ABSTRACT
Growing interests in natural and organic meat products have led to increasing application of fruits and vegetables, containing antimicrobial and antioxidant components as natural preservatives, in processed meats. The aim of this study was assessed the utilization of raisin as a natural preservative in goat meat (chevon), and thereby the impacts on quality parameters of restructured chevon jerky and its stability were evaluated. Incorporating 15% raisin increased (P < .01) darkness (lower CIE L*), but decreased (P < .01) redness and yellowness (lower CIE a* and b*) of chevon jerky. Furthermore, all instrumental color values of experimental jerky decreased (P < .01) over a 10-week storage period. Compared with jerky prepared with or without sodium nitrite (NaNO2), jerky prepared with raisin had significantly higher shear values, but lower water activity, pH and aerobic microbial counts. Furthermore, as the storage time progressed, pH values decreased (P < .01) in all jerky types, whereas the aerobic counts significantly increased. Either raisin or NaNO2 added to jerky decreased (P < .05) thiobarbituric acid reactive substances (TBARS) values, which were not changed over the 10-week storage. Raisin jerky scored lower (P < .01) in the sensory properties of color and overall acceptability than the cured jerky. However, texture and flavor sensory characteristics of raisin jerky were not differentiated from the cured jerky by the sensory panel. Consequently, raisin has potential to use as a natural preservative in processed meats and it may be possible to substitute raisins for nitrites in processed meats.
Introduction
Consumers believe that food contributes directly to their health. Such expectation steadily increases the demand of natural and organic meat products from meat producers.[Citation1] According to the United States Department of Agriculture (USDA), products labeled as “natural” should be minimally processed with no artificial colorants, flavors, preservatives, or sweeteners added.[Citation2] These USDA guidelines prohibit adding synthetically manufactured preservatives, such as sodium nitrite/nitrate, in meat products labeled as “natural.” Sodium nitrite (NaNO2) is commonly used as a curing agent in processed meat to develop color and flavor characteristics of the cured meat, as well as to inhibit oxidation of lipids and growth of bacteria, mostly Clostridium botulinum.[Citation3] However, the use of nitrites for curing meat has generated extensive debates because carcinogenic nitrosamines could be produced by the interaction of amines with nitrites (NO2−) at high temperatures.[Citation4,Citation5] This concern has led to find nitrite alternatives in food ingredients; however, efforts to find the alternatives to traditional curing agents had very limited successes.[Citation6,Citation7] Many natural and organic processed meat without sodium nitrite could result in appearances and flavors appreciably less consumer desirable than those conventionally produced with sodium nitrite.[Citation7] Furthermore, noticeable differences are existed between the cured and uncured versions of the same processed meat products.[Citation6]
Grapes and raisins generally contain various antioxidants, including bioflavonoids, proanthocyanidins, catechin monomers, procyanidin dimers and other polyphenolic compounds.[Citation8,Citation9] Either raisins or dried grapes are commonly used in bakery products as natural preservatives, which contain high concentrations of propionic acid.[Citation10,Citation11] Furthermore, phenolic compounds and Maillard reaction products in raisins also contribute to antimicrobial activity. Several physical factors such as low water activity, acid pH, and high osmolarity also contribute to the overall antimicrobial activity of raisins.[Citation12] Raisins are used as universal ingredients in many food products because their sensory properties appear to be compatible with many other food components.[Citation13] As previously indicated, with increasing demand of natural and organic meat products, there has been consequent surging interest in natural preservatives such as raisins. Previous self-life studies indicated that incorporation of raisins into beef jerky (15%, w/w) could inhibit pathogenic bacteria and increase antioxidant potential with some limited consumer acceptability over a 10 week storage period.[Citation13]
Traditionally jerky has been made from thinly sliced whole muscle. However, whole muscle jerky is generally very lean and it is often too dry along with brittle and difficult to chew, as well as developing undesirable color.[Citation14,Citation15] Compared with sliced whole muscle jerky, comminuted jerky has softer texture properties, but higher aw and fat contents, along with more susceptive to microbial growth and lipid oxidation.[Citation16,Citation17] Consequently, ground goat meat (chevon) is an excellent raw material for preparation of low fat restructured jerky products with a consistent appearance and texture. As previously indicated, raisins also can be used as natural alternatives to curing agents because of their antimicrobial and antioxidant properties, as well as compatibility with other food components. Subsequently, there is strong possibility for development of processed meat products labeled as “natural” using ground goat meat (chevon) incorporated with natural preservatives such as raisins. Although raisins contain antimicrobial and antioxidant compounds, their possible preservative effectiveness in ground goat meat (chevon) has not been studied due to the low product and consumption of goat meat (chevon) in the U.S. The purpose of this study was to produce restructured chevon jerky with raisin as a natural preservative and to determine the extent of raisin impacts on quality parameters of produced jerky and its stability during storage.
Materials and methods
Preparation of restructured chevon jerky
Primal leg and shoulder cuts from crossbred intact male goats, raised on pasture with a grain supplement, were obtained from the meat processing plant at Fort Valley State University (Fort valley, GA). Natural raisin pastes were provided by the California Raisin Marketing Board (Fresno, CA). Fresh meat from primal cuts was ground through a 9.5 mm grinder plate (a coarse grinding) and then re-ground through a 3.2 mm grinder plate (a fine grinding) using a commercial meat grinder (BIRO Model 7552 SS4; BIRO Manufacturing Co., Marblehead, OH). Jerky batter was prepared with ground goat meat (chevon; 9.07 kg) which was mixed with 273 g jerky seasoning (Legg’s Old Plantation Seasoning; A.C. Legg, Inc., Calera, AL) only, according to the manufacture’s direction, or the seasoning with raisin paste (15% w/w) or sodium nitrite (NaNO2; 0.015%, w/w) for 20 min using a Fleetwood meat mixer (Model MMS-50I; Skymsen Equipment LLC, Newark, NJ) and then stored under refrigeration (4°C) overnight. The refrigerated jerky batter was extruded through a flat jerky nozzle (3.00 x 0.48 cm) using a Jerky Cannon (L.E.M. Products, Inc., Harrison, OH) on trays (82.7 x 62.5 × 2.3 cm) and then placed in a pre-heated oven (Model 100XLT-1, Kemette, Corp., Charlotte, NC) and held at 93.3°C for 3.5 h. The processed jerky was then removed from the oven and air-dried for 1 h under forced airflow at ambient temperature, and then cut into 10.0 cm long strips.
Three batches of restructured chevon jerky with raisin paste, with and without sodium nitrite (NaNO2) were prepared within a same day for 6 days within 2 consecutive weeks using the same procedures described previously. Jerky strips (10.0 x 2.0 × 0.3 cm) from each batch were packed in vacuum pouches (6 strips/pouch; 30 to 50 mL of O2/m2/24 h/1 atm at 25°C; Cryovac Inc., Duncan, SC) using a vacuum packaging machine (Model UV2100-C Ultravac Koch; Koch Equipment Ultrasource, Kansas City, MO), and stored 10 wk at ambient temperature under fluorescent light (150–200 lux). Physicochemical, microbial, and sensory properties of chevon jerky, prepared with raisin or either with or without sodium nitrite, were determined biweekly over a 10-wk storage time.
Physicochemical properties
The physical properties of color and shear forces were measured on restructured chevon jerky during a 10 wk of storage. Among the chemical properties assessed, proximate compositions and moisture to protein ratios (MRP) were determined using only fresh (0 wk) jerky samples. However, other chemical properties, such as water activity (aw), pH, 2-thiobarbituric acid reactive substances (TBARS) and fatty acid composition were analyzed over the 10 wk of ambient storage.
The CIE L* a* b* color coordinate values were measured from the surface of jerky samples from each batch over the 10 wk storage using HunterLab color units (Minolta Chromameter, Model CR-200, Minolta, Japan) with illuminant D65 as a light source. After 0, 2, 4, 6, 8, or 10 wk of storage, jerky strips from individual batches were removed from vacuum pouches, and randomly selected 6 strips. Measurements were taken at 3 different areas (top, middle, and bottom) from each selected strip. Shear values of the jerky strips were also measured using a TA-XT2 texture analyzer equipped with a Warner-Bratzler shear attachment (Texture Technologies Corp., Scarsdale, NY) as described by Lee et al.[Citation18] Prepared individual jerky samples (2.0 x 2.0 × 0.3 cm) from the strips used in color measurements were placed at right angles to the blade. The texture analyzer was set with a 5-g load cell and a crosshead speed of 200 mm/min.
The proximate composition of jerky samples was determined from fresh (0 wk) samples only, which was analyzed using the AOAC Official Methods.[Citation19] Moisture-to-protein ratios (MPR) were also calculated as the average percent moisture divided by the average percent protein in fresh (0 wk) samples jerky. The water activity and pH of raisin treated, cured and uncured chevon jerky samples were determined in triplicate during the 10 wk storage. Each sample was minced into pieces approximately 1 mm2 in size. The water activity of jerky samples was determined in triplicate with a chilled-mirror dewpoint water activity meter (Aqualab model 3TE, Pullman, WA). Each minced sample (2.0 g) was then stirred into 10 mL of deionized water to measure pH (pH model 501, Orion Research, Inc., Boston, MA). 2-Thiobarbituric acid reactive substances (TBARS) were determined in triplicate from raisin treated, cured and uncured jerky (10.0 g) stored for 0, 2, 4, 6, 8, or 10 wk according to the distillation method of Shahidi et al.[Citation20] The absorbance of the extracted and prepared analyte was measured at 532 nm using a Shimadzu (Model UV-2401 PC) spectrophotometer (Shimadzu Corp., Columbia, MD) during the TBARS analysis. TBARS were calculated from a standard curve of malondialdehyde (MDA) and expressed as mg MDA/kg sample.
Total lipids were extracted from each jerky samples (on week 0, 2, 4, 6, 8, and 10 of storage) by means of the chloroform/methanol (2:1 v/v) method of Lee et al.[Citation21] The extracted lipids were saponified and esterified according to the AOAC Official Method 969.33[Citation22] for the preparation of fatty acid methyl esters (FAME). The prepared FAME were analyzed using a Thermo Electronic (Austin, TX) gas chromatography (Model TRACE GC Ultra) equipped with an automatic sampler Model AS-3000 (Thermo Electronic Corp., Austin, TX). A 0.25-mm i.d. with 0.25 µm film thickness by 60-m long fused silica SP-2380 capillary column (Supelco, Inc., Bellefonte, PA) was used to separate the methyl esters that were detected with a flame ionization detector (FID), with the following conditions: injection temperature, 240°C; initial temperature, 130°C; 4°C/min up to 220°C; helium flow, 1.5 mL/min; and, split ratio of 30:1. The identification of individual FAME from the sample was done by matching their retention time with those of known FAME standards (Alltech Associates, Inc., Deerfield, IL; Sigma-Aldrich Corp. Bellefonte, PA) and the relative weight percent of individual FAME in each sample was also calculated using their corrected areas according to the AOCS for fatty acid analysis.[Citation22]
Microbial counts
A 10-g sample from raisin treated, cured, or uncured chevon jerky stored for 0, 2, 4, 6, 8, or 10 wk was aseptically removed from vacuum packages and the 3MTM Petrifilm plate techniques were used to enumerate microbial loads on jerky samples as recommended by the manufacturer.[Citation23] Appropriate sample dilutions were inoculated on Petrifilm plates (3MTM Microbiology Products, St. Paul, MN) to determine, E. coli and total coliform (3 M™ PetrifilmTM E.coli/Coliform Count Plates), yeast and mold (3 M™ PetrifilmTM Yeast and Mold Count Plates), and aerobic plate counts (3 M™ PetrifilmTM Aerobic Count Plates) as prescribed by the supplier. Microbial colonies were counted and expressed as colony forming units (CFU) per gram of sample.
Sensory evaluation
On week 0, 2, 4, 6, 8, and 10 of storage, chevon jerky strips, prepared with raisin or either with or without sodium nitrite, were removed from vacuum packages and cut into 2 cm long strips (2 x 2 × 0.3 cm) for sensory evaluations. Ten experienced panelists were seated in individual booths and presented six jerky samples (3 strips/sample) in random orders on paper plates coded with 3-digit random numbers. Samples were evaluated on 8-point scales with 1 “dislike extremely” and 8 “like extremely” for color, flavor, texture, and overall acceptability.[Citation24] Panelists rinsed their mouths with room temperature bottled spring water between samples.[Citation25]
Statistical analysis
All data were analyzed as a randomized block design (RBD), blocked on day, with factorial treatment arrangement using the MIXED SAS procedure (SAS Institute Inc., Cary, NC). The effects of processing treatment (raisin, cured and uncured) and storage time (0, 2, 4, 6, 8, and 10 weeks), as well as their interaction were considered to be fixed. Significant differences among means were determined by the Least-Squares Means generated and statistically separated using the PDIFF (p-values for difference) option, protected by the ANOVA F-test (P ≤ .05).
Results and discussion
Physical properties of restructured chevon jerky
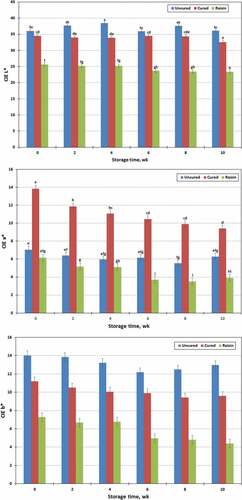
With the incorporation of raisin, distinct changes were observed in the color of the jerky products (). Jerky prepared with raisin had lower (P < .01) CIE L* (lightness; 24.42 vs 33.95 or 36.96 ± 0.310), a* (redness; 4.59 vs 11.09 or 6.23 ± 0.23) and b* (yellowness; 5.81 vs. 10.11 or 13.12 ± 0.33) values than that prepared either with or without sodium nitrite. Furthermore, uncured (without NaNO2) jerky was significantly lighter (higher CIE L* value; P < .01) and yellower (higher CIE b* value; P < .01) than all other jerky types, whereas cured (with NaNO2) jerky was noticeably redder (higher CIE a* value; P < .01). The lower CIE L* value (darker) of raisin treated jerky compared to cured or uncured jerky was related to high amounts of reducing sugars, such as glucose and fructose, presented in the raisin jerky.[Citation9] Thermal degradation of simple sugars presented in raisin might occur by two different non-enzymatic browning reaction pathways during the jerky processing.[Citation26,Citation27] Maillard reaction takes place in the presence of amino acids and caramelization occurs when simple sugars are heated at high temperatures. The resultants of non-enzymatic reactions might derive to develop the darker color appearance in the raisin jerky. Furthermore, cured jerky revealed a darker color (lower CIE L* value) then uncured jerky suggestion the presence of more darkish-colored metmyoglobin.[Citation28] Compared to uncured and raisin treated jerky products, cured jerky possessed the higher CIE a* value suggesting it had the noticeable amount of reddish-cured pigment, whereas raisin jerky had the lowest CIE a* value. Furthermore, compared to uncured and raisin jerky products, cured jerky might be more acceptable to the consumer because of development of a desirable red color.[Citation29] Cured meat color develops in a series of complicated reactions until nitric oxide (NO)-myglobin is formed.[Citation30] Subsequently, the heat stable cured color complex such as nitrosylhemochromogen is developed by heating NO-myglobin.[Citation29,Citation30] All CIE L*, a* and b* values of the jerky were influenced (P < .01) by storage time. The CIE L* (lightness) values of jerky were significantly decreased (P < .01) after storing for 10 wk (CIE L* = 32.04 to 30.67 ± 0.37). Compared to the fresh jerky (wk 0), the CIE a* (redness) and b* (yellowness) values of the jerky were also decreased (P < .01) after storing for 2 (CIE a* = 9.01 to 7.81 ± 0.27) and 4 wk (CIE b* = 10.83 to 10.02 ± 0.36), respectively. Then there were further decreases of CIE a* and b* values of the jerky until wk 10 (6.53 and 8.98, respectively) with some variation. Heat stable cured color such as nitrosylhemochromogen pigment fades upon exposure to UV light.[Citation29,Citation31] Furthermore, several intrinsic and extrinsic factors affect nitrite curing reactions such as meat system, pH, the amount of reductants present, temperature and time.[Citation29,Citation30] In the present study, the redness (CIE a* values) of cured chevon jerky decreased (P < .01) over 10 wk storage under fluorescence light that could be explained by cured color fading over the time of storage. The CIE L* values of all jerky samples decreased (P < .01) as storage time progressed with some variation suggesting the presence of more darkish-colored metmyoglobin and less reddish-colored nitrosylhemochromogen pigment. Sindelar et al.[Citation7] reported that the CIE b* values of jerky were increased with over time in general. However, the present study did not agree with these findings. Significant interactions (P < .01) were found between processing treatment (raisin, cured and uncured) and storage time (0, 2, 4, 6, 8, and 10 wk) for CIE L* and a * values. However, no interaction (P > .10) was observed in jerky processing and storage time for CIE b* values. The CIE L* values of uncured jerky were increased (P < .01) over the first 4 wk of storage, but the values did not change in cured or raisin treated jerky. However, the CIE L* values of all three jerky products (raisin, cured and uncured) were decreased (P < .01) as the storage time progressed thereafter. The CIE a* values of cured jerky were gradually decreased (P < .01) over the 10 wk of storage, whereas the CIE a* values of uncured jerky did not change until wk 8. As indicated in , raisin treated jerky decreased (P < .01) the CIE a* values at wk 6; however, no further changes were observed in the CIE a* values of the jerky after 6 wk.
Figure 1. Changes of CIE L*, a*, and b* values in chevon jerky prepared with raisin or with/without sodium nitrite (NaNO2), and stored at ambient temperature for 10 wk; bar bearing unlike letters are different (P < .05).
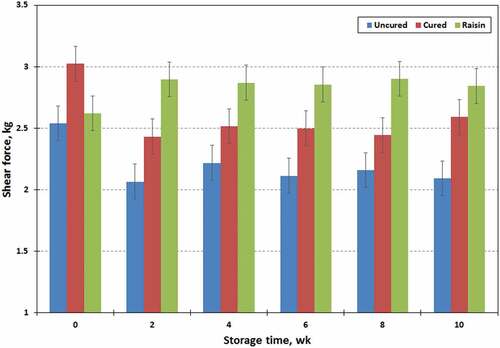
Miller[Citation32] pointed out that Warner–-ratzier shear force (WBSF) values were highly correlated with overall tenderness of muscle. The WBSF values of restructured chevon jerky were influenced (P < .01) by processing treatments (). The WBSF values (2.83, 2.20, and 2.58 ± 0.061 kg, respectively) were high in jerky prepared with raisin, low in uncured jerky, and intermediate in cured jerky. However, neither storage time (2.46 to 2.73 ± 0.061 kg) nor processing treatment x storage time affected (P > .10) the WBSF values in experimental chevon jerky products. Texture in the jerky products has an important role in quality perception, and determines market attractiveness of this type of products.[Citation32] In general, shear force values can be affected by water activity, moisture content and cooking yield.[Citation31,Citation33] Furthermore, the moisture contents of jerky products have an important effect on textural properties. Yang et al.[Citation34] found that the shear force values of cured whole muscle beef jerky samples decreased during storage. However, such relationship was not presented in the present study.
Chemical properties of restructured chevon jerky
Significant differences (P < .01) were found in the proximate compositions of jerky prepared with raisin or with/without NaNO2. Raisin, cured (with NaNO2) and uncured (without NaNO2) chevon jerky contained 45.1, 49.5, and 49.7 ± 0.64% protein; 32.8, 36.2, and 36.5 ± 1.35% moisture; 1.17, 1.32, and 1.25 ± 0.08% ash; and, 6.3, 7.2, and 8.1 ± 0.436% fat, respectively. Chevon jerky products in this study are classified as intermediate moisture foods (IMF) because of their lowed moisture contents. According to Jose et al.,[Citation35] commercial IMF contain 20–40% moisture with high amounts of protein (over 45%) and sodium chloride (NaCl; approximately 6.0%). Moisture content has a decisive effect on the stability and sensory properties of IMF; moreover, approximately 35% to 38% moisture is required for adequate sensory properties of jerky products.[Citation32,Citation36] Based on the proximate compositions of chevon jerky samples, the average MPR values did not exceed 0.75; therefore, the restructured chevon jerky satisfied the USDA-FSIS standard of identity for jerky products (moisture-to-protein ratio; MPR ≤ 0.75:1).[Citation37]
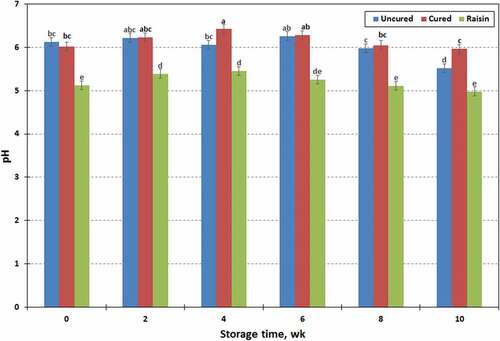
There were significant differences in the water activity (aw) of three different types of chevon jerky (). The aw values were high in uncured jerky, low in raisin jerky, and intermediate in cured jerky (0.90, 0.79, and 0.88 ± 0.007, respectively). High reducing sugar concentrations in raisins may contribute to increase osmotic pressure, and may decrease the water activity in the experimental chevon jerky. The aw values were also influenced (P < .01) by the storage time that increased (P < .05) after 4 wk of storage, but did not change thereafter (aw ranged from 0.84 to 0.87). However, processing treatment x storage time interaction was not found in the water activity (aw) of chevon jerky samples (P > .10). Water activity (aw) values less than 0.85 should control the growth of all pathogenic bacteria of concern for human health.[Citation37,Citation38] The USDA guidelines require that jerky products have aw value of ≤ 0.80 to be considered as shelf-stable.[Citation37] The resulting jerky products should be microbiologically safer, but might have unacceptable textures and flavors.[Citation32,Citation35] In the present study, water activity (aw) of fresh chevon jerky (wk 0) was within the range of 0.78–0.87 (). Such jerky products require additional hurdles such as low pH, preservatives, reduced oxidation-reduction potential and vacuum packaging, to extend shelf-life with excellent storage stability.[Citation39] In general, water activity is important for determining microbial growth rates. Furthermore, it is also an important quality control characteristic which has a potent effect on the color, texture, and fat rancidity of jerky products.[Citation32,Citation34,Citation39] Both pH and water activity of vacuum-packaged meats are known to decrease with storage time.[Citation40,Citation41] However, in this study, the water activities of each jerky variety increased over the 10 wk storage time with some variation. Jose et al.[Citation35] reported that the average pH for intermediate moisture (IM) meat products was in the broad range of 4.72–6.73. Furthermore, pH values of jerky generally ranged from 5.73 to 5.76. Processing treatment and storage time, as well as their interaction significantly affected (P < .01) the pH values of chevon jerky samples (). The pH values (6.16, 5.22, and 6.03 ± 0.077, respectively) were high in jerky prepared with sodium nitrite, low in raisin jerky, and intermediate in uncured jerky. The pH is a fundamental factor affecting the physicochemical properties of meat products, since it influences freshness, water-holding capacity, binding ability, tenderness, color, and texture of meat.[Citation33] The pH value of raisin jerky decreased because raisin contains substantial high amounts of organic acids. Food products that have an acidic pH can create a stressful environment for microorganism.[Citation39,Citation41] Furthermore, low water activity in foods can also produce a strong inhibitory effect. Consequently, incorporating raisins into jerky products could enhance the antimicrobial properties of jerky.[Citation13] The pH values of jerky samples in the present study decreased over the 10 wk storage with some variation that ranged from 5.98 to 5.49 ± 0.082. The pH values of both raisin and cured jerky samples increased after storing for 4 wk, then decreased with storage time thereafter. However, the pH values of uncured jerky samples did not change over 8 wk of storage but values decreased after storing for 10 wk.
Figure 3. Changes in water activity (aw) values of raisin, cured and uncured chevon jerky products during 10 wk of storage at ambient temperature; points with no common letters are different (P < .05).
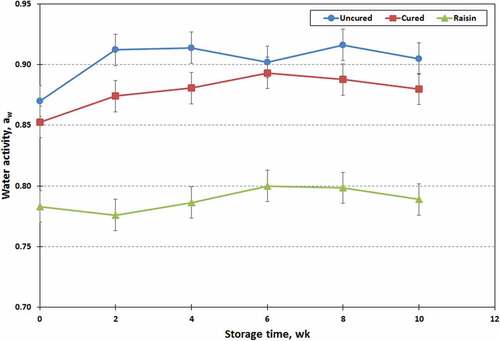
Figure 4. Changes in pH values of raisin, cured and uncured chevon jerky products during 10 wk of storage at ambient temperature; points with no common letters are different (P < .05).
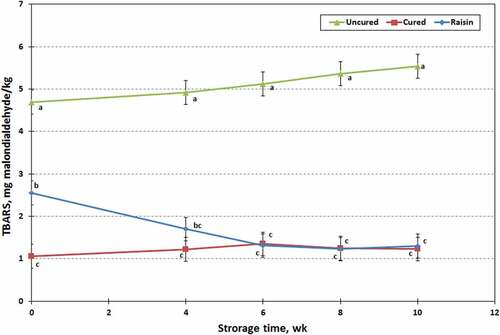
2-Thiobarbituric acid (TBA) values reflect the aldehyde levels in meat products, which is an indication of fat rancidity. Accordingly, TBA values can be used to index the degree of freshness of meat products and are therefore widely used to measure lipid oxidation in meat products.[Citation20,Citation42] Significant differences (P < .01) were found in 2-Thiobarbituric acid reactive substances (TBARS) values for restructured chevon jerky products (). The uncured jerky had higher (P < .05) TBARS values (5.12 vs 1.22 or 1.61 ± 0.127 mg MDA/kg) than cured or raisin jerky. However, storage time did not influence (P > .10) the TBARS values, which ranged from 2.59 to 2.77 ± 0.156 mg MDA/kg. However, interaction was presented (P < .01) between processing treatment and storage time for TBARS values in jerky products. No changes were observed in the TBARS values of jerky prepared with sodium nitrite (NaNO2) over the 10 wk storage (1.06 to 1.35 ± 0.270 mg MDA/kg). However, the TBARS values of raisin treated jerky samples decreased over the first 6 wk of storage (2.55 to 1.35 ± 0.270 mg MDA/kg), but did not change thereafter. In the present study, incorporating raisin into restructured chevon jerky resulted in lowered TBAR values during 10-wk storage, compared to uncured jerky samples. Raisins contains a noticeable amount of polyphenolic antioxidants; moreover, reducing sugars such as glucose, fructose and sucrose are also presented, which might be nearly as effective as polyphenolic antioxidants presented in raisins to maintain low TBAR values over the 10-wk storage due to Maillard reaction products.[Citation13,Citation14] The proposed mechanisms for antioxidant activity of Maillard reaction products include hydroperoxide reduction, inactivation of free radicals formed during oxidative degradation of unsaturated fatty acids, oxygen scavenging and chelating heavy metal ions.[Citation43] According to Yang et al.,[Citation34] the TBARS values of the cured beef and pork jerky increased as the storage time progressed. However, this trend was not followed in the present study, and this may be due to the differences in oxygen pressures within packs of jerky because the beef and pork jerky was packaged without vacuum in the previous studies. It is generally accepted that TBARS values increase in meat with increasing storage time.[Citation32,Citation34] Lipid oxidation in dried meat products such as jerky might be occurred in two different phases. The first phase leads to the formulation of peroxide during drying, and the second phase occurs during prolong storage of the final product that produces a rancid taste due to lipolysis.[Citation14,Citation34,Citation43] TBARS values were lower for the raisin treated and cured jerky products due to the direct addition of raisin and sodium nitrite during jerky processing. It is normally accepted that TBARS value increases in meat with increasing storage time, although the pattern TBARS increase in different species is not well known. Also, there was a proportional increase of lipid oxidation as water activity value decreases.
Figure 5. 2-Thiobarbituric acid reactive substances (TBARS) of raisin, cured and uncured chevon jerky products, stored over 10 wk at ambient temperature; points with no common letters are different (P < .05).
Twenty-three fatty acids were identified in lipids extracted from the jerky samples, which consisted of nine saturated (C10:0, C12:0, C13:0, C14:0, C15:0, C16:0, C17:0, C18:0, and C20:0), 7 monounsaturated (C14:1n5, C16:1 trans, C16:1n7, C17:1n8, C18:1 trans, C18:1n9, and C20:1n9), and 7 polyunsaturated (C18:2 iso 1, C18:2 iso 2, C18:2 iso 3, C18:2n6, C18:3n3, C18:3n6, C20:4n6) fatty acids (). Among the saturated fatty acids (SFA), neither processing treatment nor storage time significant (P > .10) influenced the percentage weight of individual SFA; moreover, there were no significant (P > .10) interactions found between processing treatment and storage time. Similar trends were also found in individual monounsaturated fatty acids (MUFA) and polyunsaturated fatty acids (PUFA). The major three fatty acids in jerky samples were palmitic (C16:0), stearic (C18:0), and oleic (C18:1n9) acids, which accounted for 76.40, 75.34 and 76.72% of total fatty acids in cured, uncured, and raisin treated fresh (wk 0) jerky samples, respectively. Current recommendations for the PUFA:SFA ratio for healthy food products are around 0.45.[Citation44,Citation45] The PUFA:SFA ratios in the current study were lower than the recommended ratio, being 0.11 for restructured fresh (wk 0) chevon jerk samples. The SFA such as lauric (C12:0), myristic (C14:0), and palmitic (C16:0) acids raise the LDL-cholesterol concentrations in blood, and increase the risk of cardiovascular disease.[Citation8,Citation46] These three SFA comprised 24.73, 24.96, and 24.51% of total fatty acids in the cured, uncured, and raisin treated fresh jerky (wk 0) in this present study, respectively. Nutritional guidelines have suggested that the consumption of saturated and trans-fatty acids needs to decrease and the consumption of n-3 PUFA is required to increases in order to achieve a n-6/n-3 ratio in the diet of approximately 5:1 or less.[Citation44,Citation47] The ratio of n-6/n-3 PUFA in fresh jerky prepared with raisin or with/without sodium nitrate (NaNO2) were 4.17, 4.40 or 3.98, respectively, in the present study. In general, fats from goat meats are higher in PUFA such as linoleic (C18:2n6), α-linolenic (C18:3n3), and arachidonic (C20:4n6) acids, than those from other red meats.[Citation44,Citation48] Hence, jerky from goat meat (chevon) might be more susceptible to lipid oxidation than that from beef because of a higher concentration of PUFA in chevon. Either raisin or sodium nitrite can act as an antioxidant to delay the development of oxidative rancidity. This prevention occurred in the present study according to the TBAR values determined between treated (raisin or sodium nitrite) and untreated (no sodium nitrite) chevon jerky samples (). Higher concentrations of PUFA such as linoleic (C18:2n6), α-linolenic (C18:3n3), and arachadonic (C20:4n6) acids in raisin treated and cured jerky were expected because of the protective effect of adding polyphenols and reducing sugars, and nitrites in terms of stabilizing polyunsaturated fats, respectively. However, such effects were not found in the present study. The degree of lipid oxidation is related to not only the amounts of unsaturated fats present but also other processing factors such as temperature, time, oxygen exposure, oxygen removal from packs, and the addition of antioxidants and/or reducing agents.[Citation49]
Table 1. Fatty acid profiles of chevon jerky prepared with raisin or with/without sodium nitrite (NaNO2), stored at ambient temperature for 10 wk.
Microbial counts of restructured chevon jerky
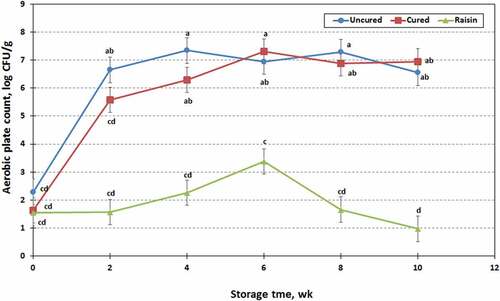
Changes in aerobic plate counts of raisin treated, cured and uncured chevon jerky samples during 10 wk of storage are shown in . Processing treatment and storage time, as well as their interaction significantly influenced (P < .01) the aerobic plate counts of chevon jerky samples. The raisin-treated jerky had significantly lower aerobic plate counts (1.89 vs 5.77 or 6.18 ± 0.264 log10CFU/g) than cured or uncured jerky, whereas no significant differences were found in the aerobic plate counts between cured and uncured jerky samples. The aerobic plate counts of all three different types of chevon jerky products increased (P < .01) with storage time, and ranged from 1.82 to 5.88 ± 0.278 log10CFU/g. The aerobic plate counts of raisin and cured jerky samples progressively increased (P < .01) over the first 6 wk of storage. No further changes were observed in the cured jerky samples thereafter. However, the aerobic plate counts were further decreased (P < .01) in raisin-treated jerky samples after 6 wk storage. In uncured jerky samples, aerobic plate counts were increased over the first 4 wk of storage, but counts did not change thereafter. Coliform and E. coli, as well as yeast and mold, were not detected in the present study. The low microbial levels in the raisin jerky might be related to its low moisture content and water activity (aw ranged from 0.78 to 0.80).[Citation34,Citation37] It is widely accepted that many food spoilage bacteria are unable to multiply at aw value below 0.95 and growth of most microorganisms is retarded or inhibited below aw at 0.90.[Citation34,Citation50] However, Haas and Herman[Citation51] found several types of spoilage bacteria capable of growing at minimum aw values of 0.84 to 0.87 in intermediate moisture food. Nitrite is added to control spore-forming pathogenic bacteria, most likely Clostridium botulinum. Bayne and Michener[Citation52] reported that no effect on the control of naturally occurring spoilage bacteria present in frankfurters whether or not nitrite was included.
Sensory properties of restructured chevon jerky
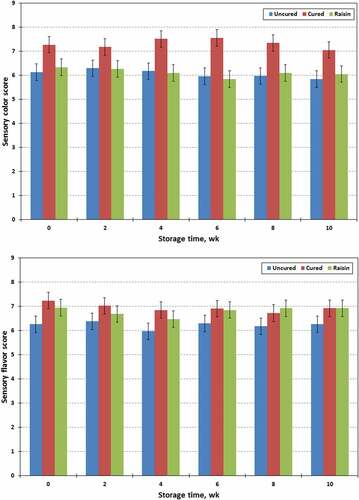
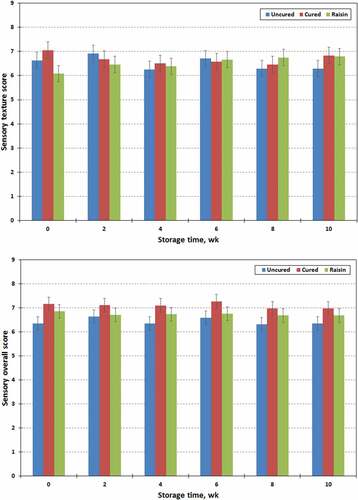
Albright et al.[Citation53] reported that texture, color, and flavor are the most important sensory attributes of jerky products. The sensory properties of chevon jerky prepared with raisin or with/without sodium nitrite stored for 10 wk are presented in . Cured jerky samples had higher (P < .01) sensory scores for color (7.32 vs 6.11 or 6.06 ± 0.266) than raisin treated or uncured equivalents. However, significant differences in the sensory flavor attributes were only found in treated (raisin and cured) and untreated jerky products. Furthermore, cured jerky also had a higher (P < .01) overall acceptability (7.10 vs 6.73 and 6.43 ± 0.208) compared to raisin treated and uncured jerky products. However, subjective texture properties (6.68 to 6.51 ± 0.261) of jerky samples were not influenced (P > .1) by processing treatment. All four sensory attributes of chevon jerky were not influenced (P > .1) by storage time and processing treatment x storage time. Perhaps color is a critical importance fact for consumer acceptance of meat products and nitrite is responsible for the fixation of a desirable shaded pink color.[Citation29] In the present study, sensory panel members clearly differentiated between the colors of cured and uncured or raisin treated chevon jerky products, and preferred cured jerky color compared to raisin treated and uncured equivalents. According to the instrumental color measurements, cured jerky had a higher CIE a* value (redness) than uncured or raisin treated jerky (). Another important function of nitrite is creating a unique flavor profile that is distinguished from products not containing nitrite.[Citation6,Citation54] In the present study, sensory panel members easily detected the difference in flavor between chevon jerky prepared with or without sodium nitrite (NaNO2), whereas the panelists could not differentiate flavors in jerky prepared with raisin and NaNO2.
Conclusion
Sodium nitrite is generally used in processed meats as a curing ingredient to develop color and flavor that is associated with cured meats, as well as to retard rancidity and microbial growth. High prevalence of producing carcinogenic byproducts from nitrites has increased pressure to find effective, alternative curing agents, one of which is using raisin, a known to have antimicrobial and antioxidant properties, in processed meats to provide similar cured meat characteristics as those with nitrite-added ones. Raisin has been reported to be an effective bioactive ingredient against lipid oxidation and microbial growth when used in processed meats. The antimicrobial properties of raisin in restructured goat meat (chevon) jerky might be derived from lowered water activity (aw), moisture and pH, as well as polyphenolic compounds. Incorporating raisin significantly reduced the microbial load on the restructured chevon jerky with similar levels of lipid oxidation prevention as the nitrite-added jerky. Restructured chevon jerky formulated with 15% raisin also significantly changed instrumental color and texture properties. Furthermore, the raisin jerky consistently scored lower in the sensory properties of color and overall acceptability than the cured jerky. However, texture and flavor sensory characteristics of raisin jerky were not differentiated from the cured jerky by the sensory panel. Consequently, raisin has potential to use as a natural preservative in processed meats and it may be possible to substitute raisins for nitrites in processed meats. However, additional research is needed to investigate ways to further improve the sensory properties of processed meats when they prepared with raisin.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Bacus, J.; Natural Ingredients for Cured and Smoked Meats. In Proceedings of the 59th Reciprocal Meat Conference: Champaign-Urbana, IL, USA, 2006; 77–78.
- USDA. Food Standards and Labeling Policy Book. www.fsis.usda.gov/OPPDE/larc/Policies/Labeling_Policy_Book_082005.pdf (accessed April 05, 2019).
- Ingram, M.;. The Microbiological Effects of Mitrite. In Processing of the International Symposium on Nitrite in Meat Products; Tinbergen, B. J., Krol, B., Eds.; Pudoc Scientific Publisher: Wagening, The Netherlands, 1974; pp 63–75.
- Chow, C.; Hong, C. Dietary Vitamin E and Selenium and Toxicity of Nitrite and Nitrate. Toxicology. 2002, 180(2), 195–207. DOI: 10.1016/S0300-483X(02)00391-8.
- Yuan, Y.; Meng, W.; Yutian, M.; Fang, C.; Xiaosong, H. Determination of Eight Volatile Nitrosamines in Meat Products by Ultrasonic Solvent Extraction and Gas Chromatography-Mass Spectrometry Method. Int. J. Food Prop 2015, 18(6), 1181–1190. DOI: 10.1080/10942912.2014.898652.
- Sebranek, J. G.; Bacus, J. N. Cured Meat Products without Direct Addition of Nitrate or Nitrite: What are the Issues? Meat Sci 2007, 77(1), 136–147. DOI: 10.1016/j.meatsci.2007.03.025.
- Sindelar, J. J.; Terns, M. J.; Meyn, E.; Boles, J. A. Development of a Method to Manufacture Uncured, No-nitrate/nitrite-added Whole Muscle Jerky. Meat Sci 2010, 86(2), 298–303. DOI: 10.1016/j.meatsci.2010.04.028.
- Siró, L.; Kápolna, E.; Kápolna, B.; Lugasi, A. Functional Food: Product Development, Marketing and Consumer Acceptance-A Review. Appetite. 2008, 51, 456–467.
- Vasavada, M. N.; Cornforth, D. P. Evaluation of Antioxidant Effects of Raisin Paste in Cooked Ground Beef, Pork, and Chicken. J. Food Sci 2006, 71(4), C242–C246. DOI: 10.1111/j.1750-3841.2006.00026.x.
- Scotter, M. J.; Thorpe, S. A.; Reynolds, S. L.; Wilson, L. A.; Sreutt, P. R. Survey of Baked Goods for Propionic Acid and Propionates. Food Addit. Contam 1996, 13, 133–139. DOI: 10.1080/02652039609374391.
- Smith, J.; Daifas, D.; El-Khoury, W.; Koukoutsis, J.; El-Khoury, A. Shelf Life and Safety Concerns of Bakery Products: A Review. Crit. Rev. Food Sci. Nutr 2004, 44, 19–55. DOI: 10.1080/10408690490263774.
- Ziemke, W. H.;. Raisins and raisin products: mold retarding properties and raisin conditioning. Am Inst Baking Tech Bulletin. . 1980, Vol. II. Issue 2:1-6.
- Bower, C. K.; Schilke, C. K.; Daeschel, M. D. Antimicrobial Properties of Raisins in Beef Jerky Preservation. J. Food Sci 2003, 68, 1484–1489. DOI: 10.1111/j.1365-2621.2003.tb09671.x.
- Kuo, J. C.; Ockerman, H. W. Effect of Salt, Sugar and Storage Time on Microbiological, Chemical and Sensory Properties of Chinese Style Dried Pork. J. Food Sci 1985, 50, 1384–1389. DOI: 10.1111/j.1365-2621.1985.tb10482.x.
- Miller, A. J.; Strange, E. D.; Whiting, R. C. Improved Tenderness of Restructured Beef Steaks by a Microbial Collagenase Derived from Vibrio B-30. J. Food Sci 1989, 54, 855–857. DOI: 10.1111/j.1365-2621.1989.tb07898.x.
- Hegenbart, S. S. M.; http://www.foodproductdesign.com/archive/1999/0199ap.html (accessed Dec. 29, 2019)
- Quinton, R. D.; Cornforth, D. P.; Hendricks, D. G.; Brennand, C. P.; Su, Y. K. Acceptability and Composition of Some Acidified Meat and Vegetable Stick Products. J. Food Sci 1997, 62(6), 1250–1254. DOI: 10.1111/j.1365-2621.1997.tb12255.x.
- Lee, J. H.; Kouakou, B.; Kannan, G. Chemical Composition and Quality Characteristics of Chevon from Goats Fed Three Different Post-weaning Diets. Small Ruminant Res 2008, 75(2–3), 177–184. DOI: 10.1016/j.smallrumres.2007.10.003.
- AOAC. Official Methods of Analysis of the AOAC International, 18th ed.; Association of Official Analytical Chemists, International: Gaithersburg, MD, 2015.
- Shahidi, F.; Rubin, L. J.; Diosady, L. L.; Wood, D. F. Effect of Sulfanilamide on the TBA Values of Cured Meats. J. Food Sci 1985, 50(1), 274–275. DOI: 10.1111/j.1365-2621.1985.tb13332.x.
- Lee, J. H.; Vanguru, M.; Moore, D. A.; Kannan, G.; Terrill, T. H.; Kouakou, B. Flavor Compounds and Quality Parameters of Chevon as Influenced by Sericea Lespedeza Hay. J. Agric. Food Chem 2012, 60(15), 3934–3939. DOI: 10.1021/jf2050125.
- AOCS. Official Methods and Recommended Practices of the American Oil Chemists’ Society, 5th ed.; American Oil Chemists’ Society: Champaign, 1993.
- The 3MTM Products. Manufacture’s Interpretation Guide; The 3MTM Microbiology Products: St. Paul, MN, 1999; pp 1–64.
- Penfield, M. P.; Campbell, A. M. Experimental Food Science, 3rd ed.; Penfield, M. P., Campbell, A. M., Eds.; Academic Press: San Diego, CA, 1990; pp. 1–541.
- Choi, J.; Jeong, J.; Han, D.; Choi, Y.; Kim, H.; Lee, M.; Lee, E.; Park, H.; Kim, C. Effects of Pork/Beef Levels and Various Casings on Quality Properties of Semi-dried Jerky. Meat Sci 2008, 80(2), 278–286. DOI: 10.1016/j.meatsci.2007.11.028.
- Wijewickreme, A. N.; Krejpcio, Z.; Kitts, D. D. Hydroxyl Scavenging Activity of Glucose, Fructose, and Ribose-lysine Model Maillard Products. J. Food Sci 1999, 64(3), 457–461. DOI: 10.1111/j.1365-2621.1999.tb15062.x.
- Antony, S. M.; Han, I. Y.; Rieck, J. R.; Dawson, P. I. Antioxidative Effect of Millard Reaction Products Added to Turkey Meat during Heating by Addition of Honey. J. Food Sci 2002, 67(5), 1719–1724. DOI: 10.1111/j.1365-2621.2002.tb08712.x.
- Cassens, R. G.; Greaser, M. L.; Lee, M. Reactions of Nitrite in Meat. Food Technol 1979, 33, 46–57.
- Cornforth, D. P.; Jayasingh, P. Chemical and Physical Characteristics of Meat/Color and Pigment. In Encyclopedia of Meat Sciences; Jensen, W. K., Devine, C., Dikeman, M., Eds.; Elsevier Ltd.: Oxford, UK, 2004; pp 249–256.
- Sebranek, J. G.;. Basic Curing Ingredients. In Ingredients in Meat Products; Tarte, R., Ed.; Springer Science and Business Media: New York, NY, 2009; pp 1–24.
- Houser, T. A.; Sebranek, J. G.; Núñez Maisonet, W.; Cordray, J. C.; Wiegand, B. R.; Ahn, D. U.; Lee, E. J. The Effects of Irradiation at 1.6 KGy on Quality Characteristics of Commercially Produced Ham and Pork Frankfurters over Extended Storage. J. Food Sci 2005, 70(4), 262–266. DOI: 10.1111/j.1365-2621.2005.tb07200.x.
- Miler, M. F.; Keeton, J. T.; Cross, H. R.; Leu, R.; Gomez, F.; Wilson, J. J. Evaluation of the Physical and Sensory Properties of Jerky Processed from Beef Heart and Tongue. J. Food Qual 1988, 11, 63–70.
- Guerrero, L.; Gou, P.; Arnau, J. The Influence of Meat pH on Mechanical and Sensory Textural Properties of Dry-cured Ham. Meat Sci 1999, 52(3), 267–273. DOI: 10.1016/S0309-1740(98)00175-2.
- Yang, H.; Hwang, Y.; Joo, S.; Park, G. The Physicochemical and Microbiological Characteristics of Pork Jerky in Comparison to Beef Jerky. Meat Sci 2009, 82(3), 289–294. DOI: 10.1016/j.meatsci.2009.01.029.
- Jose, F. S.; Rafael, G.; Miguel, A. C. Water Activity of Spanish Intermediate Moisture Meat Products. Meat Sci 1994, 38(2), 341–350. DOI: 10.1016/0309-1740(94)90122-8.
- Yang, C. Y.; Lee, S. H. An Evaluation of Quality of the Marketing Jerky in Domestic. I. Investigation of Outward Appearance, Food Additives, Nutrient Content and Sanitary State. Korean J Food Nutr. 2002, 15, 197–202.
- USDA-FSIS. Compliance Guideline for Meat and Poultry Jerky Produced by Small and Very Small Plants. http://www.fsis.usda.gov/PDF/Compliance_Guideline_Jerky.pdf (accessed March 27, 2019).
- Porto-Fett, A. C.; Call, J. E.; Luchansky, J. B. Validation of Commercial Process for Inactivation of Escherichia Coli O157:H7, Salmonella Typhimurium, and Listeria Monocytogenes on the Surface of Whole Muscle Beef Jerky. J. Food Prot 2008, 71(5), 918–926. DOI: 10.4315/0362-028X-71.5.918.
- Yamaguchi, N.; Naito, S.; Okada, Y.; Nagase, A. Effect of Oxygen Barrier of Packaging Material on Food Preservation. In Annual Report of the Food Research Institute; Yamaguchi, N., Naito, S., Okada, Y., Nagase, A., Eds.; Aichi Prefecture Government: Japan, 1986; Vol. 27, pp 69–73.
- Papadopoulos, L. S.; Miller, R. K.; Acuff, G. R.; Vanderzant, C.; Cross, H. R. Effect of Sodium Lactate on Microbial and Chemical Composition of Cooked Beef during Storage. J. Food Sci 1991, 56(2), 341–347. DOI: 10.1111/j.1365-2621.1991.tb05276.x.
- Blixt, Y.; Borch, E. Comparison of Shelf-life of Vacuum-packed Pork and Beef. Meat Sci 2002, 60(4), 371–378. DOI: 10.1016/S0309-1740(01)00145-0.
- Gray, J. L.;. Measurement of Lipid Oxidation: A Review. J. Am. Oil Chem. Soc 1978, 55(6), 539–546. DOI: 10.1007/BF02668066.
- Wijewickreme, A. N.; Kitts, D. D.; Durance, T. D. Reaction Conditions Influence the Elementary Composition and Metal-chelating Affinity of Non-dialyzable Model Maillard Reaction Products. J. Agric. Food Chem 1997, 45(12), 4577–4583. DOI: 10.1021/jf970041n.
- Wood, J. D.; Richardson, R. I.; Nute, G. R.; Fisher, A. V.; Campo, M. M.; Kasapidou, E.; Sheard, P. R.; Enser, M. Effects of Fatty Acids on Meat Quality: A Review. Meat Sci 2003, 66(1), 21–32. DOI: 10.1016/S0309-1740(03)00022-6.
- Webb, E. C.; Casey, N. H.; Simela, L. Goat Meat Quality. Small Ruminant Res 2005, 60(1–2), 153–166. DOI: 10.1016/j.smallrumres.2005.06.009.
- Noakes, M. N.; Nestle, P. J.; Clifton, T. M. Modifying the Fatty Acids Profile of Dairy Products through Feedlot Technology Lowers Plasma Cholesterol of Humans Consuming the Products. Am. J. Clin. Nutr 1996, 63(1), 42–46. DOI: 10.1093/ajcn/63.1.42.
- Wolfram, G.;. Dietary Fatty Acids and Coronary Heart Disease. Eur J Med Res 2003, 8(8), 321–324.
- Griinari, J. M.; Bauman, D. E. Biosynthesis of Conjugated Linoleic Acid and Its Incorporation into Meat and Milk in Ruminants. In Advances in Conjugated Linoleic Acid Research Vol 1; Yurawecz, M., Mossoba, M., Kramer, J., Pariza, M., Nelson, G., Eds.; American Oil Chemists’ Society Press: Champaign, 1999; pp 180–200.
- Ramarathnam, N.;. The Flavor of Cured Meat. In Flavor of Meat, Meat Products and Seafoods, 2nd ed.; Shahidi, F., Ed.; Blackie Academic and Professional: London, UK, 1998; pp 290–319.
- Leistner, L.;. Shelf-stable Products and Intermediate Moisture Foods Based on Meat. In Water Activity: Theory and Applications to Foods; Rockland, L. B., Beuchat, L. R., Eds.; Marcel Dekker: New York, NY, 1987; pp 295–328.
- Haas, G. J.; Herman, E. B. Bacterial Growth in Intermediate Moisture Food Systems. Lebensmittel-Wissenschaft und-Technologie. 1978, 11, 47–78.
- Bayne, H. G.; Michener, H. D. Growth of Staphylococcus and Salmonella on Frankfurters with and without Sodium Nitrite. J. Appl. Microbiol 1975, 30(5), 844–849. DOI: 10.1128/am.30.5.844-849.1975.
- Albright, S. N.; Kendall, P.; Avens, J. S.; Sofo, I. N. Pretreatment Effect on Inactivation of Escherichia Coli O157:H7 Inoculated Beef Jerky. Lebensmittel-Wissenschaft und-Technologie. 2003, 36(4), 381–389. DOI: 10.1016/S0023-6438(03)00042-2.
- Pegg, R. B.; Shahidi, F. The Color of Meat. In Nitrite Curing of Meat: The N-Nitrosamine Problem and Nitrite Alternatives; Pegg, R. B., Shahidi, F., Eds.; Food and Nutrition Press: Trumbull, CT, 2000; pp 23–66.