ABSTRACT
The main goal of any structural elucidation process is to achieve the highest level of confidence possible. This is usually attained using identical standards that can confirm the unequivocal structure of a metabolite. Nevertheless, the use of standards is not always possible given the great structural diversity among bioactive compounds. To overcome this problem, informatics tools can be used, such as the open source “CFM-ID” for structure elucidation, along with a meticulous mass fragmentation analysis as an initial step for putative identification. Herein, the “CFM-ID” tool was used on three Colombian fruits especially rich in phenolic compounds: Bactris gasipaes, Borojoa patinoi, and Myrciaria dubia. Thus, we obtained the characterization of new metabolites. To complete this picture, the bioactive content was measured using in-vitro assays (antioxidant, anti-inflammatory, and neuroprotective) with promising results, and was eventually related to the twenty-six new compounds reported here for the first time.
Introduction
Structural elucidation is a central and fundamental step in the search for bioactive molecules given the direct relationship between structure and function [Citation1–3] Within this process, mass spectrometry plays an essential role. [Citation4,Citation5] This technique is compatible with several separation systems, such as the different types of chromatography or capillary electrophoresis .[Citation6] Moreover, it has higher accessibility in terms of cost and infrastructure compared to elucidation techniques such as NMR. [Citation7] Thus, mass spectrometry is the initial and often the only tool, especially for the identification of molecules derived from plants or food. [Citation8,Citation9]
In any elucidation process, there are four types of molecules, that will depend on whether the molecule is already known in detail and whether its presence is expected[Citation10] associated with five levels of accuracy .[Citation11] According to the Metabolomics Standards Initiative (MSI),[Citation12] the levels are level zero, for which an unequivocal identification of the molecule has been made in three dimensions; level one, an identification has been made with a high level of confidence in two dimensions; level two, a probable structure has been encountered; level three, a possible structure is assigned; and level four, the structure is unknown. On the other hand, a high structural diversity is also expected. For example, in phenolic compounds, more than 800 structures have been reported in different plants and food matrices[Citation13] where several structural modifications are possible due to variables such as maturity,[Citation14] agricultural techniques,[Citation15] storage conditions,[Citation16] among others,[Citation17,Citation18] which may produce structural changes like different hydroxylation patterns, modifications by methylation, glycosylation, esterification, or the presence of alkyl groups,[Citation1,Citation2] modifications that make it necessary to use identical standards to confirm the unequivocal presence of a molecule .[Citation3,Citation4] Nevertheless, in many cases, it is not possible to acquire all the corresponding standards, either because they are not yet synthesized,[Citation5] they are unstable,[Citation6] or very expensive. Therefore, all these variables can convert the structural elucidation process into a challenging puzzle.
In this regard, several approaches can be used in order to obtain the highest confidence level, such as mass match on databases libraries, adduct formation, neutral loss analysis, isotope ratio analysis, pathway, and network analysis, along with orthogonal information such as UV/Vis data, ion mobility, hydrogen/deuterium exchange, or chemical derivatization .[Citation7] However, the starting point for elucidation is usually made by informatics tools .[Citation8] Thus, the identification process can be enhanced if several approaches are combined with informatics tools to achieve an unequivocal structure assignment .[Citation7,Citation8] Computational tools allow to predict mass fragmentation patterns based on heuristical approaches for molecules with no MS/MS data available[Citation9,Citation10] Among the in-silico approaches for structure analysis based on fragmentation data, we find the CFM (competitive fragmentation modeling) analysis,[Citation9] based on a probabilistic model created from experimental fragmentation data. The method identifies all the possible fragments produced by the breakage of bonds in small molecules, calculates the probabilities for each one, and predicts the spectra .[Citation9,Citation11] An online open source of this method is the Competitive Fragmentation Modeling for metabolite Identification Software[Citation12] which allows the accurate prediction of the ESI MS/MS spectrum for small molecules and the interpretation of the MS/MS fragmentation patterns by assigning the losses to a possible candidate molecule both in positive and negative mode .[Citation13] This software has proven to be useful for the identification of flavonols, flavones, and flavonoid glycosides .[Citation14] The information obtained is processed by comparison with previously reported fragments (in this case for phenolic compounds), allowing solid assignment identification without the need for an identical standard. Computational tools are an interesting and useful approach for the identification of plant-derived molecules given the profuse presence of bioactive molecules in plants and fruit matrices and the structural diversity that may limit the use of standards for their identification .[Citation15]
Within the great family of bioactive compounds, “polyphenols” are well known as antioxidant agents[Citation16–18] helpful in relieving oxidative stress, a phenomenon associated with cardiovascular diseases, diabetes, cancer, and neurodegenerative processes[Citation19] and lately related to the prevention and protection against COVID-19 .[Citation20] Endemic Amazon fruits have been proven to be a rich source of phenolics,[Citation21–24] as is the case of the fruits considered for this study. The first one, Borojoa patinoi, from the family of the Rubiaceae, genus Albertia,[Citation25] is grown in the Pacific region of Colombia and is highly consumed as a juice .[Citation26] Borojoa patinoi has shown moderate antioxidant,[Citation27] antibacterial,[Citation28] and antimicrobial activities .[Citation27] Extracts from this fruit have been studied by common extraction methods and only a few compounds have been identified .[Citation27] However, the present elucidation approach allowed us to identify several additional new phenolics. The second fruit, Bactris gasipaes, is an Arecaceae family member; genus bactris is native to the Cauca’s Valley[Citation29] district and grows as a large cluster composed of 50–100 fruits with a green, yellow, or red endocarp (depending on the maturation state), a starchy mesocarp, and a hard seed .[Citation30] The oil obtained from the seeds has shown a high antioxidant capacity,[Citation31] moreover the acetonic extract of the flour produced from the fruit has been studied. Nevertheless, the antioxidant, anti-inflammatory, or neuroprotective activities have not yet been investigated in whole fruit extracts. Additionally, there is no study of the structural elucidation of phenolic structures in this fruit. Finally, Myrciaria dubia is an exotic fruit that belongs to the family Myrtacea, genus Myrciaria,[Citation32] with a strong acid taste, and a berry-like shape .[Citation33] Despite low local production, it shows a high potential for commercialization and distribution. Several studies have shown the high antioxidant capacity of this fruit with different extraction approaches[Citation34,Citation35] There is no anti-inflammatory or neuroprotective activity reported.
The search for bioactive molecules is a process in which mass spectrometry plays a preponderant role. The main problem with this process is the lack of precise standards that help to confirm the mass fragment analysis. In this study, we used a diverse set of available phenolic standards and the CFM-ID (Competitive Fragmentation Modeling for metabolite Identification) software in the elucidation analysis. This was carried out according to the pseudo-molecular ion peaks and the fragmentation patterns. Specifically, using the pseudo-molecular ion peak, several candidates were selected based on the chromatographic retention time. Finally, the assignment was made using the fragments obtained in each case, fitting the mass fragmentation pattern with the CFM tool, and comparing them with previously reported fragments. Additionally, we quantified the total phenolic content as well as the antioxidant, neuroprotective, and anti-inflammatory assessments of methanolic extracts of the aforementioned fruits.
Materials and methods
Chemical and reagents
The reagents, 2,2-Diphenyl-1-picrylhydrazyl (DPPH), 2,2′-Azino-bis (3-ethylbenzothiazoline-6-sulfonic acid) diammonium salt (ABTS), linolenic acid, lipoxidase from glycine max (soybean), xylenol orange disodium salt, acetylcholinesterase (AchE), acetylthiocholine iodide (ATCI), 5,5′-dithiobis (2-nitrobenzoic acid) (DTNB), 5-chlorogenic acid (5-CGA), quercetin hydrate, gallic acid, apigenin, 7-hydroxicoumarin, pelargonidin and physostigmine were purchased from Sigma-Aldrich (St. Louis, Missouri, USA). The water used in the experiments (type I ultra-pure) was obtained from a smart RO Healforce water purification system (Bio-meditech Holdings Limited, Shanghai-China). The LC-MS grade solvents used were methanol (Burdik & Jackson, Muskegon, MI. USA) and Formic Acid (Sigma- Aldrich, St. Louis, MO, USA).
Fruit material
The samples of Bactris gasipaes and Borojoa patinoi were acquired in the Buenaventura’s City market located in Cauca’s Valley district of Colombia. The Myrciaria dubia sample was obtained in the Leticia’s city market in the Amazona’s district. Fifty grams of each fruit were cut into small pieces and then macerated with liquid nitrogen. The processed material was stored at −80°C until further use.
Polyphenol extracts preparation
Ten grams of the freeze-grind powder were suspended in 50 mL of MeOH-HCOOH (99:1) and shaken for 30 min in an ice bath in the dark .[Citation36] The mixture was centrifuged for 15 min at 4800 rpm; the insoluble residue was re-extracted three times with a fresh aliquot of the same mixture. The yellow extracts were pooled and concentrated to dryness under rotary evaporation (Buchi Rotavapor V-850, New Castle, DE, USA) at 35°C. For the identification analysis the extract was reconstituted with 2 mL of MeOH and filtered through a nylon membrane of 0.22 µm (Whatman ®, Sigma- Aldrich, St. Louis, MO, USA), for the in-vitro analysis the extract was reconstituted with 50 mL of MeOH and filtered through a nylon membrane of 0.45 µm (Whatman ®, Sigma- Aldrich, St. Louis, MO, USA).
Total phenolics (Folin-Ciocalteu method)
The total phenolic content was quantified using the Folin-Ciocalteu assay .[Citation37] Based on a redox reaction in which the pH is previously increased to favor the production of phenolate ions, which reduce Mo (VI) to Mo (V), readily measurable at 725 nm. Using gallic acid as reference standard, appropriate volumes were taken to obtain final concentrations over the range 120 ppm to 440 ppm. The solution standard/extract was mixed with 1 mL of Na2CO3 20% for 10 min, then 200 µL of Folin-Ciocalteu reagent were added. After 30 minutes of reaction, the absorbance was measured at 720 nm against the blank. Results were expressed as equivalent mg of gallic acid (GAEs) per 100 g of dried weight (mg of GAEs/100 g of DW). The same procedure was performed on the extracts and on the standard solutions.
Liquid chromatography-tadem mass spectrometry
The HPLC-ESI-MSn analyses were carried out using a UHPLC/Focused – Dionex UltiMate 3000, equipped with a binary pump, coupled to a LCQ FleetTM Ion Trap Mass Spectrometer (all from Thermo Fisher Scientific, Walthman, MA, USA) through an ESI source. The separation was achieved on a Kinetex Luna C18 column (150 x 3.0 mm, 3 µm) (Phenomenex, Torrance, CA, USA) with a mobile phase consisting of 0.1% formic acid in water (eluent A) and methanol (eluent B) at a flow rate of 0.2 mL/min. The gradient elution went from initial 2% of B to 5% in 10 min, followed by an increase from 5% to 40% in 50 min, then to 60% in 10 min and to 100% in 10 min. This was hold for 10 more min giving a total run time of 90 min, followed by a reconditioning .[Citation38] The column oven and the sample tray were set at 4°C. The injection volume was 10 µL. The ion trap operated in full scan (150–1000 m/z), and ion tree mode to obtain fragment ions with a collision energy of 35% and an insolation width of 2 m/z. The data dependent scan mode was used for the acquisition of data. The positive ion mode ESI source parameters were optimized by direct infusion of 10 ppm of 5-CGA in 0.1 formic acid methanolic solution, to an ionization voltage of +6 kV, capillary temperature of 260°C, sheath gas flow rate of 10 arbitrary units and auxiliary gas flow rate of 5 arbitrary units. The Thermo Fisher Scientific Excalibur 3.0 software was used for data processing.
In vitro activities
DPPH+ assay
The DPPH antioxidant activity of the extract was determined according to a previously described method[Citation39] with a few modifications. Dilutions of the extract were prepared over the concentration range of 4 µg/mL to 30 µg/mL. The diluted solutions were mixed with the necessary volume of a DPPH solution 0.2 mg/mL to get the desired final volume. The mixture was shaken and allowed to react. After 45 min, the absorbance was measured in a UV spectrophotometer (Cary Varian 100) at 515 nm. Quercetin solution was used as a positive control.
ABTS+ assay
The antioxidant activity was also measured by the ABTS+ method[Citation37] with a few modifications. A 1.5 mM aqueous solution of the ABTS cationic radical was prepared from an oxidation reaction of ABTS with K2S2O8 (1:0.5). This mixture was left isolated from the light from 12 to 16 hours under an inert atmosphere. The ABTS solution was then added to different concentrations of the extract, ranging from 2 µg/mL to 12 µg/mL, and allowed to stand for 45 min at room temperature. Then, the absorbance was measured in a UV spectrophotometer (Cary Varian 100) at 745 nm. Quercetin was used as a positive control.
Anti-inflammatory assay
The assay was performed according to the ferrous oxidation−xylenol orange (FOX) method[Citation40] with some modifications. Initially, the highest possible amount of lipoperoxides is generated in such a way that can oxidize Fe+Citation2 to Fe+Citation3. Then the ferric ions will form a complex with xylenol that can be quantified at 590 nm. A volume of 8 µL of lipoxygenase (20000 U/mL) solution was incubated with different extract concentrations at 5°C for 5 min. Then 40 µL of Linoleic acid (0.6 mM) in borate buffer (50 mm, pH 9.0) were added and the mixtures kept at 5°C for 30 minutes in the dark. Subsequently, 200 µL of FOX reagent, [consisting of sulfuric acid (30 mm), xylenol orange (100 mm), and iron sulfate (II) (100 mm) in methanol/water (9:1)] were added and the absorbance measured at 590 nm. Quercetin was used as positive control.
Neuroprotective assay
This method is based on the ability of the extract to inhibit the enzyme acetylcholinesterase, which hydrolyzes acetylcholine in the synapsis. The choline can react with DTNB (5,5′-Dithiobis (2-nitrobenzoic acid) to produce a molecule with an absorbance between 400–420 nm.[Citation41] This assay was carried out following the procedure described by Konrath[Citation42] with some modifications. AChE solution (20 µL) prepared in 50 mm Tris-HCl pH 8 with 0.1% of bovine serum albumin was mixed with 750 µL of DTNB solution prepared in the same buffer with NaCl 0.1 M. The sample was added into the AChE-DNTB mixture stirring vigorously and allowed to stand for 5 min. Then, 60 µL of ATCI 1.5 mM was added and after 45 min the absorbance was measured at 415 nm. Eserine was used as positive control.
Statistical analysis
All the experiments were performed in triplicated, and the results are expressed as the means ± standard deviation. The experimental data were analyzed with the program RStudio. Version (2015) performing one-way analysis of variance (ANOVA) followed by a Fisher test. Only p-values lower than 0.05 were considered significant.
Results and discussion
Total phenolic content and in-vitro bioassays
The values found in the extracts for TPC were 31.58 mg GAE/100 g DW for Borojoa patinoi, 55.12 mg GAE/100 g DW for Bactris gasipaes and 137.34 mg GAE/100 g DW for Myrciaria dubia. According to previous data, similar values of GAE/100 g DW were reported for Borojoa patinoi,[Citation24] 65.7 mg GAE/100 g DW for Bactris gasipaes,[Citation24] 155.0 mg GAE/100 g DW for Myrciaria dubia .[Citation43] The slight differences in these values can be explained according to several variables, such as the stages of fruit maturation[Citation44] and the sample treatment, which in our case was carried out considering both the pulp and the skin .[Citation45] Additionally, the procedures used during the extraction, such as the acidification, the concentration of acid, the volume of solvent, the extraction times, temperatures, etc. may cause further differences .[Citation46] Moreover, Myriciaria dubia showed the highest TPC and the lowest EC50 values, revealing once again the potential of phenolic compounds as antioxidants for the anti-inflammatory and the neuroprotective assays (the last one first reported) for this fruit. In Bactris gasipaes, the TPC is significantly lower compared to Myriciaria dubia, despite its the IC50 in the neuroprotective assay being relatively analogous to Myriciaria dubia (). Therefore, it is possible that the inhibition of AchE in Bactris gasipaes, is not necessarily due to phenolic compounds, showing a promising potential of bioactive molecules in this fruit. Now, when we compare the TPC values for Bactris gasipaes and Borojoa patinoi a trend between the TCP and IC50 values is found. With a slightly higher TPC for Bactris gasipaes the lowest IC50 values are found, except for the anti-inflammatory test. Thus, the ability of Borojoa patinoi extract to inhibit lipoxygenase may be due to other metabolites rather than phenolic showing again a promising potential for bioactive molecules on this fruit. Finally, a correlation between all the assays with the TPC values was made, and they showed a moderate relative correlation between the antioxidant activities; ABTS and DPPH (r = 0.61 p < .05, and r = 0.67 p < .05 respectively). In the case of the neuroprotective activity, a poor correlation was found (r = 0.57 p < .05). For the anti-inflammatory assay, a strong correlation was found (r = 0.91 p < .05).[Citation47] This can be explained due to the nature of the ABTS and the DPPH radicals since they are not found in living organisms; therefore, a greater affinity for lipoperoxides can be expected by the phenolics .[Citation48]
Table 1. IC /EC 50 values and quantification of total phenolics in methanolic extracts of Borojoa patinoi, Bactris gasipaes and Myrciaria dubia.
Phenolic acids and small phenolics
We used three standards, namely Gallic Acid, Chlorogenic Acid, and 7-Hydroxy Coumarin, in order to confirm the ability of the CFM-tool to assign the mass fragments values to the assumed molecule (Table S1, Supplementary Information). A pseudo-molecular ion peak at m/z 171 was found for Gallic Acid, with a base peak ion at m/z 125 in the MSCitation2 spectrum. This fragment can be explained as the loss of formic acid ( -I).[Citation49] For Chlorogenic Acid, a mass-to-charge ratio of 355 was found for the pseudo-molecular ion with a loss of 192 uma, corresponding to Quinic Acid (-II),[Citation50] giving a linear non-aromatic fragment that can rearrange to the reported structure for this loss by the CFM tool. For Hydroxycoumarin, a pseudo-molecular peak ion at m/z 163 was found and the fragmentation yielded a base peak ion at m/z 190, which is consistent with the ones previously reported .[Citation51,Citation52] This fragmentation can be understood as the loss of CO by a retro Diels-Alder reaction .[Citation53,Citation54] The CFM tool proposes a linear non-aromatic structure obtained by a hydride shift and a cycling process (-III). As far as the fruit’s extracts are concerned, Borojoa patinoi analysis presents two peaks, 1 and 2 (Table S2, S.I), which have the same mass-to-charge ratio values, suggesting that these two compounds might be isomers of Ferulaldehyde (m/z 179)[Citation55] with successive losses of H2O (18 uma) and CO (28 uma) (Fig S2, S.I). Peak 13 has a pseudomolecular ion at m/z 123 and MSCitation2 fragments ions at m/z 81 and m/z 95 and was therefore assigned to Benzoic Acid[Citation56] (Fig S3, S.I). Peak 44 (m/z 149), for its fragmentation pattern, was confirmed as Cinnamic Acid[Citation57] (Fig S4, SI.). The large peak number 51, with a pseudomolecular ion at m/z 339 and successive losses of H2O, was assigned to p-Coumaroylquinic acid .[Citation58] The same compound was also found in Bactris gasipaes (Table S2, S.I), confirmed through the retention time, mass spectrum, and mass fragmentation. In Bactris gasipaes, the three phenolic acids found correspond to 4-Aminobenzoic Acid,[Citation48,Citation59] Acetylsalicylic Acid,[Citation60] and p-Comaroyl-quinic Acid[Citation58] (Table S2, S.I). They were all identified by comparing the fragmentation patterns to the reported literature. For 4-Aminobenzoic Acid, the fragmentation produced a base peak at m/z 92, which can be explained as the loss of water followed by the loss of C2H4 (Fig. S5, S.I). The fragmentation spectrum of Acetylsalicylic Acid shows a base peak at m/z 165 and another at m/z 136, produced by the loss of a methyl group and a carbon dioxide respectively (Fig S6, S.I). For p-Comaroyl-quinic Acid, several adducts and dimers are present: [M+ Na]+ at m/z 361, [2 M + H]+ at m/z 678 and [2 M+ Na]+ at m/z 700. The MSCitation2 spectrum of this peak yielded two fragments corresponding to the consecutive loss of two H2O molecules. The MSCitation3 spectrum of the fragment ion at m/z 321 (produced in the MSCitation2 fragmentation) shows all the characteristics ions expected for the fragmentation of such ion (Fig S7, S.I) and was therefore useful in confirming the identification. In the base-peak chromatogram of Myrciaria dubia (Table S2, S.I), peak 1 corresponds to a pseudo-molecular ion at m/z 175 which fragmented to m/z 157 and 143 (due to the loss of H2O and CH3OH, respectively) and was assigned to Shikimic Acid[Citation61,Citation62] (Fig S8, S.I). Peak 3 with an m/z of 193 was assigned to Quinic Acid[Citation63,Citation64] with losses of CO2 and H2O in the fragmentation spectrum (Fig S9, S.I). Peak 4 has an m/z of 355 corresponds to Chlorogenic Acid, with fragment ion at 163, which corresponds to the loss of Quinic Acid moiety (Fig S10, S.I).
Flavonols and flavonones
Standards of Quercetin and Apigenin were used to prove the capacity of the CFM-Tool in this class of compounds (Table S1, S.I). Quercetin showed a pseudo-molecular ion at 303 m/z with ion fragments at 229 and 257 m/z in the MSCitation2 spectrum obtaining them for the loss of a formic acid fragment ( -V) .[Citation65] The molecular ion mass for Apigenin was found at m/z 271 and yielded a fragment ion at m/z 153, corresponding to the Citation1,Citation3A+ fragment through the previously reported mechanism,[Citation2] also assigned by the CFM-Tool (-VI). As far as the analyses of the fruits are concerned, for Bactris gasipaes (Table S2, S.I), peak 6 showed the molecular ion as the base peak at m/z 565, together with 587 and 1151 m/z ([M+ Na]+ and [2 M+ Na]+ respectively) and it was identified as Isorhamnetin 3-(6”-malonylglucoside)[Citation66] for the peak at m/z 547 observed in the MSCitation2 spectrum, corresponding to loss of H2O (Fig S11, SI). Peak 8 had a base peak at m/z 318 and for its MSCitation2 fragments corresponding to Citation1,Citation3A+z, Citation1,Citation4B+ and 0,Citation4B+ (Fig. S12, SI) could therefore be assigned to Myricetin,[Citation36,Citation67] Quercetin was assigned as peak 9 with a m/z 303 and a MSCitation2 m/z 284 [M-H2O]+[Citation67,Citation68] together with the fragments Citation1,Citation3A+ and 0,Citation3A+ (Fig S13, S.I). Peak 12 was assigned to Rhamnetin for the m/z at 317, with the m/z at 633 corresponding to the dimer[Citation69] (Fig S14, S.I). In the chromatogram of Myrciaria dubia (Table S2, S.I), peak 3 was assigned to Kampferide[Citation70] with the pseudo molecular ion at m/z 300 and the MSCitation2 fragmentation ion at m/z 147, corresponding to the ion Citation1,Citation3A+ (Fig S15, S.I). The same fragmentation pattern already encountered in Bactris gasipaes for Myricetin, with the pseudo-molecular ion at m/z 318 producing the fragment ion at m/z 300 due to [M-H2O]+, was also found for peak 5 of Myrciaria dubia (Fig S16, S.I). Peak 6 was discovered to contained a pseudo-molecular ion at m/z 317, an adduct [M+ Na]+ at m/z 339, as well as dimers [2 M + H]+ at m/z 633 and [2 M+ Na]+ m/z 655. A loss of H2O produces an ion at m/z 299 in the MSCitation2 spectrum; therefore, the compound was assigned as Rhamnetin (Fig S14, S.I) .[Citation69] Lastly, a flavonone was found in peak 7 of Myrciaria dubia, namely Naringenin, with a pseudo-molecular ion at m/z 273 and an MSCitation2 m/z at 153 corresponding to the fragment Citation1,Citation3A+ (Fig S17, S.I) .[Citation71]
Anthocyanin and (epi)catechins
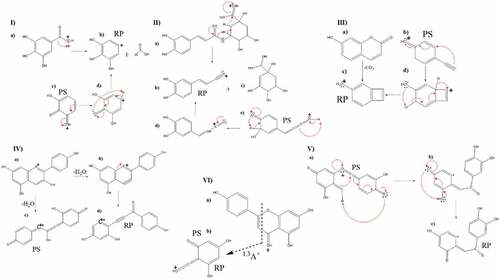
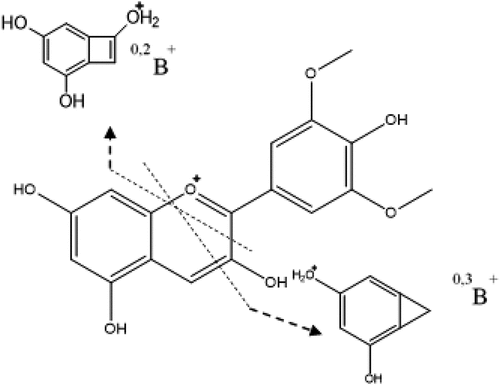
The CFM-tool was confirmed by using Pelargonidin as a standard. In the mass spectrum of this compound, a pseudo-molecular ion at m/z 271 (Table S1, S.I) was found with a fragment at 253 due to the loss of H2O. The reported fragmentation mechanism[Citation72] starts with the protonation of the hydroxyl in ring C, followed by the loss of a water molecule, which causes the formation of a triple bond, then a structure with higher stability is generated by the ring rupture. Although the CFM-ID tool does not generate the structure proposed by the reported fragmentation mechanism (-IV), an equivalent structure was obtained, in which the fragment ions are produced by a rupture in the C ring, the weakest spot of the molecule, due to the lack of aromaticity .[Citation53] In Bactris gasipaes (Table S2, S.I), peak 10 was assigned as Malvidin with a molecular ion at m/z 330 and MS2 base peak at m/z 312 (Fig S18, S.I), additionally, a fragmentation pattern was proposed in which there are two types of bond breaks in the C-ring generating several possible fragmentations for the B-ring: the 0,2B+ fragment and the 0,3B+ fragment ().[Citation73–75] This fruit also contained (Epi)-Catechin glucoside[Citation76] and Methyl-(Epi)-Catechin[Citation77] corresponding to peaks 5 and 11. The first one was identified by the loss of sugar moiety (m/z 299) and other characteristic MS2 fragment ions at m/z 333 (loss of C2H2O) and at m/z 435 (loss of H2O), from the pseudo molecular ion at m/z 453 (Fig S19, SI). The second, Methyl-(Epi)-Cate was identified using the pseudo-molecular ion at m/z 305 with MS2 fragment ions at m/z 273 (loss of CH4), 259 (loss of CH2O), 241 (successive loss of H2O and CH3OH), and 227 (successive loss of H2O and C3H8O). Neither anthocyanins nor catechins were found in Myrciaria dubia or Borojoa patinoi.
Proanthocyanidins
In Bactris gasipaes (Table S2, S.I), peak 14 with m/z 695 was tentatively assigned as Protocyanindin A5 glucoside .[Citation78] In the MSCitation2 spectrum, the peak at m/z 695 can be explained as the loss of H2O followed by the loss of C2H2; the peak at m/z 678 is due to a loss of 1,2-ethanediol and m/z 675 produced by the loss of H2O followed by loss of ethanol. The m/z 575 corresponds to the aglycone moiety, whereas m/z 453 is due to the loss of the Gallic Acid fragment followed by the loss of the glucoside moiety. The m/z 417 is caused by an initial loss of the glucoside moiety, followed by C ring fragmentation that results in a 1,3B+ fragment, whereas the m/z 288.84 is produced by the (Epi) catechin moiety (Fig S21, SI). Peak 16, with an m/z of 915, was assigned as Prodelphinidin B5 3,3’-digallate[Citation79]; in the MSCitation2 a fragment at m/z 657 was found, which can be explained as the loss of Shikimic Acid followed by the loss of Butadione. The fragment at m/z 633.25 is due to the loss of the Gallic Acid moiety and, successively, the loss of the ring B of one of the Gallocatechins in the structures. In addition, the fragment m/z 453 can be understood as the product of the rupture of the C-C bond between the gallocatechin moieties, whereas the fragment at m/z 355 is given by the fragmentation in the C ring (0–4) of one of the Gallocathechin moieties. Another fragment at m/z 339 is also due to the fragmentation in the C ring of the Gallocathechin moiety but with a different kind of rupture (1–4) and the fragment at m/z 319 comes from the fragmentation in (1–3) of the C ring in a Gallocathechin moiety (Fig S22, S.I).
Nitrogen compounds
In Bactris gasipaes (Table S2, S.I), two amino acids were found. Phenylalanine[Citation80] was assigned peak 3, with m/z 166 and MS2 fragments at m/z 149, 139, and 120, which can be explained as NH3, 2NH3, and CO2 losses, respectively[Citation80] (Fig. S23, S.I). Peak 4 was identified as Tryptophan, with m/z 205 and an MSCitation2 peak at m/z 188 due to NH3[Citation81] (Fig S24, S.I). These two amino acids were also found in Myrciaria dubia (Table S2, S.I) where the same fragmentation pattern was observed for Phenylalanine (Fig S25, S.I) and for Tryptophan (Fig S26, S.I). In this extract, an alkaloid was found with a pseudo molecular ion at m/z 232 and MSCitation2 fragment ions at m/z 214 (loss of H2O), m/z 188 (loss of C2H4O), and m/z 158 (loss of C4H10O) and was assigned as Rotundine A[Citation82] (Fig S27, S.I).
Other compounds
In Myriciaria dubia (Table S2, S.I), peak 2 with a base peak ion at m/z of 219 was assigned tentatively as Tyrosol Sulfate,[Citation83] being the MSCitation2 fragment ions found at m/z 203 (loss of H2O), m/z 200 (loss of CH4) and m/z 143 (loss of C3H6O) (Fig 28, S.I).
Conclusion
The CFM-ID fragmentation analysis tool was assessed through a wide range of phenolics and equivalent or equal structures to the previously reported mass fragmentation mechanisms were obtained, providing a further means of identification for 26 phytochemicals not previously reported on these fruits. Among these, twelve phenolic acids, nine flavonoids, two proanthocyanins, two amino acids, and one alkaloid were encountered, contributing to the chemical information on these three fruits. Furthermore, a complete study of the bioactive content of three Amazonic fruits was carried out. The Folin–Ciocalteu quantitation, the assessment of antioxidant capacities by the DPPH and ABTS methods, the neuroprotective and anti-inflammatory activities, together with their correlation values, were determined. There were positive correlations between the Total Phenolic Content and both antioxidant activity and anti-inflammatory activities. A noteworthy content of potentially interesting metabolites was found in the Myrciaria dubia extract, showing the highest values of all the assays.
Author contributions
Conceptualization, Chiara Carazzone; Methodology, Chiara Carazzone, Daniel Arias Ramirez; Investigation, Daniel Arias Ramirez; Formal analysis, Daniel Arias Ramirez; Writing –Original Draft, Daniel Arias Ramirez; Writing – Review & Editing, Daniel Arias Ramirez, Chiara Carazzone; Resources, Daniel Arias Ramirez; Funding Acquisition, Chiara Carazzone; Supervision, Chiara Carazzone. All authors read and approved the final manuscript.
Supplemental Material
Download ()Acknowledgments
This research was funded by the FAPA project of Chiara Carazzone from the Faculty of Science at University of los Andes, by the grant No. 120465741393.
Data availability statement
All datasets generated for this study are included in the article/Supplementary Material
Disclosure statement
No potential conflict of interest was reported by the author(s).
Supplementary material
Supplemental data for this article can be accessed online at https://doi.org/10.1080/10942912.2022.2147539
References
- Grotewold, E. The Science of Flavonoids. 2006. DOI: 10.1007/978-0-387-28822-2.
- Tsimogiannis, D.; Samiotaki, M.; Panayotou, G.; Oreopoulou, V. Characterization of Flavonoid Subgroups and Hydroxy Substitution by HPLC-MS/MS. Molecules. 2007, 12(3), 593–606. DOI: 10.3390/12030593.
- Mandal, S. M.; Chakraborty, D. Mass Spectrometric Detection of Phenolic Acids. Natural Prod. 2013, 2047–2059. 10.1007/978-3-642-22144-6_90.
- Molyneux, R. J.; Beck, J. J.; Colegate, S. M.; Edgar, J. A.; Gaffield, W.; Gilbert, J.; Hofmann, T.; McConnell, L. L.; Schieberle, P. Guidelines for Unequivocal Structural Identification of Compounds with Biological Activity of Significance in Food Chemistry (IUPAC Technical Report). Pure Appl. Chem. 2019, 91(8), 1417–1437. DOI: 10.1515/PAC-2017-1204/PDF.
- Demarque, D. P.; Dusi, R. G.; de Sousa, F. D. M.; Grossi, S. M.; Silvério, M. R. S.; Lopes, N. P.; Espindola, L. S. Mass Spectrometry-Based Metabolomics Approach in the Isolation of Bioactive Natural Products. Sci. Rep. 2020, 10(1), 1–9. DOI: 10.1038/s41598-020-58046-y.
- Galmarini, M. V.; Maury, C.; Mehinagic, E.; Sanchez, V.; Baeza, R. I.; Mignot, S.; Zamora, M. C.; Chirife, J. Stability of Individual Phenolic Compounds and Antioxidant Activity during Storage of a Red Wine Powder. Food Bioprocess. Technol. 2012, 6(12), 3585–3595. DOI: 10.1007/S11947-012-1035-Y.
- De Vijlder, T.; Valkenborg, D.; Lemière, F.; Romijn, E. P.; Laukens, K.; Cuyckens, F. A Tutorial in Small Molecule Identification via Electrospray Ionization-Mass Spectrometry: The Practical Art of Structural Elucidation. Mass Spectrom. Rev. 2018, 37(5), 607–629. DOI: 10.1002/MAS.21551.
- Scheubert, K.; Hufsky, F.; Böcker, S. Computational Mass Spectrometry for Small Molecules. J. Cheminform. 2013, 5(3), 1–24. DOI: 10.1186/1758-2946-5-12/FIGURES/8.
- Blaženović, I.; Kind, T.; Ji, J.; Fiehn, O. Software Tools and Approaches for Compound Identification of LC-MS/MS Data in Metabolomics. Metabolites. 2018, 8(2), 31. DOI: 10.3390/METABO8020031.
- Pluskal, T.; Uehara, T.; Yanagida, M. Highly Accurate Chemical Formula Prediction Tool Utilizing High-Resolution Mass Spectra, MS/MS Fragmentation, Heuristic Rules, and Isotope Pattern Matching. Anal. Chem. 2012, 84(10), 4396–4403. DOI: 10.1021/AC3000418.
- Hufsky, F.; Böcker, S. Mining Molecular Structure Databases: Identification of Small Molecules Based on Fragmentation Mass Spectrometry Data. Mass Spectrom. Rev. 2017, 36(5), 624–633. DOI: 10.1002/MAS.21489.
- Allen, F.; Pon, A.; Wilson, M.; Greiner, R.; Wishart, D. CFM-ID: A Web Server for Annotation, Spectrum Prediction and Metabolite Identification from Tandem Mass Spectra. Nucleic Acids Res. 2014, 42(W1), W94–W99. DOI: 10.1093/nar/gku436.
- Djoumbou-Feunang, Y.; Pon, A.; Karu, N.; Zheng, J.; Li, C.; Arndt, D.; Gautam, M.; Allen, F.; Wishart, D. S. Cfm-Id 3.0: Significantly Improved Esi-Ms/Ms Prediction and Compound Identification. Metabolites. 2019, 9(4), 72. DOI: 10.3390/metabo9040072.
- Wang, F.; Liigand, J.; Tian, S.; Arndt, D.; Greiner, R.; Wishart, D. S. CFM-ID 4.0: More Accurate ESI-MS/MS Spectral Prediction and Compound Identification. Anal. Chem. 2021, 93(34), 11692–11700. DOI: 10.1021/ACS.ANALCHEM.1C01465/SUPPL_FILE/AC1C01465_SI_001.PDF.
- Akimoto, N.; Ara, T.; Nakajima, D.; Suda, K.; Ikeda, C.; Takahashi, S.; Muneto, R.; Yamada, M.; Suzuki, H.; Shibata, D., et al. FlavonoidSearch: A System for Comprehensive Flavonoid Annotation by Mass Spectrometry. Sci. Rep. 2017, 7(1), 1–9. DOI: 10.1038/s41598-017-01390-3.
- Ozcan, T.; Akpinar-Bayizit, A.; Yilmaz-Ersan, L.; Delikanli, B. Phenolics in Human Health. Int. J. Chem. Eng. Appl. 2014, 5(5), 393–396. DOI: 10.7763/IJCEA.2014.V5.416.
- Pandey, K. B.; Rizvi, S. I. Plant Polyphenols as Dietary Antioxidants in Human Health and Disease. Oxid. Med. Cell. Longev. 2009, 2(5), 270. DOI: 10.4161/OXIM.2.5.9498.
- De Mello Andrade, J. M.; Fasolo, D. Polyphenol Antioxidants from Natural Sources and Contribution to Health Promotion. Polyphenols in Human Health and Disease. 2014, 1, 253–265. DOI: 10.1016/B978-0-12-398456-2.00020-7.
- Betteridge, D. J. What Is Oxidative Stress? Metabolism. 2000, 49(2 Suppl 1), 3–8. DOI: 10.1016/S0026-0495(00)80077-3.
- Gligorijevic, N.; Radomirovic, M.; Nedic, O.; Stojadinovic, M.; Khulal, U.; Stanic-Vucinic, D.; Velickovic, T. C. Molecular Mechanisms of Possible Action of Phenolic Compounds in COVID-19 Protection and Prevention. Int. J. Mol. Sci. 2021, 22, 22. DOI: 10.3390/IJMS222212385.
- Avila-Sosa, R.; Montero-Rodríguez, A. F.; Aguilar-Alonso, P.; Vera-López, O.; Lazcano-Hernández, M.; Morales-Medina, J. C.; Navarro-Cruz, A. R. Antioxidant Properties of Amazonian Fruits: A Mini Review of in Vivo and in Vitro Studies. Oxid. Med. Cell. Longev. 2019, 2019, 1–11. DOI: 10.1155/2019/8204129.
- Peixoto Araujo, N. M.; Arruda, H. S.; Marques, D. R. P.; de Oliveira, W. Q.; Pereira, G. A.; Pastore, G. M. Functional and Nutritional Properties of Selected Amazon Fruits: A Review. Food Res. Int. 2021, 147, 110520. DOI: 10.1016/J.FOODRES.2021.110520.
- Osorio, C. Almanza, O. Antioxidant Activity of Anthocyanin-Rich Colombian Tropical Fruits. In ACS Symposium Series; 2013. 10.1021/bk-2013-1129.ch005.
- Contreras, J.; Calderón, L.; Guerra, E.; García, B. A. C. Phenolic Content and Vitamin C in Pulp, Peel and Seed from 24 Exotic Fruits from Colombia. Food Res. Int. 2011, 44(7), 2047–2053. DOI: 10.1016/j.foodres.2010.11.003.
- Sotelo, D.; Casas, I.; F, N.; Camelo, M. G. Borojó (Borojoa Patinoi): Source of Polyphenols with Antimicrobial Activity. Vitae. 2010, 17(3), 329–336.
- Ricker, M.; Jessen, J. H.; Daly, D. C. El caso paraBorojoa patinoi en la región del Chocó, Colombia. Econ. Bot. 1997, 51(1), 39–48. DOI: 10.1007/BF02910402.
- Chaves-López, C.; Usai, D.; Donadu, M. G.; Serio, A.; González-Mina, R. T.; Simeoni, M. C.; Molicotti, P.; Zanetti, S.; Pinna, A.; Paparella, A. Potential of Borojoa Patinoi Cuatrecasas Water Extract to Inhibit Nosocomial Antibiotic Resistant Bacteria and Cancer Cell Proliferation in Vitro. Food Funct. 2018, 9(5), 2725–2734. DOI: 10.1039/c7fo01542a.
- López, C. C.; Mazzarrino, G.; Rodríguez, A.; Fernández-López, J.; Pérez-Álvarez, J. A.; Viuda-Martos, M. Assessment of Antioxidant and Antibacterial Potential of Borojo Fruit (Borojoa Patinoi Cuatrecasas) from the Rainforests of South America. Ind. Crops Prod. 2015, 63, 79–86. DOI: 10.1016/j.indcrop.2014.10.047.
- Leterme, P.; Garc??a, M. F.; Londo??o, A. M.; Rojas, M. G.; Buldgen, A.; Souffrant, W. B. Chemical Composition and Nutritive Value of Peach Palm (Bactris Gasipaes Kunth) in Rats. J. Sci. Food Agric. 2005, 85(9), 1505–1512. DOI: 10.1002/jsfa.2146.
- Smith, N. Bactris Gasipaes. Palms and People in the Amazon 2015, 177–193.
- Radice, M.; Viafara, D.; Neill, D.; Asanza, M.; Sacchetti, G.; Guerrini, A.; Maietti, S. Chemical Characterization and Antioxidant Activity of Amazonian (Ecuador) Caryodendron Orinocense Karst. and Bactris Gasipaes Kunth Seed Oils. J. Oleo Sci. 2014, 63(12), 1243–1250. DOI: 10.5650/jos.ess14007.
- Hernández, M. S.; Carrillo, M.; Barrera, J.; Fernández-Trujillo, J. P. Camu-Camu (Myrciaria Dubia Kunth McVaugh). Postharvest Biology and Technology of Tropical and Subtropical Fruits 2011, 352–375e. DOI: 10.1533/9780857092762.352.
- Akter, M. S.; Oh, S.; Eun, J.-B.; Ahmed, M. Nutritional Compositions and Health Promoting Phytochemicals of Camu-Camu (Myrciaria Dubia) Fruit: A Review. Food Res. Int. 2011, 44(7), 1728–1732. DOI: 10.1016/j.foodres.2011.03.045.
- Genovese, M. I.; Da Silva Pinto, M.; De Souza Schmidt Gonçalves, A. E.; Lajolo, F. M. Bioactive Compounds and Antioxidant Capacity of Exotic Fruits and Commercial Frozen Pulps from Brazil. Food Sci. Technol. Int. 2008, 14(3), 207–214. DOI: 10.1177/1082013208092151.
- De Souza Schmidt Gonçalves, A. E.; Lajolo, F. M.; Genovese, M. I. Chemical Composition and Antioxidant/Antidiabetic Potential of Brazilian Native Fruits and Commercial Frozen Pulps. J. Agric. Food Chem. 2010, 58(8), 4666–4674. DOI: 10.1021/jf903875u.
- Mascherpa, D.; Carazzone, C.; Marrubini, G.; Gazzani, G.; Papetti, A. Identification of Phenolic Constituents in Cichorium Endivia Var. Crispum and Var. Latifolium Salads by High-Performance Liquid Chromatography with Diode Array Detection and Electrospray Ioniziation Tandem Mass Spectrometry. J. Agric. Food Chem. 2012, 60(49), 12142–12150. DOI: 10.1021/jf3034754.
- Contreras-Calderón, J.; Calderón-Jaimes, L.; Guerra-Hernández, E.; García-Villanova, B. A. C. Phenolic Content and Vitamin C in Pulp, Peel and Seed from 24 Exotic Fruits from Colombia. Food Res. Int. 2011, 44(7), 2047–2053. DOI: 10.1016/j.foodres.2010.11.003.
- Carazzone, C.; Mascherpa, D.; Gazzani, G.; Papetti, A. Identification of Phenolic Constituents in Red Chicory Salads (Cichorium Intybus) by High-Performance Liquid Chromatography with Diode Array Detection and Electrospray Ionisation Tandem Mass Spectrometry. Food Chem. 2013, 138(2–3), 1062–1071. DOI: 10.1016/j.foodchem.2012.11.060.
- Molyneux, P. The Use of the Stable Free Radical Diphenylpicryl-Hydrazyl (DPPH) for Estimating Antioxidant Activity. Songklanakarin Journal of Science and Technology, 2004, 26 (December 2003), 211–219. 10.1287/isre.6.2.144.
- DeLong, J. M.; Prange, R. K.; Hodges, D. M.; Forney, C. F.; Bishop, M. C.; Quilliam, M. Using a Modified Ferrous Oxidation-Xylenol Orange (FOX) Assay for Detection of Lipid Hydroperoxides in Plant Tissue. J. Agric. Food Chem. 2002, 50(2), 248–254. DOI: 10.1021/jf0106695.
- Owokotomo, I. A.; Ekundayo, O.; Abayomi, T. G.; Chukwuka, A. V. In-Vitro Anti-Cholinesterase Activity of Essential Oil from Four Tropical Medicinal Plants. Toxicol. Rep. 2015, 2, 850–857. DOI: 10.1016/j.toxrep.2015.05.003.
- Konrath, E. L.; Neves, B. M.; Lunardi, P. S.; Passos, C. D. S.; Simões-Pires, A.; Ortega, M. G.; Gonalves, C. A.; Cabrera, J. L.; Moreira, J. C. F.; Henriques, A. T. Investigation of the in Vitro and Ex Vivo Acetylcholinesterase and Antioxidant Activities of Traditionally Used Lycopodium Species from South America on Alkaloid Extracts. J. Ethnopharmacol. 2012, 139(1), 58–67. DOI: 10.1016/j.jep.2011.10.042.
- Chirinos, R.; , et al. Antioxidant Compounds and Antioxidant Capacity of Peruvian Camu Camu (Myrciaria Dubia (H.B.K.) McVaugh) Fruit at Different Maturity Stages. Food Chem. 2010, 120(4), 1019–1024. DOI: 10.1016/j.foodchem.2009.11.041.
- Bravo, K.; Sepulveda-Ortega, S.; Lara-Guzman, O.; Navas-Arboleda, A. A.; Osorio, E. Influence of Cultivar and Ripening Time on Bioactive Compounds and Antioxidant Properties in Cape Gooseberry (Physalis Peruviana L.). J. Sci. Food Agric. 2015, 95(7), 1562–1569. DOI: 10.1002/jsfa.6866.
- Lago-Vanzela, E. S.; Da-Silva, R.; Gomes, E.; García-Romero, E.; Hermosín-Gutiérrez, I. Phenolic Composition of the Edible Parts (Flesh and Skin) of Bordô Grape (Vitis Labrusca) Using HPLC–DAD–ESI-MS/MS. J. Agric. Food Chem. 2011, 59(24), 13136–13146. DOI: 10.1021/jf203679n.
- Złotek, U.; Mikulska, S.; Nagajek, M.; Świeca, M. The Effect of Different Solvents and Number of Extraction Steps on the Polyphenol Content and Antioxidant Capacity of Basil Leaves (Ocimum Basilicum L.) Extracts. Saudi J. Biol. Sci. 2016, 23(5), 628–633. DOI: 10.1016/j.sjbs.2015.08.002.
- Gonçalves, S.; Romano, A. Inhibitory Properties of Phenolic Compounds against Enzymes Linked with Human Diseases. 2017, Phenolic Compounds - Biological Activity. DOI: 10.5772/66844.
- Prior, R. L.; Wu, X.; Schaich, K. Standardized Methods for the Determination of Antioxidant Capacity and Phenolics in Foods and Dietary Supplements. J. Agric. Food Chem. 2005, 53(10), 4290–4302. DOI: 10.1021/jf0502698.
- El Sayed, A. M.; Basam, S. M.; El-Naggar, E.-M. B. A.; Marzouk, H. S.; El-Hawary, S. LC–MS/MS and GC–MS Profiling as Well as the Antimicrobial Effect of Leaves of Selected Yucca Species Introduced to Egypt. Sci. Rep. 2020, 10(1), 17778. DOI: 10.1038/s41598-020-74440-y.
- Willems, J. L.; Khamis, M. M.; Mohammed Saeid, W.; Purves, R. W.; Katselis, G.; Low, N. H.; El-Aneed, A. Analysis of a Series of Chlorogenic Acid Isomers Using Differential Ion Mobility and Tandem Mass Spectrometry. Anal. Chim. Acta. 2016, 933, 164–174. DOI: 10.1016/j.aca.2016.05.041.
- Noman, L.; Oke-Altuntas, F.; Zellagui, A.; Sahin Yaglioglu, A.; Demirtas, I.; Cardoso, M.; Akkal, S.; Gherraf, N.; Rhouati, S. A Novel Benzimidazole and Other Constituents with Antiproliferative and Antioxidant Properties from Thymelaea Microphylla Coss. Et Dur. Nat. Prod. Res. 2017, 31(17), 2032–2041. DOI: 10.1080/14786419.2016.1274888.
- Li, Z.; Liu, J.; Zhang, D.; Du, X.; Han, L.; Lv, C.; Li, Y.; Wang, R.; Wang, B.; Huang, Y. Nuciferine and Paeoniflorin Can Be Quality Markers of Tangzhiqing Tablet, a Chinese Traditional Patent Medicine, Based on the Qualitative, Quantitative and Dose-Exposure-Response Analysis. Phytomedicine. 2018, 44, 155–163. DOI: 10.1016/j.phymed.2018.02.006.
- Justino, G. C.; Borges, C. M.; Helena Florêncio, M. Electrospray Ionization Tandem Mass Spectrometry Fragmentation of Protonated Flavone and Flavonol Aglycones: A Re-Examination. Rapid Commun. Mass Spectrom. 2009, 23(2), 237–248. DOI: 10.1002/rcm.3869.
- Yi, T.; Zhu, L.; Tang, Y. N.; Zhang, J. Y.; Liang, Z. T.; Xu, J.; Zhao, Z. Z.; Yu, Z. L.; Bian, Z. X.; Yang, Z. J., et al. An Integrated Strategy Based on UPLC-DAD-QTOF-MS for Metabolism and Pharmacokinetic Studies of Herbal Medicines: Tibetan “Snow Lotus” Herb (Saussurea Laniceps), a Case Study. J. Ethnopharmacol. 2014, 153(3), 701–713. DOI: 10.1016/j.jep.2014.03.031.
- Barnaba, C.; Larcher, R.; Nardin, T.; Dellacassa, E.; Nicolini, G. Glycosylated Simple Phenolic Profiling of Food Tannins Using High Resolution Mass Spectrometry (Q-Orbitrap). Food Chem. 2018, 267, 196–203. DOI: 10.1016/j.foodchem.2017.11.048.
- Huang, H. C.; Lin, M. K.; Hwang, S. Y.; Hwang, T. L.; Kuio, Y. H.; Chang, C. I.; Ou, C. Y.; Kuo, Y. H. Two Anti-Inflammatory Steroidal Saponins from Dracaena Angustifolia Roxb. Molecules. 2013, 18(8), 8752–8763. DOI: 10.3390/molecules18088752.
- Bah, M.; Chérigo, L.; Cardoso Taketa, A. T.; Fragoso-Serrano, M.; Hammond, G. B.; Pereda-Miranda, R. I. I.-V. I. I. Pentasaccharides from the Seeds of Ipomoea Intrapilosa. J. Nat. Prod. 2007, 70(7), 1153–1157. DOI: 10.1021/np0701529.
- Tanaka, Y.; Yanagida, A.; Komeya, S.; Kawana, M.; Honma, D.; Tagashira, M.; Kanda, T.; Shibusawa, Y. Comprehensive Separation and Structural Analyses of Polyphenols and Related Compounds from Bracts of Hops (Humulus Lupulus L.). J. Agric. Food Chem. 2014, 62(10), 2198–2206. DOI: 10.1021/jf405544n.
- Seraglio, S. K. T.; Valese, A. C.; Daguer, H.; Bergamo, G.; Azevedo, M. S.; Gonzaga, L. V.; Fett, R.; Costa, A. C. O. Development and Validation of a LC-ESI-MS/MS Method for the Determination of Phenolic Compounds in Honeydew Honeys with the Diluted-and-Shoot Approach. Food Res. Int. 2016, 87, 60–67. DOI: 10.1016/j.foodres.2016.06.019.
- Wabaidur, S. M.; Alothman, Z. A.; Khan, M. R. A Rapid Method for the Simultaneous Determination of L-Ascorbic Acid and Acetylsalicylic Acid in Aspirin C Effervescent Tablet by Ultra Performance Liquid Chromatography-Tandem Mass Spectrometry. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2013, 108, 20–25. DOI: 10.1016/j.saa.2013.01.070.
- Chen, S. D.; Lu, C. J.; Zhao, R. Z. Qualitative and Quantitative Analysis of Rhizoma Smilacis Glabrae by Ultra High Performance Liquid Chromatography Coupled with LTQ OrbitrapXL Hybrid Mass Spectrometry. Molecules. 2014, 19(7), 10427–10439. DOI: 10.3390/molecules190710427.
- De Marino, S.; Festa, C.; Zollo, F.; Rusolo, F.; Capone, F.; Guerriero, E.; Costantini, S.; De Felice, V.; Iorizzi, M. Phytochemical Profile of Juniperus Oxycedrus Ssp. Oxycedrus Berries: A New Monoterpene Glucoside and Evaluation of the Effects on Cancer Cell Lines. Phytochem. Lett. 2014, 10, 152–159. DOI: 10.1016/j.phytol.2014.08.015.
- Mihajlovic, L.; Radosavljevic, J.; Burazer, L.; Smiljanic, K.; Cirkovic Velickovic, T. Composition of Polyphenol and Polyamide Compounds in Common Ragweed (Ambrosia Artemisiifolia L.) Pollen and Sub-Pollen Particles. Phytochemistry. 2015, 109, 125–132. DOI: 10.1016/j.phytochem.2014.10.022.
- Liu, M. H.; Zhang, Q.; Zhang, Y. H.; Lu, X. Y.; Fu, W. M.; He, J. Y. Chemical Analysis of Dietary Constituents in Rosa Roxburghii and Rosa Sterilis Fruits. Molecules. 2016, 21(9), 1204. DOI: 10.3390/molecules21091204.
- Wei, X. H.; Yang, S. J.; Liang, N.; Hu, D. Y.; Jin, L. H.; Xue, W.; Yang, S. Chemical Constituents of Caesalpinia Decapetala (Roth) Alston. Molecules. 2013, 18(1), 1325–1336. DOI: 10.3390/molecules18011325.
- Lin, L. Z.; Harnly, J. M. Phenolic Compounds and Chromatographic Profiles of Pear Skins (Pyrus Spp.). J. Agric. Food Chem. 2008, 56(19), 9094–9101. DOI: 10.1021/jf8013487.
- Mäkilä, L.; Laaksonen, O.; Alanne, A. L.; Kortesniemi, M.; Kallio, H.; Yang, B. Stability of Hydroxycinnamic Acid Derivatives, Flavonol Glycosides, and Anthocyanins in Black Currant Juice. J. Agric. Food Chem. 2016, 64(22), 4584–4598. DOI: 10.1021/acs.jafc.6b01005.
- Saldanha, L. L.; Vilegas, W.; Dokkedal, A. L. Characterization of Flavonoids and Phenolic Acids in Myrcia Bella Cambess. Using FIA-ESI-IT-MSn and HPLC-PAD-ESI-IT-MS Combined with NMR. Molecules. 2013, 18(7), 8402–8416. DOI: 10.3390/molecules18078402.
- Agar, O. T.; Dikmen, M.; Ozturk, N.; Yilmaz, M. A.; Temel, H.; Turkmenoglu, F. P. Comparative Studies on Phenolic Composition, Antioxidant, Wound Healing and Cytotoxic Activities of Selected Achillea L. Species Growing in Turkey. Molecules. 2015, 20(10), 17976–18000. DOI: 10.3390/molecules201017976.
- Seibert, J. B.; Bautista-Silva, J. P.; Amparo, T. R.; Petit, A.; Pervier, P.; Dos Santos Almeida, J. C.; Azevedo, M. C.; Silveira, B. M.; Brandão, G. C.; de Souza, G. H. B., et al. Development of Propolis Nanoemulsion with Antioxidant and Antimicrobial Activity for Use as a Potential Natural Preservative. Food Chem. 2019, 287, 61–67. DOI: 10.1016/j.foodchem.2019.02.078.
- Zhu, J.-X.; Qin, -J.-J.; Zhang, F.; Chang, R.-J.; Ren, J.; Cheng, X.-R.; Zeng, Q.; Jin, H.-Z.; Zhang, W.-D. Chemical Constiuents of Euonymus Acanthocarpus. Chem. Nat. Compd. 2013, 49(2), 383–387. DOI: 10.1007/s10600-013-0616-y.
- Vagula, J. M.; Sinosaki, N. M.; Ribeiro, M. A. S.; Magon, T.; Bertozzi, J.; Meurer, E. C.; Santos, O. O.; Visentainer, J. V. Simple and Fast Method for Identification and Quantification of Anthocyanidins in Berries by Ultra Performance Liquid Chromatography-Mass Spectrometry. J. Braz. Chem. Soc. 2018, 29(1), 38–44. DOI: 10.21577/0103-5053.20170110.
- Barnes, J. S.; Nguyen, H. P.; Shen, S.; Schug, K. A. General Method for Extraction of Blueberry Anthocyanins and Identification Using High Performance Liquid Chromatography-Electrospray Ionization-Ion Trap-Time of Flight-Mass Spectrometry. J. Chromatogr. A. 2009, 1216(23), 4728–4735. DOI: 10.1016/j.chroma.2009.04.032.
- Barnes, J. S.; Schug, K. A. Structural Characterization of Cyanidin-3,5-Diglucoside and Pelargonidin-3,5-Diglucoside Anthocyanins: Multi-Dimensional Fragmentation Pathways Using High Performance Liquid Chromatography-Electrospray Ionization-Ion Trap-Time of Flight Mass Spectrometry. Int. J. Mass Spectrom. 2011, 308(1), 71–80. DOI: 10.1016/j.ijms.2011.07.026.
- Montoro, P.; Tuberoso, C. I. G.; Perrone, A.; Piacente, S.; Cabras, P.; Pizza, C. Characterisation by Liquid Chromatography-Electrospray Tandem Mass Spectrometry of Anthocyanins in Extracts of Myrtus Communis L. Berries Used for the Preparation of Myrtle Liqueur. J. Chromatogr. A. 2006, 1112(1–2), 232–240. DOI: 10.1016/j.chroma.2005.11.055.
- Mirali, M.; Purves, R. W.; Vandenberg, A. Profiling the Phenolic Compounds of the Four Major Seed Coat Types and Their Relation to Color Genes in Lentil. J. Nat. Prod. 2017, 80(5), 1310–1317. DOI: 10.1021/acs.jnatprod.6b00872.
- Spencer, J. P.; Schroeter, H.; Kuhnle, G.; Srai, S. K.; Tyrrell, R. M.; Hahn, U.; Rice-Evans, C. Epicatechin and Its in Vivo Metabolite, 3’-O-Methyl Epicatechin, Protect Human Fibroblasts from Oxidative-Stress-Induced Cell Death Involving Caspase-3 Activation. Biochem. J. 2001, 354(Pt 3), 493–500. DOI: 10.1042/0264-6021:3540493.
- Hatano, T.; Miyatake, H.; Natsume, M.; Osakabe, N.; Takizawa, T.; Ito, H.; Yoshida, T. Proanthocyanidin Glycosides and Related Polyphenols from Cacao Liquor and Their Antioxidant Effects. Phytochemistry. 2002, 59(7), 749–758. DOI: 10.1016/S0031-9422(02)00051-1.
- Lakenbrink, C.; Engelhardt, U. H.; Wray, V. Identification of Two Novel Proanthocyanidins in Green Tea. J. Agric. Food Chem. 1999, 47(11), 4621–4624. DOI: 10.1021/jf9813081.
- Kim, M. S.; Nam, M.; Hwang, G. S. Metabolic Alterations in Two Cirsium Species Identified at Distinct Phenological Stages Using UPLC-QTOF/MS. Phytochem. Anal. 2018, 29(1), 77–86. DOI: 10.1002/pca.2716.
- Taamalli, A.; Arráez-Román, D.; Abaza, L.; Iswaldi, I.; Fernández-Gutiérrez, A.; Zarrouk, M.; Segura-Carretero, A. LC-MS-Based Metabolite Profiling of Methanolic Extracts from the Medicinal and Aromatic Species Mentha Pulegium and Origanum Majorana. Phytochem. Anal. 2015, 26(5), 320–330. DOI: 10.1002/pca.2566.
- Jeong, S. J.; Miyamoto, T.; Inagaki, M.; Kim, Y. C.; Higuchi, R. R. A.-C. Three Novel Sesquiterpene Alkaloids from Cyperus Rotundus. J. Nat. Prod. 2000, 63(5), 673–675. DOI: 10.1021/np990588r.
- García-Villalba, R.; Tomás-Barberán, F. A.; Fança-Berthon, P.; Roller, M.; Zafrilla, P.; Issaly, N.; García-Conesa, M. T.; Combet, E. Targeted and Untargeted Metabolomics to Explore the Bioavailability of the Secoiridoids from A Seed/Fruit Extract (Fraxinus Angustifolia Vahl) in Human Healthy Volunteers: A Preliminary Study. Molecules. 2015, 20(12), 22202–22219. DOI: 10.3390/molecules201219845.