?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
This study proposes a strategy for screening and validating of antioxidant compounds and components from leaves of yacon (Smallanthus sonchifolius Poepp. and Endl.) by variable selection based on weight analysis. The theoretical basis of variable selection lies in that the varied quantity of variables will influence the activity results of samples. The ethyl acetate fraction (sample 0) with high DPPH scavenging activity was further separated using silica gel chromatographic column to obtain 17 subfractions (samples 1–17). The 18 samples contain different compounds exhibited different DPPH radical scavenging activities. Two components A and B with time range of 2.00–12.00 min and 53.00–64.00 min on the chromatogram were selected by variable selection, respectively. Simultaneously, a large number of compounds with different retention times (RTs) were screened out. Five predicted compounds, including chlorogenic acid, methyl caffeate, ethyl caffeate, homoeriodictyol, quercetin 3,7-dimethyl ether were isolated and verified by DPPH radical scavenging ability assay. The radical scavenging rates of those compounds were higher than that of ethyl acetate fraction and the positive control butylated hydroxyltoluene (BHT). Meanwhile, components A and B also show strong antioxidant activity. The radical scavenging activity of component A is higher than that of compounds which contained in component A, indicating the existence of synergistic antioxidant activity of compounds. The results of variable selection show that the proposed method is simple and reliable in screening the most active components and compounds. The method could be used for screening of compounds and components from other herbal plants with other activities.
Introduction
Yacon (Smallanthus sonchifolius Poepp. and Endl.) is a tuber crop native of Andean highlands of South America under the Asteraceaea family.[Citation1,Citation2] Yacon cultivation has been expanded to many countries with varying climates, such as New Zealand, Japan, Brazil, Korea, China in the last decades,[Citation3] and it has been planted sporadically in Hainan, Fujian, Yunnan and Guizhou provinces of China for a long time. Due to the superior environmental conditions, Guizhou province is an ideal area for the cultivation of yacon in China. Usually, yacon is cultivated as a root vegetable, its tubers contain high amounts (40%–70% dry weight) of fructooligosaccharides (FOS).[Citation4–6] FOS may decrease blood glucose levels and are considered to work as prebiotics by improving the intestinal microflora balance and promoting the growth of probiotic organisms.[Citation7] Besides, a wide variety of compounds including different phenolic acids (protocatechuic, chlorogenic, caffeic and ferulic acids), essential oil, ent-kaurenoic acid, sesquiterpene lactones and related diterpenoid substances are known from tubers and leaves of yacon.[Citation8,Citation9] Those compounds exhibit various activities, such as antidiabetic, antifungal, antimicrobial, antioxidant and anticancer properties.[Citation8,Citation10–12]
Oxygen-centered free radicals and other reactive oxygen species (ROS) are an entire class of highly reactive molecules derived from the metabolism of oxygen.[Citation13] Many human diseases, including accelerated aging, cancer, cardiovascular disease, neurodegenerative disease and inflammation, are linked to excessive amounts of ROS.[Citation14] Antioxidants are vital substances that protect human body from damages caused by free radical-induced oxidative stress. However, use of synthetic antioxidants in food products is under strict regulation due to the potential health hazards caused by such compounds.[Citation15] Many medicinal plants are rich in antioxidant components with high activities and low side-effects. Phenolic compounds, carotenoids, flavonoids, cinnamic acids, benzoic acids, folic acid, ascorbic acid, tocotrienols etc., are some of the antioxidants produced by the plant for sustenance.[Citation16]
Previous reports demonstrate that the leaves and tubers of yacon are promising source of natural antioxidant. Its leaves and tubers contained a high amount of phenolic compounds and showed significant antioxidant activities.[Citation17,Citation18] Those results indicate that a certain correlation between phenolic content and antioxidant activity exists, but, in many cases, there are also antioxidant activity in yacon that may be attributable to other unidentified substances. It’s important to find potential antioxidants from the leaves of yacon. Bioassay-guided fractionation is the conventional phytochemical approach to screen active compounds from herbal medicine. But because the target compounds are not clear, the method need to continuously separate and verify the activity of a large number of compounds, resulting in time-consuming and laborious shortcomings in the screening process of active compounds.[Citation19,Citation20] The aim of the present study is to propose a strategy for rapid screening of antioxidant activity compounds and components from yacon leaves by variable selection based on weight analysis. The theoretical basis of weight analysis lies in that the varied quantity of bioactive compounds will influence the activity results of samples. As a result, it is necessary to separate herbal medicine into components with different content of compounds for weight analysis. The contents of highly active compounds have greater impacts on the activity of components, while the contents of lowly active compounds have little or no impacts on the activity of components. Therefore, according to the activity differences of components and the differences of relative contents of compounds in each component obtained by chromatographic analysis, the variable selection based on weight analysis can be used to quickly screen out the potential active compounds from herb medicine. Then, the screened active compounds or components were prepared and verified by activity assays. Compared with traditional phytochemical approach, the method proposed in this paper will greatly speed up the screening of active compounds from herb medicine.
Materials and methods
Reagents and materials
1,10-diphenyl-2-picrylhydrazyl (DPPH), butylated hydroxyltoluene (BHT) and HPLC-grade acetonitrile was purchased from Sigma-Aldrich (St. Louis, MO, USA). HPLC-grade water was purified using a Milli-Q water purification system (Millipore, Bedford, USA). All other analytical grade chemicals were obtained from Kemio Chemical Co. (Tianjin, China). The leaves of yacon (Smallanthus sonchifolius Poepp. and Endl.) were collected from Zheng’an County, Guizhou Province of China during September 2019 and were identified by Dr. Yu-Jin Zhang from the Department of Pharmacognosy of the School of Pharmacy, Zunyi Medical University (Zunyi, China). The leaves were air-dried locally in a dark room at room temperature (about 25°C) and were oven-dried (50°C) to acquire constant weight in short time. The samples of yacon leaves were stored in refrigerator at −20°C before use.
Extraction and separation
The dried yacon leaves were grounded into fine powder using a herb grinder (Joyoung, Shandong, China) prior to extraction. An amount of 2.0 kg of the leaves were extracted with 70% EtOH three times under reflux. The extracts were combined and concentrated under reduced pressure at 50°C. A total of 374 g crude extract (CE) was obtained. The CE (373 g) was diluted in 1.0 L water and successively extracted with petroleum ether (PE), ethyl acetate (EA) and n-butanol (NB). After removing the solvents, four fractions were obtained. The yields of PE, EA, NB and water fractions were 15.13, 44.60, 50.80, 167.31 g, respectively. About 1.0 g of EA fraction was accurately weighted and set as sample 0, the remaining EA fraction (43.60 g) was subjected to further chromatographic separation on a 80–100 mesh silica gel column, eluting with a series of PE, PE/EA, EA EA/MeOH and MeOH solvent to afford 17 subfractions (samples 1–17), the specific samples information were summarized in .
Table 1. Samples information of ethyl acetate fraction separated by silica gel column chromatography.
DPPH radical scavenging ability assay
The radical scavenging activity was evaluated in vitro based on the reduction of the stable DPPH free radical.[Citation21] Briefly, 0.1 μM solution of DPPH in EtOH was prepared. Samples were diluted and analyzed at various concentrations. An aliquot of 2.0 ml of DPPH radical solution was mixed with 1.0 ml of test samples and left in the dark. Absorbance at 517 nm was measured after 10 min. BHT was used as positive control. The percentage of DPPH scavenging capacity was calculated at each concentration according to the equation below:
Where A0 is the absorbance of the blank control, and As is the absorbance of the tested sample.
Chromatographic conditions
HPLC analyses of EA fraction (sample 0) and 17 subfractions (samples 1–17) were performed on an Agilent 1260 Infinity HPLC system (Milford, MA, USA) with a binary pump, diode array detection system, automatic plate-sampler, and thermostatically controlled column compartment. Chromatographic separations were performed on an Agilent Eclipse Plus C18 column (4.6 × 250 mm, 5 μm). The mobile phase consisted of acetonitrile (A) and 0.5% acetic acid aqueous solution (B). The linear gradient elution was optimized as follows: 0–40 min, 30–60% A; 40–50 min, 60–60% A; 50–51 min, 60–95% A; 51–60 min, 95–95% A; 60–61 min, 95–30% A; 61–75 min, 30–30%A. The flow rate was kept at 1.0 mL min−1. The column temperature was set to 20°C and online UV measurements were recorded at 230 nm. An aliquot of 10 µL of each sample solution was injected onto the HPLC system. Samples were filtered through a 0.22 μm filter membrane prior to HPLC analysis.
Chromatographic peak alignment
Time shifts are inevitable in liquid chromatography. To correct time shifts, our previous work has developed a data processing software of ChromP. The correlation optimized warping (cow) algorithm[Citation22,Citation23] module of ChromP was used for peak alignment of samples from liquid chromatography. The detailed parameter settings of COW in our test were: Segments: 5; Stack: 1; Correlation Power: 1; Fix Maximum Correction: 0; Force Equal Segment: 0.
Preprocessing of antioxidant activity data
Eighteen samples 0–17 listed in were divided into two groups according to their SC50 values of the DPPH radical scavenging activities. The specific classification method is as follows: The components with SC50 (The antioxidant concentrations corresponding to 50% radical scavenging efficiencies, the determination of SC50 values were calculated using probit analysis in SPSS 18.0.) value less than that of sample 0 (EA Fr.) are defined as group 0 (highly active group), while the components with SC50 value greater than that of sample 0 are defined as group 1 (lowly active group). Then, the compound differences between samples of group 0 and group 1 were compared by their respective overlapping chromatograms.
Weight analysis
Weight analysis is a variable selection method by comparing intragroup variance and intergroup variance, which is suitable for analysis data of two groups, namely, highly active group and lowly active group. The variance weights of individual variables were calculated using the following equation:[Citation24,Citation25]
Here nC and nT represent the samples number of highly active group and lowly active group, respectively. It is obvious that nC + nT = n (total number of samples). The weight of the jth variable (variable of the jth time point) could be obtained using EquationEq. 2(2)
(2) , xcj,xc’j represent the signal intensity of j variable of different samples in groups C (highly active group), while xtj and xt’j are the signal intensity of j variable of different samples in group T (lowly active group). The signal intensity of each variable in chromatograms is the concentration of each variable. The formula of variable weight defines the contribution of j variable by caculating the ratio of the inter-group variance and the intra group variance of j variable. The inter-group variance value will be calculated by the expression of numerator in EquationEq. 2
(2)
(2) , while the intra-group variance value will be calculated by the expression of denominator in EquationEq. 2
(2)
(2) . The larger the weight value of variable j is, the larger the classification effect of the variable j on the active group is. Then it is most likely to be a potential active compound. The weight analysis was carried out by the weight analysis module of MultiDA software that developed for the routine metabolomics/metabonomics data analysis in our previous work.[Citation26]
Verification
The selected latent bioactive components and compounds were prepared and verified by DPPH radical scavenging ability assay. For latent active components, samples 9–12 and samples 1–6 mentioned were combined and dissolved in methanol, respectively. After centrifugation, two supernatants were sampled on a YMC C18 semi-preparative chromatographic column (10 mm × 250 mm, 5 μm), and separated by 90% methanol with isocratic elution on a LC 3000 HPLC apparatus (Beijing Tong Heng Innovation Technology Co., Ltd, China), respectively. Components with high polarity with retention time within 5 min were collected and condensed to obtain component A from samples 9–12, and components with low polarity with retention time between 10 and 15 min were collected and condensed to obtain component B from samples 1–6. For preparation of the latent compounds, the method was established in our previous work.[Citation27] Generally, the latent compounds were isolated from EA fraction of yacon leaves by preparative HPLC technologies, and their structures were identified by NMR spectral data analysis. At the same time, when analyzed under the same chromatographic conditions, the retention times of these compounds were consistent with that of the highly active compounds obtained by our proposed screening method.
Statistical analysis
Data were reported as mean ± SD from triplicate determinations. Statistical analysis was performed with Student’s t-test. A difference was considered statistically significant, when P < .05. All the statistical tests were performed on the statistical software (SPSS version 18.0).
Results and discussion
Chromatographic profiles of test samples
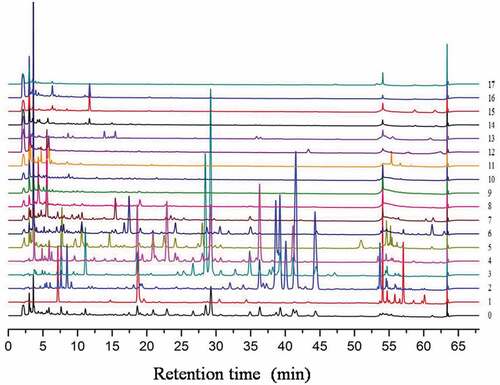
is liquid chromatograms diode array detector (DAD) data of 18 samples 0–17 after baseline correction and alignment by ChromP. It is obvious from that the separation of silica gel column chromatography resulted in obvious compounds difference between 18 samples. A total of 12500 time points were collected within 75 min, each time point represents a unique a variable, the varied quantity of variables will influence the activity results of those samples. Variables content variance is necessary for variable selection by weight analysis.
DPPH Radical Scavenging Activities
The DPPH is a stable free radical, which is a suitable model for estimating free radical scavenging activities of antioxidants.[Citation28] The dose–response curves of the DPPH radical scavenging activities of CE and four fractions of the yacon leaves are plotted in . The SC50 values of CE, PE fraction, EA fraction, NB fraction, water fraction and BHT were 58.04 ± 2.93, 66.25 ± 3.16, 15.28 ± 0.56, 20.50 ± 1.31, 29.09 ± 1.03 and 6.66 ± 0.24 μg/ml, respectively. According to these SC50 values, it can be seen that the EA fraction show the strongest DPPH radical scavenging ability (P < .05). Therefore, the EA fraction was further isolated by silica gel column chromatography to screen compounds or components with high antioxidant activity. Then samples 1–17 were obtained, their DPPH radical scavenging abilities were investigated and shown in . The SC50 values of samples 1–17 were listed in . The results of SC50 values indicated that samples 5–8 with lower SC50 values showed stronger DPPH radical scavenging ability. The DPPH radical scavenging activity of sample 5 was even higher than that of BHT. The different activities lie in the bioactive compounds quantity varied in different samples, which is the basis of variable selection.
Figure 2. DPPH radical scavenging activities of butylated hydroxyltoluene (BHT), crude extract (CE), petroleum ether (PE), ethyl acetate (EA) and n-butanol (NB) and Water fractions (a) and samples 0–17 separated by silica gel column chromatography from ethyl acetate fraction (b, c and d) of the leaves of Smallanthus sonchifolius Poepp. and Endl. Values are mean ± SD (n = 3) of three determinations.
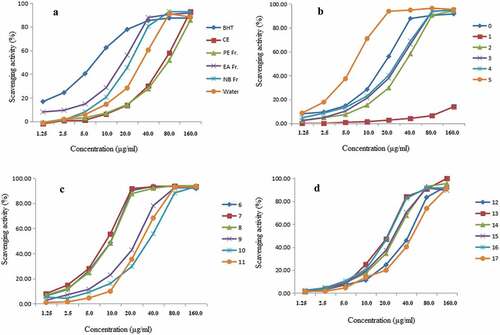
Table 2. The SC50 values of Samples a 1–17 and BHT.
Classification of Samples
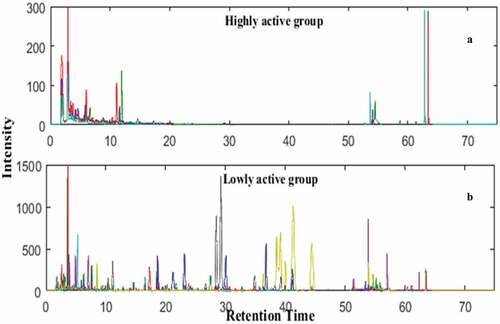
According to the SC50 value of EA fraction (15.28 ± 0.56 μg/ml), Samples 1–17 were divided into two groups. Among them, samples 5–8 were classified into group 0 (highly active group), and the remaining samples were attributed in group 1 (lowly active group). are overlapped chromatography of group 0 and group 1, respectively. There are obvious differences between their overlapped chromatogram in . The compounds of highly active group are mainly distributed in the chromatogram in the time range of 2.00–12.00 and 53.00–64.00 min (). There are only a few compounds with retention time (RT) in the 12.00–53.00 minutes range in highly active group, while the lowly active group contains a large number of compounds in this time range, indicating most compounds aroud time of 12.00–53.00 min may have less or even negative contribution to the DPPH radical scavenging activity.
Results of weight analysis
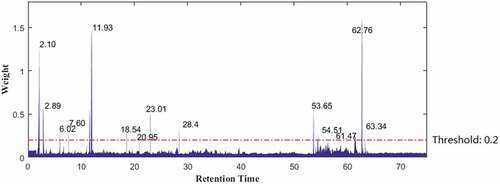
After setting the threshold of weight to 0.2, the variable selection to select compounds and components with strong antioxidant activities was performed on the liquid chromatogram data through weight analysis. The weight analysis results of the highly active group and the lowly active group are shown in . It can be seen that the weight values of variables in 2.00–12.00 min (active component A) and 53.00–64.00 min (active component B) are relatively large on the whole, namely, the contribution of the compounds during those two time periods to the DPPH radical scavenging activity may be large. Similarly, when it comes to single compound, a large number of compounds with different RTs show high weight values, indicating that these compounds may be potential antioxidant active substances. Overall, the results of weight analysis are basically consistent with the results of samples classification. The difference is that weight analysis can screen specific compounds with high activity through a large number of data operations. According to the RTs of the selected compounds, the peak area corresponding to the RT is relatively smaller, indicating that the active substances are not the main compounds in yacon, but the compounds with low content (See sample 0). Due to the low content of latent compounds, it brings certain degree of difficulties and challenges to our separation and preparation work.
Validation of latent components and compounds
In order to prepare the selected compounds and components with high radical scavenging activities, some samples rich in those compounds were combined and further separated on a semi-preparative chromatographic system to obtain latent active compounds and components. Two active components A (2.00–12.00 min) and B (53.00–64.00 min) were prepared as depicted in extraction and separation section. Five latent compounds, including chlorogenic acid (1), methyl caffeate (2), ethyl caffeate (3), homoeriodictyol (4), 3,7-dimethyl ether quercetin (5) were prepared. The RTs of those compounds are consistent with the prediction and show strong activity of free radicals 2–3 times higher than that of BHT. The RTs of potential active compounds and components and their respective SC50 values are shown in . Compounds 1–3 are phenolic acids, which were reported showing strong antioxidant activity in previous studies.[Citation29]
Table 3. Retention times and SC50 values of latent compounds and components.
The RTs of compounds 1, 2 and 3 were 2.89, 7.60, 11.93 min, respectively, which were contained in the time period of component A. The scavenging activity of the prepared active component A is higher than that of the three compounds, indicating that there is a certain synergistic effect between the monomers in the active component A. Two compounds 4 and 5 of flavonoids are obtained from yacon for the first time in our previous work,[Citation27] suggesting that the high antioxidant compounds in the leaves of yacon are not only phenolic acids, but also flavonoids.
Conclusion
Rapidly screening of bioactive, inactive or toxic components is important for modernization and quality control of herbal medicine, the target compounds are not clear in the traditional bioassay-guided phytochemical approach, resulting in blindness in the screening process of active compounds. The purpose of this work is to screen the variables that significantly related to radical scavenging activity by the variable selection based on weight analysis. By comparing the weight values of variables, the effective target compounds with specific RTs and components in a certain period of time could be revealed by our proposed screening method. Subsequently, only the target compounds and components need to be isolated, identified and verified by activity assays. This screening method will save the time of separating ineffective compounds and components, which will greatly improve the speed and accuracy of screening. Two bioactive components and five compounds were selected and isolated from yacon (Smallanthus sonchifolius) leaves by variable selection based on weight analysis. As expected, their activity is superior to BHT, a commonly used synthetic antioxidant. The SC50 value of the selected component A in the time range of 2.00–12.00 min, is smaller than that of the selected compounds chlorogenic acid (RT:2.89 min, 1), methyl caffeate(RT: 7.6 min, 2), ethyl caffeate (RT: 11.7 min, 3). Therefore, there might be synergy effect between compounds in the selected component. The result is in accord with those reported in related references. Consequently, our proposed strategy for screening active compounds from complicated plant extract lays methodological foundation for rapid screening of active candidates from medicinal plants and edible plants.
Author Contributions
In this paper, Xiao-Yan Yuan designed the experiments and wrote the paper; Lan Yu and Ya-Ping Zhou contributed reagents and materials; Zi-Yao Li, Xiao-Lan Chen, Xiao-Qian Ding performed the experiments; Qian-Xu Yang analyzed the data. All authors approved the final manuscript.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Campos D.; Betalleluz-Pallardel I.; Chirinos R.; Aguilar-Galvez A.; Noratto G.; Pedreschi R. Prebiotic Effects of Yacon (Smallanthus Sonchifolius Poepp. & Endl), a Source of fructooligo-saccharides and Phenolic Compounds with Antioxidant Activity. Food Chem. 2012, 135, 1592–1599.
- Gallo M.; Cavalcanti B. C.; Barros F. W. A.; Moraes M.; Pupo M. T. Chemical Constituents of Papulaspora Immersa, an Endophyte from Smallanthus Sonchifolius (Asteraceae), and Their Cytotoxic Activity. Chem. Biodivers. 2010, 7, 2941–2950.
- Geyer M.; Manrique I.; Degen L.; Beglinger C. Effect of Yacon (Smallanthus Sonchifolius) on Colonic Transit Time in Healthy Volunteers. Digestion. 2008, 78, 30–33.
- Zheng X.; Kuo G.; Deng D. Q.; Chen G. R.; Kang T. G.; Shi Y. Y.; Li X. T.; Dong F. A New Hexenol Glycoside from Leaves of Smallanthus Sonchifolius. Nat. Prod. Res. 2010, 24, 1592–1597.
- Ojansivu I.; Ferreira C. L.; Salminen S. Yacon, a New Source of Prebiotic Oligosaccharides with a History of Safe Use. Trends Food Sci. Tech. 2011, 22, 40–46.
- Fernández E. C; Rajchl A.; Lachman J.; Čížková H.; Kvasnička F.; Kotíková Z.; Milella L.; Voldřich M. Impact of Yacon Landraces Cultivated in the Czech Republic and Their Ploidy on the Short and long-chain Fructooligo Saccharides Content in Tuberous Roots. LWT-Food Sci. Techno. 2013, 54, 80–86.
- Pedreschi R.; Campos D.; Noratto G.; Chirinos R.; Cisneros-Zevallos L. Andean Yacon Root (Smallanthus Sonchifolius Poepp. Endl) Fructooligo Saccharides as a Potential Novel Source of Prebiotics. J.Agr. Food Chem. 2003, 51, 5278–5284.
- Ford D. C.; Ulloa J. L.; Catalán C. A. N.; Grau A.; Martino V. S.; Muschietti L. V.; Merfort I. The Sesquiterpene Lactone Polymatin B from Smallanthus Sonchifolius Induces Different Cell Death Mechanisms in Three Cancer Cell Lines. Phytochemistry. 2015, 117, 332–339.
- Takenaka M.; Yan X.; Ono H.; Yoshida M.; Nagata T.; Nakanishi T. Caffeic Acid Derivatives in the Roots of Yacon (Smallanthus Sonchifolius). J. Agr. Food Chem. 2003, 51, 793–796.
- Choi J. G.; Kang O. H.; Lee Y. S.; Oh Y. C.; Chae H. S.; Obiang-Obounou B.; Park S. C.; Shin D. W.; Hwang B. Y.; Kwon D. Y. Antimicrobial Activity of the Constituents of Smallanthus Sonchifolius Leaves against methicillin-resistant Staphylococcus Aureus. Eur. Rev. Med. Pharmaco. 2010, 14, 1005–1009.
- Valentova K.; Cvak L.; Muck A.; Ulrichova J.; Simanek V. Antioxidant Activity of Extracts from the Leaves of Smallanthus Sonchifolius. Eur. J. Nutr. 2003, 42, 61–66.
- Russo D.; Valentão P.; Andrade P.; Fernandez E.; Milella L. Evaluation of Antioxidant, Antidiabetic and Anticholinesterase Activities of Smallanthus Sonchifolius Landraces and Correlation with Their Phytochemical Profiles. Int. J. Mol. Sci. 2015, 16, 17696–17718.
- Lee S. E.; H. Hwang J.; Ha J.S.; Jeong H.S.; Kim J. H. Screening of Medicinal Plant Extracts for Antioxidant Activity. Life Sci. 2003, 73, 167–179.
- Yuan X. Y.; Gao M. Z.; Xiao H. B.; Tan C. Y.; Du Y. G. Free Radical Scavenging Activities and Bioactive Substances of Jerusalem Artichoke (Helianthus Tuberosus L.) Leaves. Food Chem. 2012, 133, 10–14.
- Wettasinghe M.; Shahidi F. Antioxidant and Free radical-scavenging Properties of Ethanolic Extracts of Defatted Borage (Borago Offcinalis L.) Seeds. Food Chem. 1999, 67, 399–414.
- Bharti R.; Ahuja G.; Ganapathy S.; Dakappa S. S. A Review on Medicinal Plants Having Antioxidant Potential. J. Pharm. Res. 2012, 5, 4278–4287.
- Sugahara S.; Ueda Y.; Fukuhara K.; Kamamuta Y.; Matsuda Y.; Murata T.; Kuroda Y.; Kabata K.; Ono M.; Igoshi K.; Yasuda S. Antioxidant Effects of Herbal Tea Leaves from Yacon (Smallanthus Sonchifolius) on Multiple Free Radical and Reducing Power Assays, Especially on Different Superoxide Anion Radical Generation Systems. J. Food Sci. 2015, 80, C2420–C2429.
- Khajehei F.; Hartung J.; Graeff-Hönninger S. Total Phenolic Content and Antioxidant Activity of Yacon (Smallanthus Sonchifolius Poepp. and Endl.) Chips: Effect of Cultivar, Pre-Treatment and Drying. Agriculture. 2018, 8, 183.
- Rauf A.; Uddin G.; Khan H.; Arfan M.; Siddiqui B. S. Bioassay-guided Isolation of Antibacterial Constituents from Diospyros Lotus Roots. Nat. Prod. Res. 2015, 30, 426–428.
- Erenler R.; Meral B.; Sen O.; Elmastas M.; Aydin A.; Eminagaoglu O.; Topcu G. Bioassay-guided Isolation, Identification of Compounds from Origanum Rotundifolium and Investigation of Their Antiproliferative and Antioxidant Activities. Pharm. Biol. 2017, 55, 1646–1653.
- Amarowicz R.; Karamac M.; Weidner S.; Abe S.; Shahidi F. Antioxidant Activity of Wheat Caryopses and Embryos Extracts. J. Food Lipids. 2002, 9, 201–210.
- Nielsen N. P. V.; Carstensen J. M.; Smedsgaard J. Aligning of Single and Multiple Wavelength Chromatographic Profiles for Chemometric Data Analysis Using Correlation Optimised Warping. J. Chromatogr 1998, A(805), 17–35.
- Tomasi G.; Berg F.; Andersson C. Correlation Optimized Warping and Dynamic Time Warping as Preprocessing Methods for Chromatographic Data. J. Chemometr. 2004, 1, 231–241.
- Yang Q.X; Cheng M. C.; Wang L.; Kan X. X.; Zhu X. X.; Xiao H. B. Antitumor Components Screening of Stellera Chamaejasme L. under the Case of Discrete Distribution of Active Data. Acta Pharm. Sin. 2014, 49, 927–931.
- Yi L. Z.; He J.; Liang Y. Z.; Yuan D. L.; Chau F. T. Plasma Fatty Acid Metabolic Profiling and Biomarkers of Type 2 Diabetes Mellitus Based on GC/MS and PLS-LDA. FEBS Lett. 2006, 580, 6837–6845.
- Yang Q. X.; Zhang L. X.; Wang L. X.; Xiao H. B. MultiDA: Chemometric Software for Multivariate Data Analysis Based on Matlab. Chenometr. Intell. Lab. 2012, 116, 1–8.
- Elansary H. O.; Ashmawy N. A. Study on Chemical Constituents of Ethyl Acetate Fraction of Smallanthus Sonchifolius Leaves. J.Zunyi Med. Univ. 2017, 40, 609–612.
- Simonovska B.; Vovk I.; Andrenšek S.; Valentová K.; Ulrichová J. Essential Oils of Mint between Benefits and Hazards. J. Essent. Oil Bear. Pl. 2013, 16, 429–438.
- Yuan X. Y.; Chen X. L.; Liao Y.; Ma Z. L.; Yu L. Investigation of Phenolic Acids in Yacon (Smallanthus Sonchifolius) Leaves and Tubers. J. Chromatogr. A. 2003, 1016, 89–98.