?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
Ethiopian Hibiscus rosa-sinensis Linn contains a variety of bioactive phytochemicals, which possess several biological activities. This study aims to analyze the antibacterial and antioxidant activity of aqueous crude flower, leaf, and bark extracts of Hibiscus rosa-sinensis, as well as to see the geographical effects and method development of Co2Res2/Glassy carbon electrode. The secondary metabolites of Hibiscus rosa-sinensis extracts were investigated by maceration techniques. Disc diffusion methods are used for antimicrobial activities against a total of four human pathogens bacteria such as E. coli, K. pneumonia, S. aureus, and S. epidermidis, respectively. Antioxidant activities were assessed using differential pulse voltammetry (DPV), whereas, the phytoconstituents of Hibiscus rosa-sinensis extracts were profiled using FTIR and UV-Vis analysis. The phytochemical investigations showed that phenols, flavonoids, and quinines were observed more exist in crude flower, whereas quinines and flavonoids moderately exist in leaf, but steroids, terpenoids, and cardiac glycosides have not existed in leaf and bark extracts of Hibiscus rosa-sinensis, respectively. The plant extracts showed a growth inhibitory effect against S. aureus and S. epidermidis in the range of 6.33 ± 0.33 to 11.50 ± 0.29 mm, whereas a growth inhibitory effect was recorded against K. pneumonia and E. coli in the range of 6.67 ± 0.33 to 13.00 ± .58 mm, respectively. The XRD analysis confirmed that Co(II) based MOF, Co2Res2 was a nanosize structure with an average crystal size of 27.17 nm. Furthermore, the DPV showed that the observed net modified current peaks and potentials were the most significant compared to the pure net current peaks and the potential of aqueous fresh flower, leaf, and bark extracted from Hibiscus rosa-sinensis. These extracts of Hibiscus rosa-sinensis have shown an improved potential source of natural antioxidants and as well as antibacterial activities. Chemically and thermally stable porous crystalline MOF, Co2Res2 serves as catalytic reactions to increase current and decrease potential in modified GCE, this might be due to the synergetic cumulative effect of the presence of bioactive ingredients and Co2Res2 catalyst.
Abbreviations: ABS: Acetate buffer solution; ANOVA: Analysis of variance; ATR: Attenuated total reflectance; CFU/ml: Colony forming unit per milliliter: Co2Res2: Co(II) based metal-organic framework (ResH2: resorcinol); DPV: Differential plus voltammetry: FTIR; Fourier transforms infrared radiation; GCE: Glassy carbon electrode; JCPDS: A joint committee on power diffraction standards; MOF: Metal-organic framework; PBS: Phosphate buffer solution; SPSS: Statistical package for the social sciences; UV-Vis: Ultraviolet-visible spectroscopy; XRD: X-ray diffraction
Introduction
Medicinal plants are now getting more attention than ever because they have the potential for countless benefits to society, especially in the line of medicine and pharmacology. The medicinal value of these plants lies in bioactive phytochemical constituents that produce definite physiological action on the human body.[Citation1] Plants contain secondary metabolites also known as phytochemical, natural bioactive compounds, or plant constituents, that are not directly involved in the normal growth, development, or reproduction of organisms but often play an important role in plant defense. Some of the most important bioactive phytochemical constituents are alkaloids, essential oils, flavonoids, tannins, terpenoids, saponins, phenolic compounds, and many more.[Citation2] They are found in plants, such as vegetables, fruits, flowers, leaves, and roots that work with nutrients and fiber to act as a defense system against disease.[Citation3]
Hibiscus rosa-sinensis is a member of the family Malvaceae (the mallows, is a family of flowering plants) which is considered an herbal tea and an important medicinal family, it is an evergreen woody, glabrous, showy shrub estimated 5–8 feet (height is a smooth shrub), and widely cultivated in the tropics as an ornamental plant in public and home places and has several forms with varying colors of flowers. The red-flowered variety is more preferred for medicinal trust.[Citation4,Citation5] The plant is widely cultivated in central and western Sudan, Syria, Southern Iraq, Egypt, China, Ethiopia, and many other countries. They thrive in mildly warm climates that witness the herbal plant’s widespread growth in the southern Mediterranean and the tropics, including the horn of Africa. Some other studies proved that Hibiscus rosa-sinensis has many medicinal benefits, for example, hibiscus tea drink lowers high blood pressure and increases the speed of blood circulation (lowers blood pressure in a group of pre-hypertensive and mildly hypertensive adults), and strengthens the heart.[Citation6] Furthermore, the use of herbs in the treatment and management of various forms of diseases and ailments is becoming popular worldwide. This could be due to the accumulating scientific evidence that some of these herbs possess the medicinal properties sought by the traditional people, they are also available at a reasonable cost and are easily accessible compared to modern pharmaceutical agents.[Citation7] Recently, interest has increased considerably in finding naturally occurring antioxidants for use in medicinal materials to replace synthetic antioxidants which are being restricted due to their side effects such as carcinogenicity.[Citation8] Since natural antioxidants contained in spices and herbs help to reduce oxidative stress, which is caused by a high concentration of free radicals in cells and tissues, can be induced by various negative factors, such as gamma, UV, and X-ray radiation, psycho-emotional stress, polluted food, adverse environmental conditions, intensive physical exertion, smoking, alcoholism, and drug addiction.[Citation9]
In another way, MOFs have received extensive interest under their exciting properties including large surface areas, ordered pore structure, stability, tenability, and multiple coordination sites as well. They have many applications in various areas including catalysis, electrochemical sensor, adsorption, and drug delivery.[Citation10] Additionally, in the electrochemical method, voltammetric techniques have attracted more attention since it possesses the merits of simple operation, fast response, and low cost, which offers the opportunity for portable, cheap, and rapid methodologies. They have been extremely useful in the analysis of a variety of samples, measuring blood levels, metabolites, and urinary excretion of drugs following low doses, especially when coupled with chromatographic methods.[Citation11,Citation12] Several previous studies reported that HRSL contained some phytochemical constituents.[Citation13] However, medicinal plants are extremely influenced by environmental conditions (e.g., high and low temperature, climate change, global warming, UV irradiation, intense sunlight and shade, ozone, carbon dioxide, drought, salinity, nutrient deficiency (soil type), agrochemicals, waste, heavy metals, weeds, pests, and pathogen infections) in regulating secondary metabolisms and the metabolic yield of biologically active molecules of the aromatic plants. From a literature review, negligible scientific studies have been conducted to date to validate the simultaneous use of the Ethiopian Hibiscus rosa-sinensis as antibacterial and antioxidant activity. Because of the toxicity of other organic solvents such as alcohol, which causes blindness, and their antibacterial and antioxidant activities,[Citation14,Citation15] people in Ethiopia have only information about this plant used for gardening and fencing purposes but do not have any awareness of plants widely used as a source of herbal tea and medicine.[Citation16] Therefore, the main objective of this study was to evaluate the antibacterial and antioxidant activity of aqueous flower, leaf, and bark extracts of Hibiscus rosa-sinensis and the use of Co(II)-based metal-organic framework (Co2Res2, ResH2: resorcinol) modified with glassy carbon electrode for determination of electrochemical behaviors. During extraction, water was used as the solvent using the maceration technique, since water is the universal solvent, not toxic, cheap, readily available, and the safest as compared to organic solvents.
Materials and methods
Hibiscus rosa-sinensis sample collections
This study was conducted at Bahir Dar University, college of natural science, department of chemistry, Ethiopia. At the beginning of January 2021, the flowers, leaves, and barks of Hibiscus rosa-sinensis were collected from Bahir Dar University on the main campus, Bahir Dar, Ethiopia. The plant species was guided by the informants and authenticated by the senior taxonomist at the Department of Botany, Bahir Dar University (voucher number: MB-M28-2021). Each part of Hibiscus rosa-sinensis washed and cleaned samples were dried at room temperature and then stored in tight polythene bags by labeling flower, leaf, and bark samples using sticker paper and keeping them in a cool and dry place (desiccators) until the experiments have completed on July 2021.
Preparation of aqueous crude flower, leaf, and bark extracts of Hibiscus rosa-sinensis
The sample of flower, leaf, and bark of Hibiscus rosa-sinensis aqueous crude extracts (bioactive ingredients) was prepared using maceration techniques according to the literature with a slight modification as follows photographically (Appendix 1)[Citation16,Citation17:] The fresh flower, leaf, and bark sample of Hibiscus rosa-sinensis were first cut and collected by a plastic bag. After that, the samples were two times washed with running tap water to remove dust and other debris. And then the samples were dried to constant weight under shade at room temperature, obtained each 20 g of flower, leaf, and bark was ground by using a mortar and pestle and screened through a 250 µM sieve to get a uniform texture, and stored in tight polythene bags labeled as sample A (flower), sample B (leaf), and sample C (bark) and keep it in a cool and dry place (desiccator) until use. Form each sieved samples A, B, and C five different mass such as 0.1 g, 0.2 g, 0.3 g, 0.4 g, and 0.5 g were weighed and added into 200 ml of Erlenmeyer flasks. 25 ml of distilled water was poured into each labeled Erlenmeyer flask and then stirred for 30 minutes using a magnetic stirrer for homogeneity of solution. The conical flasks were tightly closed with aluminum foil and the mixture solutions were left for 72 hrs using an electronic shake under room temperature. The final extracted solution of each flower, leaf, and bark sample of Hibiscus rosa-sinensis filter with bug cotton and /or Hand man No-1 filter paper. Finally, the samples were ready for antibacterial and antioxidant analysis, whereas the percentage of the yield was calculated using Eq. 1 ():
Table 1. The percentage yields of aqueous crude flower, leaf, and bark extracts of Hibiscus rosa-sinensis.
Test microorganisms used
The antibacterial activities were evaluated against a total of four human pathogens bacteria such as two gram-negative E. coli (ATCC 25922) and K. pneumonia (ATCC 43816), and two gram-positive S. aureus (ATCC 29213) and S. epidermidis (ATCC 12228), respectively. These were obtained from Amhara Public Health Institute (APHI), Ethiopia. The serial test concentrations (0.1 g, 0.2 g, 0.3 g, 0.4 g, and 0.5 g) of each extract were prepared by dissolving the sieved sample of flower, leaf, and bark in 25 mL of distilled water, the minimum and maximum test concentrations were determined based on research reports.
Antimicrobial activity of Hibiscus rosa-sinensis
Agar disc diffusion assay
The bacterial suspension was prepared from a 72 hrs pure culture of E. coli, K. pneumonia, S. aureus, and S. epidermidis. The optical densities of the suspensions were adjusted to 0.132 at 600 nm using a spectrophotometer. The number of bacterial population at this turbidity standard is 1.5 × 108 CFU/ml, approximately 0.5 ml of the bacterial suspension were aseptically inoculated to Mueller Hinton Agar medium and uniformly distributed using a spreader. Several Mueller Hinton agar plates were made and labeled according to the extracted by the name of the bacteria, standard control (tetracycline), and positive control (methanol). A bacterial suspension was prepared in sterile distilled water concerning the 0.5 McFarland Standards. The turbidity of the bacterial suspension was compared with 0.5 McFarland standard solutions, followed by a culture of 100 µl of the bacterial suspension on Mueller Hinton agar plates using a sterile glass rod spreader and allowed to remain in the incubator for 15 minutes to remove excess moisture. On each plate, equidistant discs were put on it. This is followed by incubation at 37°C for 48 hrs. The inhibition zone around each disc was recorded in millimeters (mm) with a ruler and the assay is carried out with three replications for each extracted sample to obtain consecutive results.
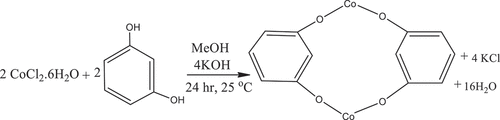
Synthesis of Co(II) based metal-organic framework, Co2Res2
The Co(II) based MOF, Co2Res2 was prepared according to the literature with a slight modification[Citation18] as follows: 4 mmol of KOH and 2 mmol of resorcinol were dissolved in 10 ml of methanol in conical flasks 1 and 2, respectively this solution was stirred for 5 min using a magnetic stirrer. 2 mmol of cobalt(II) chloride hexahydrate dissolved in 10 ml of methanol in a round bottom flask, and stirred it for 5 min using a magnetic stirrer, the purple color was observed. Solutions in conical flask 1 were slowly poured into conical flask 2 and the mixture solutions were stirred for 7 min for deprotonating, the sangria red solution was observed in conical flask 3. After that, this solution was poured into the solution contained in a round bottom flask and then vigorously stirred for 24 hrs by using a magnetic stirrer at room temperature to produce a hickory brown solution. The side product of KCl salt was separated from the final product solution through decantation and the remaining solvent was removed by using a rotary evaporator obtained dark brown crystal, Co2Res2 (yield = 44.7%) (). The proposed reaction mechanism of Co(II) based MOF, Co2Res2 is as follows:
An overall reaction would be:
Electrochemical methods
A voltammetric experiment was carried out using CH Instruments: (CHI760D electrochemical analyzer/workstation, USA) connected to a personal computer. All electrochemical experiment was performed by employing a conventional three-electrode system of a glassy carbon electrode (3 mm in diameter) and/or Co2Res2/GCE as the working electrode, whereas platinum coil and Ag/AgCl were used as an auxiliary and reference electrode respectively. All experiments were carried out at 20 ± 2°C. For differential pulse voltammetry (DPV) analysis, 0.5 g of each sieved samples flower, leaf, and bark were taken and poured into 200 ml of Erlenmeyer flask, and then 100 ml of distilled water was added to it, and shake the mixture solution for 72 hr by using electronic shaker under room temperature. The final extracted solution was filtered off with bug cotton and/or Hand man No-1 filter paper. Finally, the samples were ready for DPV analysis.
Characterization techniques
This study was conducted at Bahir Dar University, college of natural science, department of chemistry, Ethiopia. The aqueous crude flower, leaf, and bark extraction of Hibiscus rosa-sinensis, using maceration techniques. UV-Vis spectroscopy (DU-8800D spectrophotometer, optical system: Double Beam, Blazed Holographic Grating (1200 lines/mm) China), and FT-IR spectroscopy (FT/IR-6600 FTIR Spectrometer from JASCO, USA), DPV (CH Instruments: CHI760D electrochemical analyzer/ workstation, USA), preliminary qualitative phytochemical and antibacterial activities whereas, X-ray diffractometer (BRUKER D8 Advance XRD, Germany) equipped with a Cu target for generating a Cu Kα radiation (wavelength 1.5406 Å) were conducted at Addis Ababa University, Ethiopia. For UV-Vis characterization, the extracted clear solution of each sample of flower, leaf, and bark of Hibiscus rosa-sinensis was used at a lower concentration of 0.1 g/ml, but for FTIR characterization used at a high concentration of 0.5 g/ml, that means the solid crude powder of each sample was obtained by removing the aqueous solvent through the dry freezer and then ready for FTIR analysis.
Statistical data analysis
All existed the numeric values of aqueous crude flower, leaf, and bark extracts of Hibiscus rosa-sinensis data were using three measurements (mean) ± standard error was tested by One-way ANOVA (Analysis of variance) using the SPSS software for windows version 20, IBM, NY, USA. This was done after carrying out a test of homogeneity and normal distribution of each measured parameter. Mean values were compared with Tukey’s Honestly Significant Difference (HSD) test and all the statistical analyses were computed with SPSS significant difference was determined at 5% (p < .05).
Results
Preliminary phytochemical studies
Choosing the best solvent was subjected to preliminary phytochemical studies of Hibiscus rosa-sinensis to screen for the presence of secondary metabolites, this study was using water as a solvent by using standard protocols with a slight modification.[Citation19] The percent yield of extracted samples and bioactive compounds have existed in aqueous crude flower, leaf, and bark extracts of Hibiscus rosa-sinensis (, and ).
Figure 1. Qualitative preliminary phytochemical screening of aqueous crude flower, leaf, and bark extracts of Hibiscus rosa-sinensis, a) Phenol, b) Tannins, c) Flavonoids, d) Saponins, e) Steroids, f) Quinones, g) Terpenoids, and h) Cardiac glycosides.
Table 2. Qualitative phytochemical analysis and its constituents existence in aqueous crude flower, leaf, and bark extracts of Hibiscus rosa-sinensis [0.3 g/25 ml].
UV-Vis spectra analysis
The UV-Vis spectra of aqueous crude flower, leaf, and bark extracts of Hibiscus rosa-sinensis were shown in , respectively. These UV‐Vis profiles show the peaks at 276 and 327, 273, 275, and 321 nm with the absorption values 0.53 and 0.38, 0.57, 0.33, and 0.25, respectively. This indicated the presence of phenolic compounds like tannin and flavonoid compounds. Since these compounds might be used as an antioxidant, anti-inflammatory, and anti-bacterial activities.
FTIR spectra analysis
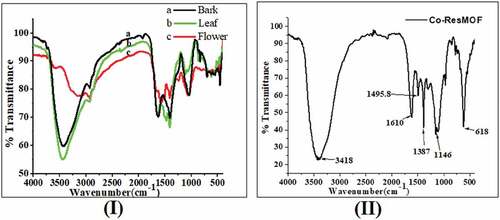
The FTIR spectra were used to identify the functional group of the active components found in aqueous crude flower (a), leaf (b), and bark (c) extracts of Hibiscus rosa-sinensis (). The FTIR absorption peaks 2500–3500 cm−1 show the presence of hydroxyl group, OH stretching in aqueous crude extracts of Hibiscus rosa-sinensis, which is common in all phenolic compounds. And also the broad peak at 3125–3447 cm−1 indicated the presence of O-H and/or N-H stretching. The peaks obtained at 2930 and 2937 cm−1 indicated the presence of C-H stretching. The peaks obtained at 1410, 1415, and 1479 cm−1 indicated the presence of O-H bending and aromatic starching. The peaks obtained at 1048, 1059, and 1117 cm−1 indicated the presence of C-O stretching. The peak obtained at 624 cm−1 indicated the presence of C-H and/or C-Br bend out of the plane. The peaks at 457 cm−1 and 544 cm−1 shifted to 618 cm−1 which indicates in the new compound formation, peaks at 681.5, 773.5, 952, and 1151.8 shifted to 1146 cm−1; peaks at 1301 and 1381 cm−1 shifted to 1387; peaks at 1490 and 1610 cm−1 shift to 1610 cm−1. So, this indicates the resorcinol ligand coordinated to the cobalt metal center, formation of Co(II) based MOF, Co2Res2.[Citation20,Citation21]
XRD analysis
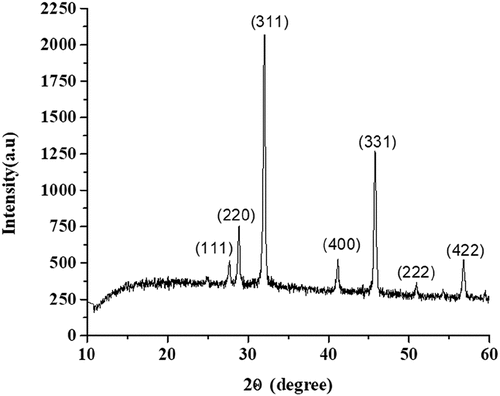
The diffraction peaks shown in were more intensive, implying a good crystalline nature of the as-synthesized Co2Res2 product (the most intense peak (311) in the XRD patterns was used to calculate the average crystalline size (D)), and the diffraction peaks at scattering angles (2θ): 27.66, 28.83, 32.02, 41.08, 45.78, 50.91, and 56.76 corresponds to the reflection from (111), (220), (311), (400), (331), (222), and (422) crystal planes for all as-synthesized powder suggesting the formation of Co-O bond in Co2Res2 nanoparticles and these planes are in agreement with the cubic phase of Co2Res2 (JCPDS file no. 75–0393 and 09–0418). The crystalline size of the compound is calculated by the Debye-Scherrer formula (Equation 2) and based on the highest diffraction peak (assigned to the 311 crystal plane). The 2θ value with a maximum intensity of the peak for the compound was found to be 32.029 which corresponds to the average crystal size D = 27.166 nm ().
Table 3. The average crystallite size (D) of as-synthesized Co2Res2.
where D is the average crystallite size, λ is the wavelength of the X-ray = 0.15406 nm for Cu target Kα radiation, β is the peak width of half-maximum (FWHM) of an XRD, and θ is the Bragg diffraction angle.
Antibacterial activities
The aqueous crude flower, leaf, and bark extracts of Hibiscus rosa-sinensis were shown the presence of antimicrobial activities against four selected pathogenic bacteria such as two gram-negative E. coli and K. pneumonia and two gram-positive S. aureus and S. epidermidis, the maximum and minimum inhibition zone were recorded in different concentration ranges (0.1–0.5 g/25 ml) after an incubation period of 72 hrs at 37°C by using disc diffusion method ().
Figure 5. A bar graph shows inhibition zone versus concentration of antibacterial activities of aqueous crude flower, leaf, and bark extracts of Hibiscus rosa-sinensis against E. coli, K. pneumonia, S. aureus, S. epidermidis, and tetracycline, respectively in different concentration ranges (0.1–0.5 g/ 25 ml).
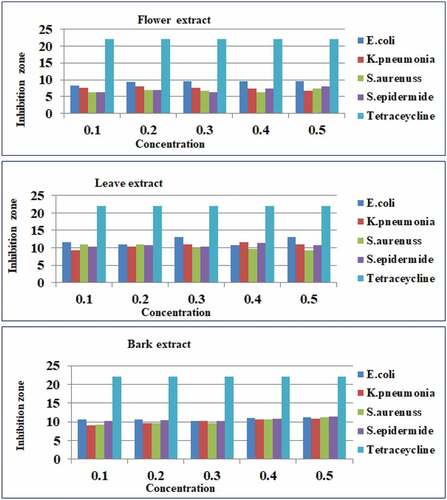
For aqueous crude flower extract of Hibiscus rosa-sinensis
The highest and the lowest zone of inhibition were recorded at 0.1 g/25 ml E. coli (8.33 ± .33 mm) and both in S. aureus and S. epidermidis (6.33 ± .33 mm); at 0.2 g/25 ml E. coli (9.33 ± .33 mm) and both in S. aureus and S. epidermidis (7.00 ± .58 mm); at 0.3 g/25 ml E. coli (9.67 ± .33 mm) and S. epidermidis (6.33 ± .33 mm); at 0.4 g/25 ml E. coli (9.67 ± .58 mm) and S. aureus (6.33 ± .33 mm); at 0.5 g/25 ml E. coli (9.67 ± .33 mm) and K. pneumonia (6.67 ± .33 mm), respectively. In this case from the given concentration ranges, E. coli had the highest zone of inhibition, whereas the lowest zone of inhibition was recorded in S. aureus and S. epidermidis, respectively.
For aqueous crude leaf extract of Hibiscus rosa-sinensis
The highest and the lowest zone of inhibition were recorded at 0.1 g/25 ml E. coli (11.67 ± .88 mm) and K. pneumonia (9.33 ± .33 mm); at 0.2 g/25 ml S. aureus (11.00 ± .58 mm) and K. pneumonia (10.33 ± .33 mm); at 0.3 g/25 ml E. coli (13.00 ± .58 mm) and S. aureus (10.00 ± .58 mm); at 0.4 g/25 ml K. pneumonia (11.67 ± .33 mm) and S. aureus (9.67 ± .33 mm); at 0.5 g/25 ml E. coli (13.00 ± .58 mm) and S. aureus (9.33 ± .33 mm), respectively.
For aqueous crude bark extract of Hibiscus rosa-sinensis
The highest and the lowest zone of inhibition were recorded at 0.1 g/25 ml E. coli (10.67 ± .33 mm) and K. pneumonia (9.00 ± .58 mm); at 0.2 g/25 ml E. coli (10.67 ± .33 mm) and both in K. pneumonia (9.67 ± .67 mm) and S. aureus (9.67 ± .33 mm); at 0.3 g/25 ml surprisingly the same values were observed in E. coli (10.33 ± .33 mm), K. pneumonia (10.33 ± .33 mm) and S. epidermidis (10.33 ± .33 mm) whereas, S. aureus (9.00 ± .58 mm); at 0.4 g/25 E. coli (11.00 ± .00 mm) and both in K. pneumonia (10.67 ± .33 mm) and S. aureus (10.67 ± .33 mm); at 0.5 g/25 ml S. epidermidis (11.50 ± .29 mm) and K. pneumonia (10.83 ± .17 mm), respectively. Therefore, generally in different concentration ranges different bacteria strains have the highest, moderate, and lowest zone of inhibition recorded, and in some cases as the concentration increased the inhibition zones also increased. The three parts of the aqueous crude extracts of Hibiscus rosa-sinensis antibacterial activities potentials increasing order were flower < bark < leaf for gram-negative and positive bacteria respectively, and this indicates leaf extract has shown more promising antibacterial activity than flower and bark for gram-negative and positive bacteria.
DPV analysis
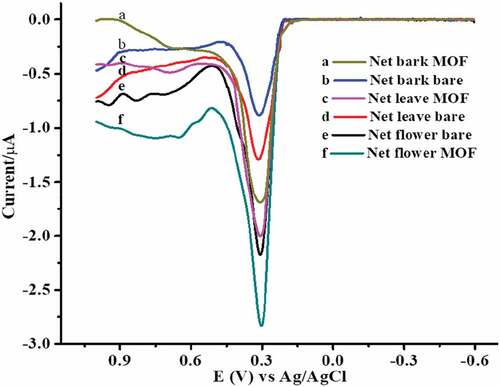
describes the electrochemical behavior of DPV in aqueous crude flower, leaf, and bark extracts of Hibiscus rosa-sinensis with unmodified and modified Co2Res2/GCE at pH 5 of ABS. They tend to readily undergo oxidation at the electrode surfaces. Hence, antioxidants that oxidize at lower potentials show higher antioxidant activity. Besides, their analytical advantages, these techniques play an important role in the study of pharmacologically active compounds and metabolites produced by different metabolic pathways involving redox reactions. These the net flower bare (e) and net flower MOF (f) were a potential decreased 0.309 V to 0.304 V and current increased 2.162 µA to 2.812 µA; net leaf-bare (d) and net leaf MOF (c) a potential decreased 0.317 V to 0.309 V and a current increased 1.277 µA to 1.983 µA; net bark bare (b) and net bark MOF (a) a potential decreased 0.312 V to 0.309 V and current increased 0.870 µA to 1.682 µA, respectively. From these results, an oxidative peak with an improved peak current and peak potential at the modified electrode relative to the unmodified electrode confirmed the catalytic effect of the Co2Res2/GCE toward oxidation of aqueous crude flower, leaf, and bark extract of Hibiscus rosa-sinensis. While the peak of the aqueous crude flower, leaf, and bark extract of Hibiscus rosa-sinensis of different parts the potential difference was negligible that indicating the sample was the same species. The best antioxidants of aqueous crude flower, leaf, and bark, extract of Hibiscus rosa-sinensis by the modified Co2Res2/GCE exhibited excellent electrochemical performance with extended linear ranges and lower detection limits.
Discussion
Medicinal plants have been a therapeutic source for a long time and plant products played an essential role in ancient medicine. Since ancient times, higher plants are sources of medicinal compounds and play a dominant role in the maintenance of human health.[Citation22] The Hibiscus rosa-sinensis plants are rich in secondary metabolites such as flavonoids, carotenoids, phenols, alkaloids, terpenoids, steroids, etc., that have been found to have excellent antioxidant as well as antimicrobial properties.[Citation23] According to the World Health Organization, 65–80% of the world population relies on traditional medicine to treat various diseases and 50% of all modern clinical drugs are of natural product origin, whereas natural products play an important role in the drug development program in the pharmaceutical industry.[Citation24,Citation25] In this study, the phytochemical analysis of an aqueous crude flower, leaf, and bark extract of Hibiscus rosa-sinensis results were shown more existence, moderately existence, and absence of active metabolite compounds such as phenols, tannins, flavonoids, saponins, steroids, quinines, terpenoids, and cardiac glycosides (). This illustrated that phenols, flavonoids, and quinines were observed more exist in crude flower, saponins were observed more exist in leaf and bark, whereas flavonoids and quinines have moderately existed in leaf, but steroids, terpenoids, and cardiac glycosides were not existed in leaf and bark extracts of Hibiscus rosa-sinensis, respectively. Our findings were similar to those reported in previous papers.[Citation26] This shows the in vitro antibacterial activity of Hibiscus rosa-sinensis flower extract against human pathogens using disc and agar diffusion methods and also ethanol and methanol used as a solvent. Their result showed in the cold extraction technique a maximum zone of inhibition against B. subtilis and E. coli were recorded at 17.00 ± 2.91 and 14.50 ± 1.71 mm, respectively. Whereas in hot extraction, E. coli and Salmonella sp. recorded 11.66 ± 3.14 and 10.60 ± 3.09 mm, respectively. So, in methanol extraction, the highest zone of inhibition was recorded against B. subtilis and E. coli at 18.86 ± 0.18 and 18.00 ± 1.63 mm, respectively, while ethanol extraction showed the utmost zone of inhibition recorded against Salmonella sp. at 20.40 ± 1.54 mm. In other studies, five different cultivars of Hibiscus rosa-sinensis were tested for antibacterial activity of four different extracts (hexane, ethyl acetate, methanol, and distilled water) against gram-positive bacteria (S. aureus, B. subtilis, S. alboniger, M. luteus, and S. epidermis) and gram-negative bacteria (P. aeruginosa, and B. bronchiseptica), this was determined by the agar diffusion method. From these, ethyl acetate leaf extract showed significant antibacterial activity against cultivar ‘orange.’ Among these four plant extracts, the ethyl acetate and methanol extracts tested showed higher antibacterial activity compared to the other extracts, while very low antibacterial activity was observed with the hexane extract.[Citation27,Citation28] However, the red-flowered variety of our result shows slightly lower than the reported data to the zone of inhibition recorded, this might be solvent effect and environmental impact. Differences may also occur due to soil content, geographic extent, time of plant collection, plant part, plant growth stage, etc. According to Copp,[Citation29] secondary metabolites of the terpenoid family possess antibacterial activity that may be related to their lipophilicity and ability to penetrate bacterial cell walls. Additionally, variations of bioactive components have been reported to differ between solvent extraction and raw material collection zones.[Citation30,Citation31]
Spectroscopic methods have become a powerful tool for secondary metabolite profiling as well as for qualitative and quantitative analysis of pharmaceutical and biological materials. The current study revealed that the aqueous crude flower (a), leaf (b), and bark (c) extracts of Hibiscus rosa-sinensis showed the presence of phenols, tannins, flavonoids, saponins, steroids, quinines, terpenoids, and cardiac glycosides (, ). This was correlated with other researchers’ FTIR results and it has shown functional groups observed from the isolated compound of the aqueous extract solution of Hibiscus rosa-sinensis, which was extracted successively with petroleum ether, chloroform, and ethanol. The reported FTIR absorptions at 3495, 3389, 3182 cm−1 (OH), 2959, 2881 cm−1 (C–H), 1697, 1687 cm−1 (C=O),1649 cm−1 (–C=C–), 1462 cm−1 (C–H), and 1438 cm−1 (C–C).[Citation32–34] The UV-Vis spectroscopic analysis confirms the presence of phenols and flavonoids in the Hibiscus rosa-sinensis extracts and correlated with related studies showing absorption peaks at 280 nm (phenolic), 320 nm (flavones), and 360 nm (phenolic acids), respectively.[Citation35] However, the polyphenol compound may not be expected to be present in its glycosylated form, this chemical form was more common in plant extract due to its higher solubility in water. It is important to note that glycosides do not have a major effect on the absorption peak within UV-Vis spectra.[Citation36] Similarly, the Co2Res2 MOF shows an absorption peak at 493 nm, this indicates the lower energy transitions ∏-∏* for the existence of aromatic groups containing C=C bonds responsible.
The electrochemical method has attracted more attention since it possesses the merits of simple operation, fast response, and low cost, which offers the opportunity for portable, cheap, and rapid methodologies. In an electrochemistry system, the given substance exhibits overlapped oxidation peaks at conventional solid electrodes, and are difficult to be distinguished. Thus, electrochemical detection approaches for those substances must be developed with suitable modification materials that can provide complete resolution of their electrochemical signals, or determine the selection of at least one substance without influence from the other. For this purpose, various kinds of electrodes modified with graphene, carbon nanotubes and gold nanoparticles[Citation37] have been constructed to improve the electrochemical performance of the sensing platform. Therefore, in this paper we reported the DPV profile of Co(II) based MOF, Co2Res2 () supported the XRD data ( and ), based on these results, we may conclude aqueous crude extracts of flower, leaf, and bark of Ethiopian Hibiscus rosa-sinensis contains phytochemical compounds with simultaneously used for antibacterial activities against the sensitive study bacteria and antioxidant activities.
Conclusion
The current studies revealed the UV-Vis, FTIR, and DPV profiles of aqueous crude flower, leaf, and bark extracts of Hibiscus rosa-sinensis. This study may thus serve as preliminary scientific validation of their antibacterial and antioxidant activities. UV-Vis spectrophotometer revealed that the presence of phenolic compounds like tannin and flavonoid compound, these compounds would be used as an antioxidant, anti-inflammatory, and anti-bacterial activities. Whereas, the FTIR spectra confirmed polyphenols and flavonoids due to O-H stretching and terpenes due to the C-H group. These compounds belong to secondary plant metabolites since metabolites help plants cope with environmental and/or external stimuli in a rapid, reversible, and ecologically meaningful manner. Because, the impacts of various environmental factors (temperature, climate change, intense sunlight and shade, carbon dioxide, salinity, nutrient deficiency, agrochemicals, heavy metals, and pathogen infections) play a crucial role in regulating the metabolic yield of these biologically active molecules. The peaks at 681.5, 952, and 1151.8 shifted to 1146 cm−1; peaks at 1301 and 1381 cm−1 shifted to 1387, etc., which indicates the resorcinol ligand coordinated to the cobalt metal center, formation of Co(II) based MOF. Moreover, the as-synthesized Co2Res2 compound was analyzed by using powder XRD and the 2θ value with a maximum intensity of the peak for the compound was found to be 32.029 which corresponds to the average crystal size D = 27.166 nm, it confirmed that particles with diameters in nano dimension size, this might be used as a catalyst for electrochemical method development. DPV results of aqueous crude flower, leaf, and bark extract of Hibiscus rosa-sinensis and unmodified and modified Co2Res2/GCE observed with potential, (V) and current (µA) in the range between 0.304 V-0.317 V and 0.870 µA −2.812 µA, respectively. Therefore, the presence of phytochemical bioactive constituents and functional groups of the indigenous Hibiscus rosa-sinensis plant has shown promising medicinal values and could be used for developing herbal medicines to target oxidative stress and infectious diseases in near future. Advanced isolation and characterization of Hibiscus rosa-sinensis plant active components is our priority next line study.
Acknowledgments
This work was supported and grant-funded by the minister of education and Bahir Dar University (BDU), Ethiopia.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Akinmoladun, A. C.; Ibukun, E. O.; Afor, E.; Obuotor, E. M.; Farombi, E. O. Phytochemical Constituent and Antioxidant Activity of Extract from the Leaf of Ocimum Gratissimum. Sci. Res. Essays. 2007, 2(5), 163–166. DOI: 10.5897/SRE.9000731.
- Edeoga, H. O.; Okwu, D. E.; Mbaebie, B. O. Phytochemical Constituents of Some Nigerian Medicinal Plants. Afr. J. Biotechnol. 2005, 4(7), 685–688. DOI: 10.5897/AJB2005.000-3127.
- Krishnaiah, D.; Sarbatly, R.; Bono, A. Phytochemical Antioxidants for Health and Medicine a Move Towards Nature. Biotechnol. Mol. Biol. Rev. 2007, 2(4), 97–104. DOI: 10.5897/BMBR2007.0009.
- Austin, D. F. Plant Resources in South-East Asia. Medicinal and Poisonous Plants 2. Economic Botany. 2002, 6(3), 297–304.
- Adhirajan, N.; Kumar, T. R.; Shanmugasundaram, N.; Babu, M. In Vivo and in Vitro Evaluation of Hair Growth Potential of Hibiscus rosa-sinensis Linn. J. Ethnopharmacol. 2003, 88(2–3), 235–239. DOI: 10.1016/s0378-8741(03)00231-9.
- Mozaffari-Khosravi, H.; Jalali-Khanabadi, B. A.; Afkhami-Ardekani, M.; Fatehi, F.; Noori-Shadkam, M. The Effects of Sour Tea (Hibiscus Sabdariffa) on Hypertension in Patients with Type II Diabetes. J. Human Hypertens. 2009, 23(1), 48–54. DOI: 10.1038/jhh.2008.100.
- Alkhalidy, H.; Wang, Y.; Liu, D. Dietary Flavonoids in the Prevention of T2D: An Overview. Nutrients. 2018, 10(4), 438. DOI: 10.3390/nu10040438.
- Kumaran, A.; Karunakaran, R. J. In Vitro Antioxidant Activities of Methanol Extracts of Five Phyllanthus Species from India. LWT-Food Sci. Technolo. 2007, 40(2), 344–352. DOI: 10.1016/j.lwt.2005.09.011.
- Patra, K.; Jana, S.; Mandal, D. P.; Bhattacharjee, S. Evaluation of the Antioxidant Activity of Extracts and Active Principles of Commonly Consumed Indian Spices. J. Environ. Pathol. Toxicol. Oncol. 2016, 35(4), 299–315. DOI: 10.1615/JEnvironPatholToxicolOncol.2016016387.
- Della-Rocca, J.; Liu, D.; Lin, W. Nanoscale metal-organic Frameworks for Biomedical Imaging and Drug Delivery. Acc. Chem. Res. 2011, 44(10), 957–968. DOI: 10.1021/ar200028a.
- Arteaga, J. F.; Ruiz-Montoya, M.; Palma, A.; Alonso-Garrido, G.; Pintado, S.; Rodríguez-Mellado, J. M. Comparison of the Simple Cyclic Voltammetry (CV) and DPPH Assays for the Determination of Antioxidant Capacity of Active Principles. Molecules. 2012, 17(5), 5126–5138. DOI: 10.3390/molecules17055126.
- Piljac-Žegarac, J.; Valek, L.; Stipčević, T.; Martinez, S. Electrochemical Determination of the Antioxidant Capacity of Fruit Tea Infusions. Food Chem. 2010, 121(3), 820–825. DOI: 10.1016/j.foodchem.2009.12.090.
- Shukla, Y. N., and Mishra, M. A Hydroxyl Acid and Sterols from Hibiscus rosa-sinensis. Indian Drugs. 2001, 38(10), 543. [Accesed date 03 June 2022]. https://www.lifesciencesite.com/lsj/life1105/001_23106life110514_1_8.pdf.
- Missoum, A. An Updated Review on Hibiscus rosa-sinensis Phytochemistry and Medicinal Uses. J. Ayurvedic herb. med. 2018, 4(3), 135–146. DOI: 10.31254/jahm.2018.4308.
- Dyrda, G.; Boniewska-Bernacka, E.; Man, D.; Barchiewicz, K.; Słota, R. The Effect of Organic Solvents on Selected Microorganisms and Model Liposome Membrane. Mol. Biol. Rep. 2019, 46(3), 3225–3232. DOI: 10.1007/s11033-019-04782-y.
- Ruban, P.; Gajalakshmi, K. In Vitro Antibacterial Activity of Hibiscus rosa-sinensis Flower Extract against Human Pathogens. Asian Pac. J. Trop. Biomed. 2012, 2(5), 399–403. DOI: 10.1016/S2221-1691(12)60064-1.
- Rai, V. M.; Pai, V. R.; Kedilaya, P. H., and Hegde, S. Preliminary Phytochemical Screening of Members of Lamiaceae Family: Leucaslinifolia, Coleus Aromaticus, and Pogestemon Patchouli. Int. J. Pharm. Sci. Rev. Res. 2013, 21(1), 131–137. [Accesed date 02 June 2022]. https://citeseerx.ist.psu.edu/viewdoc/download?.
- Ramachandran, R.; Zhao, C.; Luo, D.; Wang, K.; Wang, F. Synthesis of Copper Benzene-1, 3, 5-tricarboxylate metal-organic Frameworks with Mixed Phases as the Electrode Material for Supercapacitor Applications. Appl. Surf. Sci. 2018, 460, 33–39. DOI: 10.1016/j.apsusc.2017.11.271.
- Gul, R.; Jan, S. U.; Faridullah, S.; Sherani, S.; Jahan, N. Preliminary Phytochemical Screening, Quantitative Analysis of Alkaloids, and Antioxidant Activity of Crude Plant Extracts from Ephedra Intermedia Indigenous to Balochistan. The Sci. World J. 2017, 5873648. DOI: 10.1155/2017/5873648.
- Kowalczuk, D.; Pitucha, M. Application of FTIR Method for the Assessment of Immobilization of Active Substances in the Matrix of Biomedical Materials. Materials. 2019, 12(18), 2972. DOI: 10.3390/ma12182972.
- Salama, R. S.; Mannaa, M. A.; Altass, H. M.; Ibrahim, A. A.; Khder, A. E. Palladium Supported on a mixed-metal-organic Framework (CO-Mn-MOF-74) for Efficient Catalytic Oxidation of CO. RSC Adv. 2021, 11(8), 4318–4326. DOI: 10.1039/D0RA09970H.
- Farombi, E. O. African Indigenous Plants with Chemotherapeutic Potentials and Biotechnological Approach to the Production of Bioactive Prophylactic Agents. Afr. J. Biotechnol. 2003, 2(12), 662–671. DOI: 10.5897/ajb2003.000-1122.
- Guno, S. C.; Rohan, S. B.; Chaitanya, R. P. Analgesic Activity of Chloroform Extract of Caesalpinia Pulcherrima. J. Pharm. Res. 2009, 2(7), 1199–1200.
- Baker, J. T.; Borris, R. P.; Carte, B.; Cordell, G. A.; Soejarto, D. D.; Cragg, G. M.; Gupta, M. P.; Iwu, M. M.; Madulid, D. R.; Tyler, V. E. Natural Product Drug Discovery and Development: New Perspective on International Collaboration. J. Nat. Products. 1995, 58(9), 1325–1357. DOI: 10.1021/np50123a003.
- Sumathi, S.; Krishnaveni, M. Preliminary Screening, Antioxidant and Antimicrobial Potential of Chaetomorpha Antennina and Caulerapa Scalpelliformis in Vitro Study. Int. J. Environ. Sci. Technol. 2012, 2(3), 2319–2327. DOI: 10.6088/ijes.00202030112.
- Sobhy, E. A.; AbdElaleem, K. G.; AbdElaleem, H. G. Potential Antibacterial Activity of Hibiscus rosa-sinensis Linn Flowers Extracts. Int. J. Curr. Microbiol. Appl. Sci. 2017, 6(4), 1066–1072. DOI: 10.20546/ijcmas.2017.604.132.
- Patel, R. J.; Patel, A.; Vaghasiya, D., and Anju, N. Antimicrobial Evaluation of Hibiscus rosa-sinensis Plant Extracts against Some Pathogenic Bacteria. Bull. Environ. Sci. Res. 2012, 1(3–4), 14–17. [Aceesed date 01 June 2022]. http://besr.org.in/index.php/besr/article/view/30.
- Khan, Z. A.; Naqvi, S. A.; Mukhtar, A.; Hussain, Z.; Shahzad, S. A.; Mansha, A.; Ahmad, M.; Zahoor, A. F.; Bukhari, I. H.; Ashraf-Janjua, M. R., et al. Antioxidant and Antibacterial Activities of Hibiscus rosa-sinensis Linn Flower Extracts. Pak. J. Pharm. Sci, 2014, 27(3), 469–474, PMID: 24811803
- Copp, B. R. Antimycobacterial Natural Products. Nat. Prod. Rep. 2003, 20(6), 535–557. DOI: 10.1039/b212154a.
- Orhan, I. E.; Atasu, E.; Senol, F. S.; Ozturk, N.; Demirci, B.; Das, K.; Sekeroglu, N. Comparative Studies on Turkish and Indian Centella Asiatica (L.) Urban (Gotu Kola) Samples for Their Enzyme Inhibitory and Antioxidant Effects and Phytochemical Characterization. Ind. Crops Prod. 2013, 47, 316–322. DOI: 10.1016/j.indcrop.2013.03.022.
- Das, K. Phytochemical Evaluation and Comparative Antibiocide Efficacy of Aqueous, Ethanolic and Equal Mixture of Aqueous and Ethanolic (1:1) Bark Extract of Lannea Coromandelica L. Procured from Eastern Region of India. Int. Lett. Nat. Sci. 2014, 26, 21–31. DOI: www.scipress.com/ILNS.26.21.
- Sahayaraj, P. A.; Gowri, J.; Dharmalingam, V.; Shobana, R.; Prema, A. A. Phytochemical Screening by FTIR Spectroscopic Analysis of Leaf and Stem Extracts of Wedeliabiflora. Int. J. nano corros. sci. eng. 2015, 2(5), 322–334. DOI: 10.9734/ajrb/2021/v8i130171.
- Thummajitsakul, S.; Samaikam, S.; Tacha, S.; Silprasit, K. Study on FTIR Spectroscopy, Total Phenolic Content, Antioxidant Activity and anti-amylase Activity of Extracts and Different Tea Forms of Garcinia Schomburgkiana Leaf. LWT-Food Sci. Technol. 2020, 134, 110005. DOI: 10.1016/j.lwt.2020.110005.
- Amin, A.; Hamza, A. A. Hepatoprotective Effects of Hibiscus, Rosmarinus and Salvia on azathioprine-induced Toxicity in Rat. Life Sci. 2005, 77(3), 266–278. DOI: 10.1016/j.lfs.2004.09.048.
- Altemimi, A.; Lakhssassi, N.; Baharlouei, A.; Watson, D. G.; Lightfoot, D. A. Phytochemicals: Extraction, Isolation, and Identification of Bioactive Compounds from Plant Extracts. Plants. 2017, 6(4), 42. DOI:10.3390/plants6040042
- Tura, D.; Robards, K. Sample Handling Strategies for the Determination of Bio Phenols in Food and Plants. J. Chromatogr. A. 2002, 975(1), 71–93. DOI: 10.1016/s0021-9673(02)00879-8.
- Hu, F.; Chen, S.; Wang, C.; Yuan, R.; Yuan, D.; Wang, C. Study on the Application of Reduced Graphene Oxide and Multiwall Carbon Nanotubes Hybrid Materials for Simultaneous Determination of Catechol, Hydroquinone, p-cresol, and Nitrite. Anal. Chim. Acta. 2012, 724, 40–46. DOI: 10.1016/j.aca.2012.02.037.

![Figure 2. UV-Vis spectra of aqueous crude flower, leaf, bark extracts of Hibiscus rosa-sinensis (I) [0.5 ml extract/4.5 ml distilled water] and Co2Res2 (II).](/cms/asset/44a04cc4-b9fd-4bdd-a894-ae05abb145f7/ljfp_a_2112598_f0002_oc.jpg)