to produce carbonyl (C = O) and carboxyl (HO-C = O) groups, so that it can produce cracks or cavities in starch granules. Soto et al.[Citation30] reported that hydrothermal treatment increased the carbonyl and carboxyl content of corn starch. While ozonation effectively oxidizes starch molecules because ozone is a very strong oxidizing agent, it can break the C-C bond and change the hydroxyl group of the starch molecule into carbonyl and carboxyl groups. Satmalawati et al.[Citation14] reported that ozonation was effective in increasing the carbonyl and carboxyl content of cassava starch. The ozonation treatment, which was preceded by the MHT treatment, resulted in effective starch oxidation because the cracks in the starch granules due to the MHT treatment also facilitated the penetration of ozone to oxidize the starch.
Table 2. Effect of MHT and ozonation on the carbonyl and carboxyl content of adlay starch.
Pasting properties
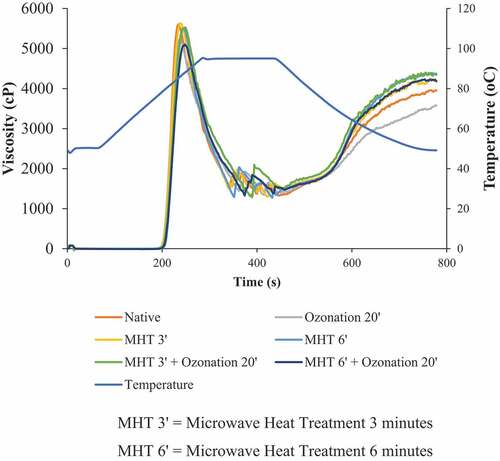
The pasting properties of native and modified adlay starch are presented in and . The initial gelatinization temperature of native adlay starch is not much different from that of modified adlay starch. However, starch modification tends to decrease the peak viscosity of adlay starch. The combination of 6 min of MHT modification followed by 20 min of ozonation showed the lowest peak viscosity. This could be due to a change in the size of the starch granules and the damage to the integrity of the adlay starch granules. Reducing the size of starch granules can reduce the peak viscosity of starch.[Citation31] This is also affected by the breaking of the C-C bond and the breakdown of the hydroxyl group into carbonyl and carboxyl groups of the starch, as shown in . Modified starch tends to decrease the breakdown viscosity of adlay starch with adlay starch with a combination of modified MHT for 6 min followed by ozonation for 20 min showing the lowest breakdown viscosity. The low breakdown viscosity indicates that the starch is more stable against shear forces during stirring. Hot temperatures cause partial gelatinization and produce a stronger starch structure against shear forces.[Citation32] Ozonation also decreases the breakdown viscosity of native adlay starch. It could be due to the increased carbonyl and carboxyl content of adlay starch. The increase in carbonyl and carboxyl levels of starch by ozone (see ) inhibits starch chain re-association, thereby reducing breakdown viscosity.[Citation33]
Table 3. Effect of MHT and ozonation on the pasting properties of adlay starch.
Modification of MHT and the combination of MHT-ozonation increased the setback viscosity of adlay starch with the highest setback viscosity of adlay starch with 6 min of MHT modification. The high setback viscosity indicates that the adlay starch is more likely to undergo retrogradation. According to Chisenga et al.,[Citation34] starch with high setback viscosity can produce a faster retrogradation rate. On the other hand, ozonation treatment showed adlay starch with the lowest setback viscosity. Ozonation makes adlay starch less susceptible to starch re-association. The increase in viscosity depended on the tendency of the starch to re-associate when the hot paste was cooled. Starch re-association was determined by the attraction between the hydroxyl groups of starch. Oxidation by ozone gas forms a carboxyl group in starch, where the carboxyl group plays a role in replacing the hydroxyl group of starch. As a result, this process limits the attractiveness between the hydroxyl groups of starch so that the starch becomes less susceptible to re-association and reduces the setback viscosity.
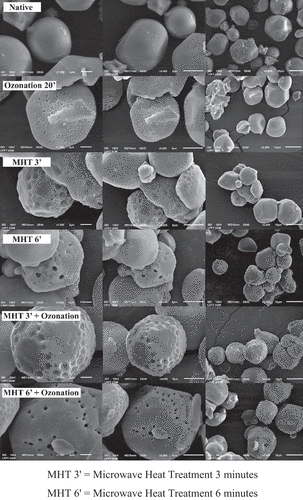
Starch granule morphology
The starch granule morphology can be seen in . The native adlay starch granules are non-uniform in shape, with a non-porous smooth surface following the adlay flour starch granules observed by Dechkunchorn and Thongngam.[Citation35] Ozonation of 20 min seems to be able to form small pores in native adlay starch granules. Oxidation by ozone gas weakens the crystalline structure of starch, causing pores and cracks on the surface of granules.[Citation13] MHT causes clearer cavities and pores on the surface of native adlay starch granules. Adlay starch granule shape also becomes irregular and coarse. This change is due to the principle of microwaves, namely dielectric properties. The dielectric properties of starch indicate the ability of starch to absorb microwave energy and convert it into heat energy. Starch begins to gelatinize after sufficient heat energy is converted from microwave energy.[Citation7] Porous and coarse starch granules show effective absorption and energy conversion because morphological changes depend on gelatinization.[Citation36]
The water content of adlay starch had previously been added up to 30% during the MHT process. The high water content determines the changes in starch morphology that occur. According to BeMiller and Huber,[Citation37] polar molecules will spin, hit and fight against each other while adjusting to the electromagnetic field of microwaves. Friction was caused by polar molecules that would generate heat energy. This phenomenon is called the dielectric relaxation phenomenon. Tao et al.[Citation7] stated that starch with low moisture content has microwave energy absorption properties and weak conversion ability. The starch granules will remain intact after the MHT process. According to Brasoveanu and Nemtanu,[Citation8] the dielectric relaxation of starch caused the starch granules to heat rapidly, so the water molecules evaporated, and high pressure was formed in the starch granules. It made the starch granules widen from the center. The hydration that occurred cannot follow the expansion of the granules, causing the starch granules to degrade and break.
Modification of the MHT combination for 6 min followed by ozonation for 20 min showed the surface with the clearest and deepest pores. It can be caused by microwave heating to form pores that were quite deep in the adlay starch granules, and the starch granules were increasingly cracked and damaged due to the oxidation of ozone gas, resulting in clearer pores.
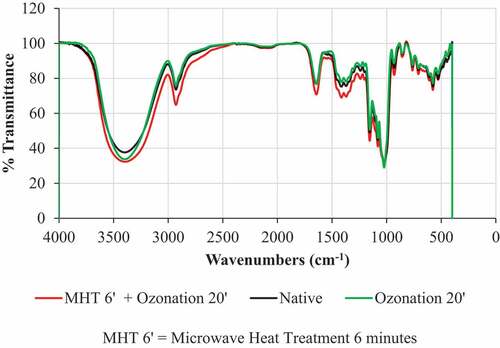
Functional group of starch
The results of the starch functional group test are presented in . Based on the results of the FTIR test, the modification was not able to form new absorptions in the FTIR spectra of adlay starch. This indicated that the starch modification carried out was not strong enough to change the chemical structure of adlay starch and form new functional groups. FTIR spectra of native adlay starch showed that adlay starch had O-H (hydroxyl) groups because there was absorption at wave number 3397–3398 cm−1. In addition, native adlay starch also showed absorption at a wave number of 1640 cm−1, which indicated the presence of a C = O group (carbonyl group) in native starch without ozonation. This is because starch is a glucose homopolymer that has a native carbonyl group and a hydroxyl group in its structure. However, the increase in hydroxyl and carbonyl groups can be seen from the absorption intensity in the FTIR spectrum. The absorption intensity of modified adlay starch was sharper than native adlay starch at wave numbers 3397–3398 cm−1 and wave numbers 1640 cm-1. Sharper absorption indicates the presence of higher carbonyl and hydroxyl levels.[Citation14] The oxidation of starch generally causes a change in the hydroxyl group to a carbonyl group and a carboxyl group so that the carbonyl and carboxyl content of starch increases. The ratio of the bands at 1045 and 1022 cm−1 was also important due to the link with the crystalline and amorphous regions in the starch.[Citation38] The results indicated that the smaller ratio in the adlay starch-modified ozonation and MHT 6 min + ozonation (with the same value, namely 0.88) compared to native starch (0.90). Thus, modified starch had a larger amorphous region than native starch. However, research on modified adlay starch showed that although there was no addition of new functional groups in modified adlay starch, there was an increase in the intensity of carbonyl groups, carboxyl groups, and the amorphous region of the starch.
Conclusion
Modification of adlay starch with a combination of Microwave Heat Treatment (MHT) and ozonation methods increased the swelling volume, solubility, and water absorption capacity of starch. The combination of MHT-ozonation modification can also change the pasting properties of adlay starch by decreasing the breakdown viscosity and peak viscosity and increasing the setback viscosity. The combination of MHT 6 min followed by ozonation 20 min produced adlay starch with better characteristics. The granules had a porous surface and the increasing carbonyl content, carboxyl content, and the amorphous region of starch. However, the modification did not lead to the formation of new functional groups in starch.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Liu, H.; Shi, J.; Cai, Z.; Huang, Y.; Lv, M.; Du, H.; Gao, Q.; Zuo, Y.; Dong, Z.; Huang, W., et al. Evolution and Domestication Footprints Uncovered from the Genomes of Coix. Mol. Plant.2020, 13(2), 295–308. DOI: 10.1016/j.molp.2019.11.009
- Cahyana, T. Y.; Nurhadi, B.; Desani, W. D.; Subroto, E. In Vitro Digestibility and the Characteristics of Puffed Products from Different Degrees of Milling and Moisture Content of Adlay Grain (Coix Lacryma – Jobi L.). Food Res 2021, 5(2), 371–378. DOI: 10.26656/fr.2017.5(2).548.
- Chaisiricharoenkul, J.; Tongta, S.; Intarapichet, K. Structure and Chemical and Physicochemical Properties of Job ’ S Tear (Coix Lacryma-Jobi L .) Kernels and Flours. Suranaree J.Sci. Technol 2011, 18(2), 109–122.
- Capule, A. B.; Trinidad, T. P. Isolation and Characterization of Native and Modified Starch from Adlay (Coix Lacryma Jobi-L.). Int. Food Res. J 2016, 23(3), 1199–1206.
- Subroto, E.; Indiarto, R.; Wulandari, E.; Astari, A. P. Modification of Porous Adlay (Coix Lacryma-Jobi L.) Starch by Ultrasonication and Ozonation. J. Teknol. Has. Pertan. 2021, 142, 117. DOI:10.20961/jthp.v14i2.54338
- Chen, J.; Wang, Y.; Liu, J.; Xu, X. Preparation, Characterization, Physicochemical Property and Potential Application of Porous Starch: A Review. Int. J. Biol. Macromol 2020, 148, 1169–1181. DOI: 10.1016/j.ijbiomac.2020.02.055.
- Tao, Y.; Yan, B.; Fan, D.; Zhang, N.; Ma, S.; Wang, L.; Wu, Y.; Wang, M.; Zhao, J.; Zhang, H. Structural Changes of Starch Subjected to Microwave Heating: A Review from the Perspective of Dielectric Properties. Trends Food Sci. Technol 2020, 99, 593–607. DOI: 10.1016/j.tifs.2020.02.020.
- Brasoveanu, M.; Nemtanu, M. R. Behaviour of Starch Exposed to Microwave Radiation Treatment. Starch/Staerke. 2014, 66(1–2), 3–14. DOI: 10.1002/star.201200191.
- Deka, D.; Sit, N. Dual Modification of Taro Starch by Microwave and Other Heat Moisture Treatments. Int. J. Biol. Macromol 2016, 92, 416–422. DOI: 10.1016/j.ijbiomac.2016.07.040.
- Adiyanti, T.; Subroto, E. Modifications Of Banana Starch And Its Characteristics : A Review. Int. J. Sci. Technol. Res 2020, 9(3), 3–6.
- Vadivambal, R.; Jayas, D. S. Changes in Quality of Microwave-Treated Agricultural Products-a Review. Biosyst. Eng 2007, 98(1), 1–16. DOI: 10.1016/j.biosystemseng.2007.06.006.
- Pandiselvam, R.; Manikantan, M. R.; Divya, V.; Ashokkumar, C.; Kaavya, R.; Kothakota, A.; Ramesh, S. V. Ozone: An Advanced Oxidation Technology for Starch Modification. Ozone Sci. Eng. 2019, 416, 491–507. DOI:10.1080/01919512.2019.1577128
- Castanha, N.; Matta Junior, M. D.; da Augusto, P. E. D. Potato Starch Modification Using the Ozone Technology. Food Hydrocoll 2017, 66, 343–356. DOI: 10.1016/j.foodhyd.2016.12.001.
- Satmalawati, E. M.; Pranoto, Y.; Marseno, D. W.; Marsono, Y. Oxidation of Cassava Starch at Different Dissolved Ozone Concentration: Effect on Functional and Structural Properties. Food Res 2020, 4(6), 1896–1904. DOI: 10.26656/fr.2017.4(6).209.
- Nath, A.; Mukhim, K.; Swer, T.; Dutta, D.; Verma, N.; Deka, B. C.; Gangwar, B. A Review on Application of Ozone in the Food Processing and Packaging. J. Food Prod. Dev. Packag. 2014, 1, 7–21.
- Román, L.; Martínez, M. M.; Rosell, C. M.; Gómez, M. Effect of Microwave Treatment on Physicochemical Properties of Maize Flour. Food Bioprocess Technol 2015, 8(6), 1330–1335. DOI: 10.1007/s11947-015-1493-0.
- Collado, L. S.; Corke, H. Heat-Moisture Treatment Effects on Sweet potato Starches Differing in Amylose Content. Food Chem 1999, 65(3), 339–346. DOI: 10.1016/S0308-8146(98)00228-3.
- Beuchat, L. R. Functional and Electrophoretic Characteristics of Succinylated Peanut Flour Protein. J. Agric. Food Chem 1977, 25(2), 258–261. DOI: 10.1021/jf60210a044.
- Smith, R. J.;. Production and Use of Hypochlorite Oxidized Starches. In Starch Chemistry and Technology; Whistler, R. L., Paschall, E. F., Eds.; Academic Press: New York, USA, 1967; pp 620–625.
- Chattopadhyay, S.; Singhal, R. S.; Kulkarni, P. R. Optimisation of Conditions of Synthesis of Oxidised Starch from Corn and Amaranth for Use in Film-Forming Applications. Carbohydr. Polym 1997, 34(4), 203–212. DOI: 10.1016/S0144-8617(97)87306-7.
- Subroto, E.; Indiarto, R.; Marta, H.; Shalihah, S. Effect of Heat Moisture Treatment on Functional and Pasting Properties of Potato. Food Res 2019, 3(October), 469–476. DOI: 10.26656/fr.2017.3(5).110.
- Lawal, O. S. Composition, Physicochemical Properties and Retrogradation Characteristics of Native, Oxidised, Acetylated and Acid-Thinned New Cocoyam (Xanthosoma Sagittifolium) Starch. Food Chem 2004, 87(2), 205–218. DOI: 10.1016/j.foodchem.2003.11.013.
- Forssell, P.; Hamunen, A.; Autio, K.; Suortti, P.; Poutanen, K. Hypochlorite Oxidation of Barley and Potato Starch. Starch‐Stärke. 1995, 47(10), 371–377. DOI: 10.1002/star.19950471002.
- Zeng, S.; Wu, X.; Lin, S.; Zeng, H.; Lu, X.; Zhang, Y.; Zheng, B. Structural Characteristics and Physicochemical Properties of Lotus Seed Resistant Starch Prepared by Different Methods. Food Chem 2015, 186, 213–222. DOI: 10.1016/j.foodchem.2015.03.143.
- Narwojsz, A.; Borowska, E. J.; Polak-Śliwińska, M.; Danowska-Oziewicz, M. Effect of Different Methods of Thermal Treatment on Starch and Bioactive Compounds of Potato. Plant Foods Hum. Nutr 2020, 75(2), 298–304. DOI: 10.1007/s11130-020-00808-0.
- Chan, H.-T.; Fazilah, A.; Bhat, R.; Leh, C.-P.; Karim, A. A. Effect of Deproteinization on Degree of Oxidation of Ozonated Starch. Food Hydrocoll 2012, 26(2), 339–343. DOI: 10.1016/j.foodhyd.2011.03.006.
- Sandhu, H. P. S.; Manthey, F. A.; Simsek, S. Ozone Gas Affects Physical and Chemical Properties of Wheat (Triticum Aestivum L.) Starch. Carbohydr. Polym 2012, 87(2), 1261–1268. DOI: 10.1016/j.carbpol.2011.09.003.
- Trinh, K. S.; Dang, T. B. Structural, Physicochemical, and Functional Properties of Electrolyzed Cassava Starch. Int. J. Food Sci 2019, 2019, 1–7. DOI: 10.1155/2019/9290627.
- Handarini, K.; Hamdani, J. S.; Cahyana, Y.; Setiasih, I. S. Gaseous Ozonation at Low Concentration Modifies Functional, Pasting, and Thermal Properties of Arrowroot Starch (Maranta Arundinaceae). Starch/Staerke. 2020, 72(5–6), 1900106. DOI: 10.1002/star.201900106.
- Soto, D.; Urdaneta, J.; Pernia, K. Characterization of Native and Modified Starches by Potentiometric Titration. J. Appl. Chem 2014, 2014, 1–9. DOI: 10.1155/2014/162480.
- Singh, N.; Kaur, L. Morphological, Thermal, Rheological and Retrogradation Properties of Potato Starch Fractions Varying in Granule Size. J. Sci. Food Agric. 2004, 84(10), 1241–1252. DOI: 10.1002/jsfa.1746.
- Nadiah, N. I.; Uthumporn, U.; Syahariza, Z. A. Effect of Microwave Heating on Potato and Tapioca Starches in Water Suspension. Int. J. Adv. Sci. Eng. Inf. Technol. 2016, 54, 264–271. DOI:10.18517/ijaseit.5.4.502
- Liu, J.; Wang, B.; Lin, L.; Zhang, J.; Liu, W.; Xie, J.; Ding, Y. Functional, Physicochemical Properties and Structure of Cross-Linked Oxidized Maize Starch. Food Hydrocoll 2014, 36, 45–52. DOI: 10.1016/j.foodhyd.2013.08.013.
- Chisenga, S. M.; Workneh, T. S.; Bultosa, G.; Laing, M. Characterization of Physicochemical Properties of Starches from Improved Cassava Varieties Grown in Zambia. AIMS Agric. Food. 2019, 44, 939–966. DOI:10.3934/agrfood.2019.4.939
- Dechkunchorn, M.; Thongngam, M. Characterization of Flour, Starch and Protein from White and Black Adlay Cultivars. Kasetsart J. Nat. Sci 2016, 11(3), 31–40.
- Oyeyinka, S. A.; Umaru, E.; Olatunde, S. J.; Joseph, J. K. Effect of Short Microwave Heating Time on Physicochemical and Functional Properties of Bambara Groundnut Starch. Food Biosci 2019, 28, 36–41. DOI: 10.1016/j.fbio.2019.01.005.
- BeMiller, J. N.; Huber, K. C. Physical Modification of Food Starch Functionalities. Annu. Rev. Food Sci. Technol 2015, 6(1), 19–69. DOI: 10.1146/annurev-food-022814-015552.
- Monroy, Y.; Rivero, S.; García, M. A. Microstructural and Techno-Functional Properties of Cassava Starch Modified by Ultrasound. Ultrason. Sonochem 2018, 42, 795–804. DOI: 10.1016/j.ultsonch.2017.12.048.