?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
Black soybean hull, as main by-production in black bean processing, has significant biological activity. In this study, the polysaccharide from black soybean hull (BSPS-H) was obtained by enzymatic hydrolysis, the yield was 5.3%, and its chemical composition and structural composition were further studied. The results shown that BSPS-H was mainly composed of Mannose, Galactose, and Glucose (molar ratio = 48.1: 29.7: 15.8), and might be existed in the form of pyran ring in α/β-linkage type. The anti-oxidant activity shown that BSPS-H had a certain capacity of anti-oxidation in a concentration-dependent manner. Among them, it has the best scavenging effect on DPPH free radicals. When the polysaccharide concentration was 10 mg/ml, the scavenging rate reached 94.37%. The results of anti-fatigue effect indicated that BSPS-H could prolong the duration of exhaustive swimming in mice, increased the contents of liver glycogen, reduced the content of blood urea nitrogen (BUN), enhanced the activity of lactate dehydrogenase (LDH) and reduced the accumulation of lactic acid (LA). This study provides a fundamental basis for the development and use of BSPS-H in medicine and functional foods.
Introduction
Black soybean (Glycine max (L.) Merr.) is the black seed of the leguminous plant soybean, which is widely cultivated in the United States, China, Japan and other countries .[Citation1] Black soybeans contain highly nutritious substances, including protein, lipid, polyphenols, polysaccharides, and other active ingredients which have good biological activity .[Citation2] Recent studies on black soybean suggest that peptides from this legume of black soybean can stabilize postprandial blood glucose levels in prediabetic patients,[Citation3] its polyphenols of black soybean have anti-inflammatory effects,[Citation4] its black bean extract can reduce abdominal obesity [Citation5] and polysaccharide of black soybean can protect against carbon tetrachloride-induced acute liver injury in mice .[Citation6] Black soybean hull is a by-product of black bean processing, which is used as a feed or discarded, resulting in a waste of resources .[Citation7] At present, most of the work is focused on the whole black soybean, and studies on the by-products of black soybean are fewer. Thus, exploring new ways to utilize the active components of black soybean by-products effectively has become the research highlight. Researchers have reported that black soybean hull has been used in fresh-keeping, preparation of dietary fiber, and development of solid drinks .[Citation8–10] As an effective component of the black soybean hull, the polysaccharides have been extracted by researchers in recent years. Some researchers have studied its structure, while studies on biological activity is not comprehensive enough .[Citation11–13]
Fatigue is a normal physiological phenomenon that occurs as a result of intense mental or physical activity .[Citation14] Persistent fatigue can cause chronic fatigue syndrome (CFS). There are several theories about the causes of fatigue, such as metabolite accumulation, energy failure, ion metabolism disorder, and free radical accumulation .[Citation15–17] As a natural active polymer, the polysaccharides have been found to inhibit exercise-induced fatigue in many studies, such as alleviating body fatigue by Spirulina polysaccharide by regulating hemoglobin level. After the intake of polysaccharides, the levels of blood lactic acid (LA), blood urea nitrogen (BUN), and other biochemical indices in the body can also change .[Citation18] Although previous studies have investigated the protective effect of polysaccharides prepared from black soybean on acute liver injury[Citation2] and anti-oxidant effect on hepatocytes[Citation7] in mice, the characterization and anti-fatigue activity of polysaccharides from black soybean hull have been unclear. Fatigue is a common phenomenon in today’s society. In addition, obtaining clear functional components from materials of the same origin as medicine and food could assist in maximizing the utilization value of this resource. Besides, exploring the structure-activity relationship between polysaccharide structure and antioxidant and anti-fatigue functions could help us to have a comprehensive understanding of the bean resources.
In the present study, we aimed to obtain polysaccharides from black soybean hull using enzymatic hydrolysis which was named BSPS-H. The chemical properties, monosaccharide composition, and structural composition were further studied. In addition, the anti-oxidant and anti-fatigue effects of BSPS-H were investigated using in vivo and in vitro assays. The results of the current study can improve the added value of black soybean and increase the development prospect of black soybean by-products.
Materials and methods
Materials
Dried black soybean hull was supplied by the Yushu Yuxiang bean products Co., Ltd in the Jilin Province, PR China. Cellulase and proteases were purchased from Henan Wanbang Chemical Technology Co., Ltd. The strain-producer of cellulase, flavorzyme, and acid proteinase used was Trichoderma spp., Aspergillus oryzae, and Aspergillus Niger with a specific activity of 10000 U/g, 50000 U/g, 50000 U/g, respectively. The producer of papain used was papaya single puree, and the specific activity was 30000 U/g. In addition, 1,1-diphenyl-2-trinitromethylhydrazine (DPPH) was purchased from Shanghai McLean Biochemical Technology Co., Ltd.; Nitrotetrazolium chloride (NBT), reduced coenzyme I disodium (NADH) and phenazine methylsulfate (PMS) were purchased from Shanghai Aladdin Biochemical Technology Co., Ltd, while acetonitrile, methanol, and NaCl were obtained from Beijing Chemical Plant Co., Ltd. The commercial kits used for the determination of blood urea nitrogen (BUN), hepatic glycogen (HG), lactate dehydrogenase (LDH) and blood lactic acid (LA) were procured from the Jiancheng Institute of Biotechnology (Nanjing, China).
Extraction of polysaccharides from black soybean hull
The dried black soybean hull was mixed with distilled water in a certain proportion (w/v = 1:25). Next, 3% cellulase was added to the mixture and polysaccharides were extracted at 50°C for 2 h. The pH of the reaction was then adjusted accordingly (flavor protease: pH 6, acid protease: pH 4, papain pH 6), and different proteases at 3% concentration were added into the system and the hydrolysis was continued for 2 h. The reaction system was rapidly heated to 90°C to inactivate the enzyme. The deproteinized supernatant was precipitated with a final ethanol concentration of 70% at 4°C for 12 h. After centrifugation, the precipitate was dried at 50°C to remove ethanol and then freeze-dried to obtain black soybean hull polysaccharide (BSPS-H). To improve the purity of polysaccharide, the protein in the supernatant was removed using trichloroacetic acid (TCA) method,[Citation19] and protein residue in the polysaccharide was measured by a UV spectrophotometer in the range of 200–800 nm .[Citation20]
Chemical composition of BSPS-H
Basic chemical properties: The total sugar content in BSPS-H was determined using the phenol-sulfuric acid colorimetric method .[Citation21] BSPS-H aqueous solution was reacted with 6% phenol and concentrated sulfuric acid for 30 min. The absorbance was then measured at 490 nm using a microplate reader to determine the total sugar content. The protein content of BSPS-H was estimated using the Bradford method .[Citation22] BSPS-H aqueous solution was reacted with Coomassie brilliant blue solution for 5 minutes. The absorbance of the solution was measured at 590 nm using a microplate reader. In addition, the protein content was also determined using m-hydroxybiphenyl method .[Citation23] BSPS-H aqueous solution was heated and reacted with NH₂SO₃H and concentrated sulfuric acid at 100°C for 20 min. After rapid cooling, the protein content of BSPS-H was measured by checking the absorbance using a microplate reader at 525 nm.
Monosaccharide composition: The monosaccharides composition of BSPS-H was analyzed using the method described by Zhang et al[Citation24] with some modifications. The polysaccharide sample (1 mg) was hydrolyzed initially with hydrous methanol containing 1 M HCl at 80°C for 10 h and then with 2 M trifluoroacetic acid (TFA) at 120°C for 1 h. The mixture of standards was composed of mannose (Man), glucuronic acid (GlcA), rhamnose (Rha), galacturonic acid (GalA), glucose (Glc), galactose (Gal), xylose (Xyl), arabinose (Ara) and fucose (Fuc). Next, NaOH solution was added to the dry sample and standard mixture, followed by derivatization with 1-phenyl-3-methyl-5-pyrazolone (PMP). Finally, the released monosaccharides were analyzed using high performance liquid chromatography (HPLC) (SHIMADZU Japan). HPLC analysis method: Shimadzu HPLC system (CTO-20a pump and SPD-20avd ultraviolet detector) and DIKMA Inertsil ODS-3 chromatographic column (4.6 × 150 mm); the mobile phase was Phosphate Buffer Saline (PBS: 0.2 M, pH 7.0) – acetonitrile 81:19 (V/V); the flow rate was 1.0 mL/min; and the injection volume was 20 μL. The detection wavelength was 245 nm.
FT-IR spectroscopy analysis: The functional group of BSPS-H was determined using FT-IR spectroscopy. One milligram of the sample was added with 180 mg of anhydrous potassium bromide under dry conditions and pressed into tablets. The FT-IR spectrum of BSPS-H was determined using a spectrum FT-IR in the range of 4000–500 cm−1 (Perkin Elmer, USA).
Molecular weight determination:
Dextran with different molecular weights was used as the standard, and the retention time and the logarithm of the molecular weight of dextran standard were plotted to obtain the regression curve equation between them. The molecular weight of the polysaccharide was calculated according to the regression curve equation. The sample molecular weight distribution was determined using High performance gel permeation chromatography (HPGPC) method with an high performance liquid phase system (CTO-20 pump A and RID-10 detector A) and TSK gel G-3000 PWXL column (4.6 × 150 mm). The sample was dissolved in water and filtered through a 0.22 μm water filter. The following HPGPC conditions were used; mobile phase: 0.2 M NaCl; flow rate: 1.0 mL/min, column temperature: 35°C; and injection volume: 20 μL.
Anti-oxidant activities of BSPS-H in vitro
2,2-Diphenyl-1-picrylhydrazyl radical (DPPH)-scavenging activity: The sample solutions (0.6 mL) of BSPS-H at different concentrations (0.5–10 mg/mL), mixed with DPPH solution (2.4 mL, 0.1 M) and incubated for 30 min in the dark. The OD values (A1) and dH2O (A0) were determined at 517 nm. Simultaneously, the DPPH was then replaced with absolute ethanol, and the OD value obtained was denoted as A2. The DPPH radical scavenging activity was calculated as:
Superoxide radical scavenging activity: The sample solutions (50 µL) of BSPS-H at different concentrations (0.5–10 mg/mL) were mixed with NBT solution (50 µL, 200 µM), NADH solution (50 µL, 936 µM) and PMS solution (50 µL, 120 µM), and incubated for 30 min in the dark. The OD values of the mixture (A1) and dH2O (A0) were determined at 510 nm at the same time. PMS was replaced with absolute ethanol, and the OD value was denoted as A2. The superoxide radical scavenging activity was calculated as follows:
Hydroxyl radical scavenging activity: The sample solutions (0.5 mL) of BSPS-H at different concentrations (0.5–10 mg/mL) were mixed with FeSO4 (1 mL, 6 mM), salicylic acid (1 mL, 6 mM), H2O2 (1 mL, 6 mM), and incubated for 30 min in the dark. The OD values of the mixture (A1) and dH2O (A0) were determined at 517 nm at the same time, H2O2 was replaced with absolute ethanol, and the OD value was denoted as A2. The hydroxyl radical scavenging activity was calculated as follows:
Anti-fatigue activity of BSPS-H in vivo
Experimental animals: Male and female Kunming mice, weighing 20 ± 2 g, were obtained from the Animal Experiment Center of Jilin University, China. The experiments were carried out according to the institutional regulations and national criteria for animal experimentation. The Institutional Animal Ethics Committee reviewed the entire animal protocol prior to conducting the experiments. All mice were fed adaptively under a 12 h light/12 h dark cycle and provided food pellets and tap water throughout the study. After one week of adaptation, the mice were randomly divided into 8 groups according to gender (4 groups for males and 4 groups for females, and one cage for every 5 mice) as follows: control group: treated with sterile water; high dose feeding group (BSPS-H(H)): treated with 500 mg/kg·d; and medium dose feeding group (BSPS-H(M)): treated with 200 mg/kg·d; low dose feeding group (BSPS-H(L)): treated with 100 mg/kg·d. The oral gavage treatment was performed at 9:00 every day for 4 weeks and the body weight of mice was recorded.
Weight-loaded swimming test analysis: The anti-fatigue experiment was carried out according to the test method for relieving physical fatigue in the technical specification for inspection and evaluation of health food. After 4 weeks of gavage, 30 min after the last feeding of polysaccharide, the mice with tailed load of 5% body weight lead skin were placed in a swimming box for swimming. The water temperature was 25 ± 0.1°C, and the water level was not lower than 30 cm. The swimming time was recorded from the beginning of swimming to when the mice failed to rise the surface of water to breathe within 7 s. At the end of the experiment, the mice were taken out of the water, wiped dry, and then put back into their cages.
Biochemical analysis: After the weight-loaded swimming test, blood was collected by removing the mouse eyeball. The plasma was obtained by centrifugation at 4000 rpm at 4°C for 10 min, and then stored at −80°C. The mice were dissected immediately following blood collection, and the livers were collected, rinsed with saline and weighed. The liver was stored at −80°C. The levels of LA, BUN, LDH, and HG were analyzed using commercial kits. The test results were determined by referring to the technical specification for inspection and evaluation of health food. When the weight-loaded swimming test results were positive, and any two of the three biochemical indicators of blood lactic acid, serum urea, and liver glycogen were also positive, the sample was be considered to have an anti-fatigue effect.
Statistical analysis
Data are expressed as the mean ± S.D. The statistical differences were determined by single-factor analysis of variance (ANOVA) and post hoc least significant difference (LSD) test, using the SPSS 24.0 software. The differences at p < .01 were considered statistically significant.
Results and discussion
Extraction of polysaccharides from black soybean hull
Black soybean is usually eaten or used in traditional Chinese medicine because of its nutritious value. In this study, the water-soluble polysaccharide, named BSPS-H was obtained from black soybean hull using enzyme-assisted extraction, which contained proteases and cellulase. To verify the optimal protease enzyme for the preparation of BSPS-H, different proteases, including flavorzyme, acid proteinase, and papain, were used to hydrolyze black soybean hull compounded with cellulase, and then the yield of polysaccharide was determined. As shown in , the highest yield (5.3%) of BSPS-H was obtained flavorzyme was used to extract of polysaccharides. Thus, flavorzyme was chosen as the optimum protease type to prepare BSPS-H.
Figure 1. Effect of protease types on the yield of polysaccharides from black soybean hull. Values are represented as means ± SD (n = 3).
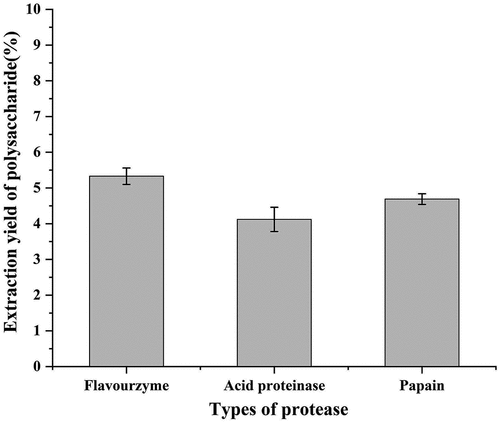
In the recent years, there have been many studies on the functional components of the black soybean. Previous studies showed that the yield of polysaccharides extracted from peeled black soybean by hot water extraction was 2.6% .[Citation25] During extraction, enzymes can decompose plant tissues under relatively mild conditions and accelerate the release of polysaccharides. Therefore, enzyme assisted extraction is an ideal polysaccharide extraction method, which can improve the yield of polysaccharides. The yield of chickpea polysaccharide reached 10% using enzyme-assisted extraction .[Citation26] In this study, the polysaccharide was extracted from black soybean hull by enzyme-assisted extraction. The extraction rate was 5.3%, which was higher than that of black soybean hull polysaccharide extracted using hot water (1.8%) .[Citation7]
Chemical composition of BSPS-H
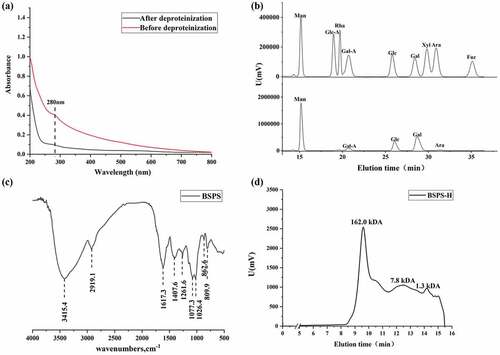
BSPS-H was prepared in large quantities under the optimal extraction condition. The chemical composition of BSPS-H, which included total sugar, uronic acid and protein was determined and listed in . The contents of total sugar and uronic acid were 42.7% and 27.5%, respectively. The polysaccharide components extracted from different parts of black soybean are different. In this study, the content of uronic acid in the crude polysaccharide of black soybean hull was 27.5%, which was higher than that of whole black soybean polysaccharide extracted using hot water extraction,[Citation27] and this phenomenon may also be related to the polysaccharide extraction method. Besides, the protein content in BSPS-H was high up to 9.2%. Therefore, the deproteinization of BSPS-H was carried out using the TCA method. A study has shown that non-germinated black soybean contains 34.68% neutral sugar and 32.88% uronic acid .[Citation28] In addition to polysaccharides, black bean seed coat contains ash substances such as calcium, iron, zinc, and phosphorus, pigments, and crude fibers .[Citation2,Citation28,Citation29] Thus, the above reasons may cause the determination of the total sugar content in the extracted black soybean crude polysaccharides to be low. As shown in , the absorption peak at 280 nm disappeared after deproteinization, demonstrating that the protein has been eliminated. Furthermore, the monosaccharide composition analysis using HPLC showed that BSPS-H was primarily composed of Man, GalA, Glc, Gal and Ara residues at a molar ratio of 48.1:5.0:15.8:29.7:1.3, respectively (/). Therefore, we concluded that BSPS-H might be a Glucogalactomannan. Another study showed that the black soybean hull polysaccharide extracted using boiling water was a galactomannan, composed of Gal, Man and Rha, and the molar ratio was 6.0:3.6:1.0 .[Citation29] A previous study also found that the BSPS-1 purified from peeled black soybean contained (1 → 6)- α- D-glucan, while BSPS-3 purified from peeled black soybean was type II arabinogalactan .[Citation25] For determination of the structural characteristics of BSPS-H, FT-IR was used to analyze its functional groups. As shown in , a typical broad band at 3415.4 cm−1 represented the stretching of the hydroxyl groups .[Citation30–32] The narrow band around 2919.1 cm−1 was attributed to the C–H stretching and bending vibrations. The band at 1617.3 cm−1 was due to the nonsymmetrical vibration of C = O, suggesting that BSPS-H contained uronic acid. The absorption at 1407.6 cm−1 was related to the presence of an internal C–H deformation. The strong absorptions at 1077.3 cm−1 and 1026.4 cm−1 were fingerprints associated with the existence of the pyranose rings in the polysaccharide fractions. The absorption at 862.6 cm−1 and 809.9 cm−1 was related to β-glycosidic and α-glycosidic bonds, respectively. Therefore, the results of FT-IR and HPLC, indicated that Man, Gal, and Glc might exist in the form of a pyran ring in an α/β-linkage .[Citation25] The molecular weight of BSPS-H is shown in . It was observed that the molecular weight distribution of BSPS-H was uneven, mainly concentrating in the three parts of 162.0 kDa, 7.8 kDa, and 1.3 kDa, which indicated that the composition of the BSPS-H was complex because it was a crude polysaccharide that was not further purified and therefore, it was probably heterogeneous.
Table 1. Chemical composition and sugar composition of BSPS-H.
In vitro anti-oxidant activity assays of BSPS-H
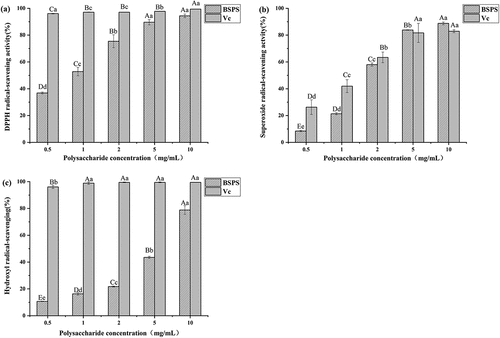
Polysaccharides from natural plants are polymeric carbohydrates composed of monosaccharides connected by glycosidic bonds .[Citation33] Different monosaccharide compositions exhibit different properties and biological activities .[Citation34] Several studies have demonstrated that oxidative stress plays an important role in the etiology of fatigue .[Citation35] Therefore, three methods, including DPPH, superoxide radical, and hydroxyl radical scavenging abilities were used to evaluate the antioxidant activity of BSPS-H. The ability of BSPS-H to scavenge DPPH radicals is shown in . With an increase in the polysaccharide concentration, the scavenging ability against DPPH radical was gradually enhanced. It could be seen that the DPPH radical scavenging ability of BSPS-H showed a concentration dependence. When the concentration of BSPS-H was below 1 mg/mL, the DPPH scavenging ability was less than 50%, which was lower than Vc. However, the DPPH scavenging ability of BSPS-H was equivalent to Vc when the concentration reached 10 mg/mL. The BSPS-H also exhibited superoxide radical-scavenging activity in a dose-dependent manner (). With an increase in concentration to more than 50%, the superoxide radical scavenging activity was even stronger than Vc. The ability of BSPS-H to scavenge hydroxyl radicals is shown in . Although the hydroxyl radical-scavenging activity of BSPS-H was stronger with an increase in concentration, and the ability of BSPS-H to scavenge hydroxyl radicals reached the maximum when the concentration was 10 mg/mL, it was still much weaker than Vc. In conclusion, BSPS-H showed a certain anti-oxidative capacity in a concentration-dependent manner.
Figure 3. In vitro anti-oxidant activities of BSPS-H. (A) DPPH-scavenging activity, (B) Superoxide radical-scavenging activity, (C) Hydroxyl radical-scavenging activity. Values are represented as means ± SD (n = 3). Values with different uppercase letters within the same sample was significantly different from each other (p < 0 .05), and values with different lowercase letters within the same sample was extremely significantly different (p < .01).
The BSPS-H extracted in this study was mainly composed of Man, Gal, and Glc, which could be speculated to be a Glucogalactomannan. Mannan is one of the main components of plant cell wall hemicellulose, and its galactomannan subfamily is very common in beans .[Citation36] A previous study showed that the polysaccharide extracted from soybean residue, a by-product of soybean processing, mainly contained Gal, Glc and Man, which had good anti-oxidant activity in vitro .[Citation37] Besides, the mannan extracted from yeast cell walls showed good scavenging ability against superoxide anion and hydroxyl radicals, and displayed certain anti-oxidant activity .[Citation38] In this study, it was found that BSPS-H exhibited anti-oxidant capacity in vitro. This anti-oxidant effect of BSPS-H might be related to the Man residue in the monosaccharide composition; however, the specific mechanism needs to be further discussed. Previous studies have shown that the human intake of dietary anti-oxidants could affect the oxidative stress caused by exercise .[Citation39] Therefore, the effect of BSPS-H on the physiological indices of exercising mice was further explored in this study.
In vivo anti-fatigue activity assays of BSPS-H
Effects of BSPS-H on the daily food intake and body weights of mice: The effect of BSPS-H on the body weight of mice is shown in . The four-week feeding test and control test showed an obvious upward trend. In addition, with an increase in the feeding weeks, the weight gain of male mice was higher than that of female mice. There was no statistically significant difference between the control and experimental groups (p > .05), and there were no adverse reactions in mice during feeding. It could be concluded that BSPS-H did not significantly affect the body weights of mice.
Table 2. The average food intake and body weight of mice in all treatment groups. Values are represented as means ± SD (n = 10).
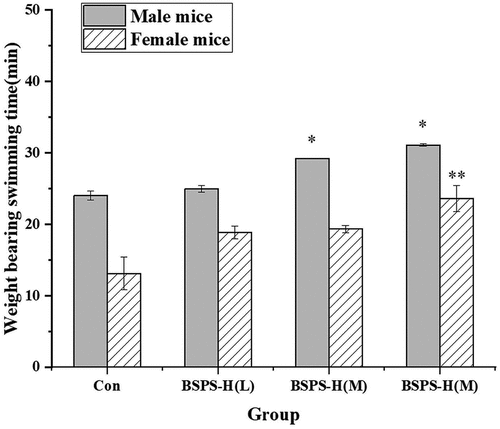
Effects of BSPS-H on mice subject to weight-loaded swimming: The mice subjected to a weight-bearing swimming experiment were used to study the relationship between fatigue and exercise endurance. The effect of BSPS-H on weight-bearing swimming time in mice is shown in . Compared with the blank control group, the weight-bearing swimming time in male and female mice was prolonged at low-dose feeding, but the difference was not statistically significant (p > .05). When fed with medium and high doses of BSPS-H, the weight-bearing swimming time in male mice was significantly prolonged (p < .05). In contrast, the weight-bearing swimming time in female mice in high-dose feeding exhibited a highly significant difference (p < .01) compared with the control group. From the above results, it could be seen that the weight-bearing swimming time in mice was significantly dependent on the concentration of feeding dose of BSPS-H. Research has shown that the length of weight-bearing swimming time indicates the degree of fatigue. Therefore, BSPS-H in medium and high doses has a potential anti-fatigue effect on mice.
Figure 4. Effects of BSPS-H during exhaustive swimming exercise (ESE) testing. Values are represented as means ± SD (n = 6). *p < .05 when compared to the control group. **p < .01 when compared to the control group.
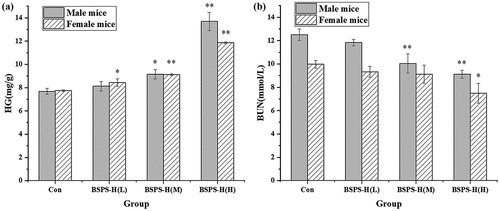
Effects of BSPS-H on HG and BUN: Glycogen is one of the main energy sources during weight-loaded swimming in mice, and the consumption of glycogen is promoted in strenuous sports .[Citation32] A previous study showed that the active components of polysaccharides could significantly improve the body’s resistance and achieve an anti-fatigue effect by promoting glycogen synthesis, reducing exercise-related metabolites, and improving the ability of anti-hypoxia .[Citation40] The HG level in mice after treatment with different doses of BSPS-H is shown in . The content of HG increased with an increase in the polysaccharide intake in the male and female mice. Furthermore, the content of HG in all experimental groups was higher than that in the control group. When the intake of BSPS-H reached 20 mg/kg per day, the level of HG in mice was significantly (p < .05) different from that in the control group. The experimental results showed that BSPS-H could increase the liver glycogen content and provide energy to the mice, to alleviate the fatigue caused by exercise. In addition, the BUN produced after long-term exercise could also indirectly reflect the fatigue degree in the body .[Citation41] The BUN in mice after exercise is shown in . The BUN content in all experimental groups was lower than that in the control group, and there were significant (p < .05) differences between them when fed with high-dose of BSPS-H. When fed with different doses of BSPS-H, it was found that the BUN content in mice decreased with an increase in polysaccharide intake, and the BUN content in male mice was higher than that in female mice. It could be speculated that the male mice had a longer swimming time, and the original degree of fatigue after swimming was greater than that of the female mice.
Figure 5. Effect of the BSPS-H on the mice after swimming. (A) Hepatic glycogen-HG, (B) Blood urea nitrogen-BUN. Values are represented as means ± SD (n = 6). *p < .05 when compared to the control group. **p < .01 when compared to the control group.
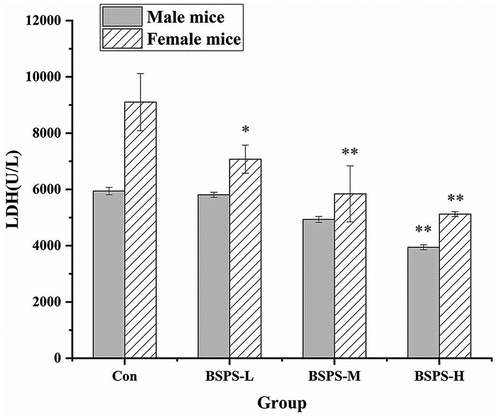
Effects of BSPS-H on LDH and LA: LDH promotes the transformation of lactate in the blood, which can indirectly reflect the fatigue degree of the body .[Citation42] As shown in , the activity of LDH in the blood of mice after swimming was concentration dependent on the dose of BSPS-H. There was a significant (p < .01) difference in LDH activity between the experimental and control group when there was an intake of high-dose BSPS-H, and the LDH in the blood of female mice was higher than the male mice. Furthermore, the LA contents in mice, which were the product of glycolysis under hypoxia in high-intensity sports was detected by kits. As shown in , the LA values in mice increased initially and then decreased after swimming. The area of the LA curve in the male and female mice in high-, medium-, and low-dose feeding groups was significantly lower than that in the blank control group. There was a significant difference in LA content between high-dose feeding and blank control groups (p < . 05). However, there was no significant difference in LA values between male and female mice. Therefore, it could be concluded that feeding polysaccharide might affect the LA content in mice, and the gender of mice had no effect on the LA value. A polysaccharide MP obtained from Lepidium meyenii Walp showed anti-fatigue activity, mainly composed of Ara, Man, Glc, and Gal. The biochemical indices of mice fed with MP changed after exhaustive swimming. The content of BUN decreased, the content of HG increased, and the contents of LA and LDH decreased in the mice .[Citation30] The BSPS-H, which was mainly composed of Man, Glc, and Gal, extracted in this study had anti-fatigue activity similar to the polysaccharide extracted by Tang et al .[Citation43] Thus, it can be inferred that BSPS-H may achieve the anti-fatigue effect by supplementing energy metabolites and reducing the accumulation of free radicals.
Table 3. Effect of the test sample on blood lactic acid (LA) level of mice (mmol/L, n = 6,) BSPS-H(L): low dose group; BSPS-H(M): medium dose group; BSPS-H(H): high dose group. Values are represented as means ± SD (n = 6).
Conclusion
In conclusion, a type of black soybean hull polysaccharide, named BSPS-H, was prepared using enzymatic hydrolysis, and its basic composition, anti-oxidant and anti-fatigue activities were studied. The BSPS-H was composed of Man, Gal, and Glc, in the mole ratio 48.1: 29.7: 15.8, and structural composition of BSPS-H might exist in the form of a pyran ring in an α/β-linkage. The BSPS-H showed superior anti-oxidant in vitro, and its anti-oxidant activity might be related to the monosaccharide composition. In addition, BSPS-H also showed superior anti-fatigue activity in vivo, which could prolong the weight-bearing swimming time of mice and cause changes in the biochemical indices in vivo. Our study can provide a fundamental basis for the comprehensive use of black soybean by-products, foster deep processing technology of black soybean that is relatively backward and extend the black soybean industry chain.
Practical applications
The chemical composition, anti-oxidant activity and anti-fatigue activity of polysaccharide from black soybean hull show potential applications in novel functional foods and medicine. The research in this study will improve the added value of black beans and increase the development prospect of black bean by-products.
Author contributions
Duo Liu and Zenghui Shi: Data curation, Writing - original draft; Siqi Wang: Investigation; Dongxia Gou and Liyuan Zhai: Formal analysis; Jun Zhao: Investigation; Yanbo Hu: Supervision, Writing - review &editing.
Additional information
Funding information
This research was supported by the young and middle-aged science and technology innovation and entrepreneurship outstanding talents (team) project (innovation category) of the Department of science and technology of Jilin Province (Grant number. YDZJ202101ZYTS077) and ”Climbing plan” projects of Changchun University (Grant number. ZKP202107).
Date availability statement
The original contributions presented in the study are included in the article, further inquiries can be directed to the corresponding authors.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Khosravi, A.; Razavi, S. H. Therapeutic Effects of Polyphenols in Fermented Soybean and Black Soybean Products. J. Funct. Foods. 2021, 81(1), 104467. DOI: 10.1016/J.JFF.2021.104467.
- Wen, X. Y. (2016). Extraction, characterization and protective effect on acute liver injury in mice of polysaccharides from black soybean. Doctoral dissertation University., Yangzhou https://kns.cnki.net/KCMS/detail/detail.aspx?dbname=CMFD201701&filename=1016293025.nh 5 2 2022
- Kwak, J. H.; Lee, J. H.; Ahn, C. W.; Park, S. H.; Chae, J. S.; Song, Y. D.; Han, E. N.; Lee, K. H.; Chae, J.-S. Black Soy Peptide Supplementation Improves Glucose Control in Subjects with Prediabetes and Newly Diagnosed Type 2 Diabetes Mellitus. J. Med. Food. 2010, 13(6), 1307. DOI: 10.1089/jmf.2010.1075.
- Kim, S. Y.; Wi, H. R.; Choi, S.; Ha, T. J.; Lee, B. W.; Lee, M. Inhibitory Effect of anthocyanin-rich Black Soybean Testa (Glycine Max (L.) Merr.) on the inflammation-induced Adipogenesis in a Dio Mouse Model. J. Funct. Foods. 2015, 14, 623–633. DOI: 10.1016/j.jff.2015.02.030.
- Lee, M.; Sorn, S. R.; Park, Y.; Park, H. K. Anthocyanin rich-black Soybean Testa Improved Visceral Fat and Plasma Lipid Profiles in overweight/obese Korean Adults: A Randomized Controlled Trial. J. Med. Foods. 2016, 2016, 3762. DOI: 10.1089/jmf.2016.3762.
- Jian, S.; Wen, X.; Liu, J.; Kan, J.; Qian, C., Wu, C., and Jin, C. Protective Effect of an Arabinogalactan from Black Soybean against Carbon tetrachloride-induced Acute Liver Injury in Mice. Int. J. Biol. Macromol 2018, 117, 659–664. DOI: 10.1016/j.ijbiomac.2018.05.203.
- Zhao, Y.; Hu, D. Y.; Li, Y. Q.; Li, P. C., and Zhu, X. J. Extraction and Antioxidative Activity in Jpatocyte Evaluation of Polysaccharides from Black Bean Skin 48 (17), 10–12. DOI:10.3969/j.issn.1007-1865.2021.17.006 , 2021.
- Jin, L. M.; Gao, Y.; Feng, X. X.; Hu, Y. G.; Niu, G. C.; Li, Z. J. Extraction of Polyphenols from Black Bean Peel and Preservation Effect for Strawberries. Cereals Oils 2021, 34(10), 5.
- Qiao, X. Q.;. (2019). Development of black compound solid beverage which rich in dietary fiber and vitamin. (Doctoral dissertation, Henan University Of Science And Technology) .https://kns.cnki.net/KCMS/detail/detail.aspx?dbname=CMFD201902&filename=1019658558.nh 3 12 2021
- Zhu, M. Y.; Kong, W. D.; Zhou, J.; Cao, H. Extraction of water-soluble Dietary Fiber of Black Soyabean Hull and Its Application in Food. Modern Food. 2021, 5, 3. DOI: 10.16736/j.cnki.cn41-1434/ts.2021.05.010.
- Liu, J.; Wen, X. Y.; Kan, J., and Jin, C. H. Structural Characterization of Two water-soluble Polysaccharides from Black Soybean (Glycine Max (L.) Merr.). J. Agric Food Chemi 2015, 63(1), 225–234. DOI:10.1021/jf505172m.
- Li, C.; Tian, Z. N.; Cai, J. P.; Chen, K. X.; Zhang, B.; Feng, M. Y.; Shi, Q.-T.; Li, R.; Qin, Y.; Geng, J.-S., et al. Panax Ginseng Polysaccharide Induces Apoptosis by Targeting twist/akr1c2/nf-1 Pathway in Human Gastric Cancer. Carbohydr. Polym 2014, 102(Complete), 103–109. DOI: 10.1016/j.carbpol.2013.11.016.
- Xu, F.; Ge, Y. Y.; Liu, X. C.; Zhang, Y. Y.; Wang, X. Research Progress on Nutritional Components and Biological Activities of Black Soybean. Food Nutr. China. 2019, 25(9), 7. CNKI:SUN:ZGWY.0.2019-09-013.
- Chaudhuri, A.; Behan, P. O. Fatigue in Neurological Disorders. Lancet. 2004, 363(9413), 978–988. DOI: 10.1016/S0140-6736(04)15794-2.
- Tang, W.; Jin, L.; Xie, L.; Huang, J.; Wang, N., Chu, B., Dai, X., Liu, Y., Wang, R., and Zhang, Y. . Structural Characterization and Antifatigue Effect in Vivo of Maca (Lepidium Meyenii Walp) Polysaccharide: Structural Characteristics and Antifatigue Effect of Mp. J. Food ence.2017, 82(1–3), 759–766. DOI: 10.1111/1750-3841.13619.
- Surhio, M. M.; Wang, Y.; Fang, S.; Li, J.; Ye, M. Anti-fatigue Activity of a Lachnum Polysaccharide and Its Carboxymethylated Derivative in Mice. Bioorg. Med. Chem. Lett 2017, 27(20), 4777–4780. DOI: 10.1016/j.bmcl.2017.07.034.
- Xu, J.; Li, Y.; Regenstein, J.; Su, X. In Vitro and in Vivo anti-oxidation and anti-fatigue Effect of Monkfish Liver Hydrolysate. Food Biosci 2017, 18, 9–14. DOI: 10.1016/j.fbio.2017.03.002.
- Zhu, J. M.; Zhu, Z.; Ding, M.; Liu, S.; Zou, H. Analysis of the anti-fatigue Activity of Polysaccharides from Spirulina Platensis: Role of Central 5-hydroxytryptamine Mechanisms. Food Funct 2020, 11(2), 1826–1834. DOI: 10.1039/C9FO02804H.
- Cong, H. X.; Hu, G. S.; Wang, W. C.; Yao, L.; Lu, J. Study on Protein Removal and Antioxidant Activity of Crude Polysaccharide from Nymphaea Hybrid. J. Shanghai Jiaotong Univ.(Agric Sci). 2018, 36(6), 5. DOI: 10.3969/J.1671-9964.2018.06.008.
- Li, Y. J.; Xin, Y. Z.; Xu, F. X., Zheng, M. M.; , Xi, X. Z.;Cui, X. W.; Cao, H.; Guo, H.,and Han, C. C. 2018, Maca Polysaccharides: Extraction Optimization, Structural Features and anti-fatigue Activities. Int. J. Biol. Macromol DOI: 10.1016/j.ijbiomac.2018.04.063.
- Dubois, M.; Gilles, K. A.; Hamilton, J. K.; Rebers, P. A.; Smith, F. Colorimetric Method for Determination of Sugars and Related Substances. Anal. Chem 1956, 28(3), 350–356. DOI: 10.1021/ac60111a017.
- Bradford, M. M. A Rapid and Sensitive Method for the Quantitation of Microgram Quantities of Protein Utilizing the Principle of protein-dye Binding. Anal. Biochem 1976, 72(1–2), 248–254. DOI: 10.1016/0003-2697(76)90527-3.
- Filisetticozzi, T.; Carpita, N. C. Measurement of Uronic Acids without Interference from Neutral Sugars. Anal. Biochem 1991, 197(1), 157–162. DOI: 10.1016/0003-2697(91)90372-Z.
- Zhang, X.; Yu, L.; Bi, H.; Li, X.; Ni, W.; Han, H.; Li, N.; Wang, B.; Zhou, Y.; Tai, G., et al. Total Fractionation and Characterization of the water-soluble Polysaccharides Isolated from Panax Ginseng C. A. Meyer. Carbohydr. Polym 2009, 77(3), 544–552. DOI: 10.1016/j.carbpol.2009.01.034.
- Liu, J.; Wen, X. Y.; Zhang, X. Q.; Pu, H. M.; Kan, J.; Jin, C. H. Extraction, Characterization and in Vitro Antioxidant Activity of Polysaccharides from Black Soybean. Int. J. Biol. Macromol 2015, 72, 1182–1190. DOI: 10.1016/j.ijbiomac.2014.08.058.
- Yu, R.; Li, C. C.; Lu, H.; Jiang, Y. Optimization of Enzymatic Extraction of Chickpeas Polysaccharide by Response Surface Methodology and Its Infrared Spectral Analysis. Food Sci. Technol 2016, 9, 6. CNKI:SUN:SSPJ.0.2016-09-051
- Bai, Z.; Meng, J.; Huang, X.; Wu, G.; Nie, S.; Nie, S. Comparative Study on Antidiabetic Function of Six Legume Crude Polysaccharides. Int. J. Biol. Macromol 2020, 154(7581), 25–30. DOI: 10.1016/j.ijbiomac.2020.03.072.
- Lee, A.L.; Yu, Y.P.; Hsieh, J. F.; Kuo, M. L.; Ma, Y.S.; ; , and Lu, C.P. Effect of Germination on Composition Profiling and Antioxidant Activity of the polysaccharide-protein Conjugate in Black Soybean [Glycine Max (L.) Merr.]. Int. J. Biol. Macromol 2018, 113, 601–606. DOI: 10.1016/j.ijbiomac.2018.02.145.
- Hu, B.Y.; Deng, J.C.; Yang, C.Q.; Hu, Y.; Zhang, J.; Yang, W.Y., and Liu, J. Extraction Optimization, Purification and Characterization of Polysaccharides from the Seed Coat of Black Soybean. Plos One.2017, 12(12), e0190202. DOI: 10.1371/journal.pone.0190202.
- Tang, W. M.;. (2017). Comparative Studies on Chemical Structure and Anti-fatigue Effect of Polysaccharides both from Turnip (Brassica rapa L.) and Maca (Lepidium mevenii Walp.). (Doctoral dissertation, Zhejiang University). https://kns.cnki.net/KCMS/detail/detail.aspx?dbname=CDFDLAST2017&filename=1017071316.nh 3 12 2021
- Gao, H.; Zhang, W.; Wang, B.; Hui, A.; Du, B.; Wang, T., Meng, L.; Bian, H. X.and Wu, Z. Purification, Characterization and anti-fatigue Activity of Polysaccharide Fractions from Okra (Abelmoschus Esculentus (L.) Moench). Food Funct 2018, 9, DOI: 10.1039/c7fo01821e.
- Lamou, B.; Taiwe, G.S.; Hamadou, A.; Houlray, J.; Atour, M.M., and Tan, P. V. Antioxidant and Antifatigue Properties of the Aqueous Extract of Moringa Oleifera in Rats Subjected to Forced Swimming Endurance Test. Oxidat. Med. Cell. Longevit 2016, 2016, 3517824. DOI: 10.1155/2016/3517824.
- Li, C.; Wu, G.; Zhao, H.; Dong, N.; Wu, B.; Chen, Y.; Lu, Q. Natural-Derived Polysaccharides from Plants, Mushrooms, and Seaweeds for the Treatment of Inflammatory Bowel Disease. Front. Pharmacol 2021, 12, 755. DOI: 10.3389/FPHAR.2021.651813.
- Yu, Y.; Shen, M.; Song, Q.; Xie, J. Biological Activities and Pharmaceutical Applications of Polysaccharide from Natural Resources: A Review. Carbohydr. Polym 2018, 183, 91–101. DOI: 10.1016/j.carbpol.2017.12.009.
- Xia, F.; Zhong, Y.; Li, M.; Chang, Q.; Liao, Y.; Liu, X., and Pan, R. Antioxidant and anti-fatigue Constituents of Okra. Nutrients.2015, 7(10), 8846–8858. DOI: 10.3390/nu7105435.
- Xdsa, B.; Jyy, A.; Swc, C.; Qi, W. C.; Syw, B.; Spn, A. Plant-derived Glucomannans: Sources, Preparation Methods, Structural Features, and Biological properties-science Direct. Trends Food Sci. Technol 2020, 99, 101–116. DOI: 10.1016/j.tifs.2020.02.016.
- Wang, S. Q. (2021). Study on the structure analysis and antioxidant activity of water-soluble polysaccharide from soybean dregs (Master dissertation, Changchun University). https://kns.cnki.net/KCMS/detail/detail.aspx?dbname=CMFDTEMP&filename=1021837159.nh 5 1 2022
- Yang, L.; Huang, G.; Lv, M. Extraction, Characterization and Antioxidant Activities of Mannan from Yeast Cell Wall. Int. J. Biol. Macromol 2018, 118, S0141813018319536. DOI: 10.1016/j.ijbiomac.2018.06.145.
- Wang, J. J.; Shieh, M. J.; Kuo, S. L.; Lee, C. L.; Pan, T. M. Effect of Red Mold Rice on Antifatigue and exercise-related Changes in Lipid Peroxidation in Endurance Exercise. Appl. Microbiol. Biotechnol 2006, 70(2), 247–253. DOI: 10.1007/s00253-005-0051-5.
- Zhou, S. S.; Jiang, J. G. Anti-fatigue Effects of Active Ingredients from Traditional Chinese Medicine: A Review. Curr. Med. Chem 2017, 24. DOI: 10.2174/0929867324666170414164607.
- Cui, B.; Huang, W.; Lin, Y. Experimental Study of the anti-fatigue and Anti Hypoxia Function of Phyllanthus Emblica L. in Mice. Modern Chin. Med 2008. DOI: 10.13313/j.1673-4890.2008.06.014.
- Huang, L.Z.; Huang, B.K.; Liang, J.; Zheng, C. J.; ; Han, T.; Zhang, Q.Y., and Qin, L.P. Antifatigue Activity of the Liposoluble Fraction from Acanthopanax Senticosus. Phytotherapy Res 2011, 25(6), 940–943. DOI: 10.1002/ptr.3346.
- Tang, Y.; Zhu, Z. Y.; Pan, L. C.; Sun, H.; Song, Q. Y.; Zhang, Y. Structure Analysis and anti-fatigue Activity of a Polysaccharide from Lepidium Meyenii Walp. Nat. Prod. Res 2018, 33(17), 1. DOI: 10.1080/14786419.2018.1452017.