ABSTRACT
Consumer demand for higher-quality fruit juices has resulted in the development of non-thermal processing to extend the shelf life of fruit juices. Ozonation is a novel technique, involving the production of volatile oxygen atoms capable of destroying an extensive range of microorganisms and lengthening the shelf stability of fruit juices. The purpose of this research was to examine the influence of ozonation on the physicochemical, bioactive components, and microbial properties of kinnow juice. Kinnow juice was exposed to ozone gas with fixed concentration 150 mg/h for 5, 10, and 15 minutes. Then, kinnow juice was filled in a plastic bottle and kept at 4 ± 2°C for 3 months. The mentioned parameters were examined after 30 days, 60 days, and 90 days. It was found that ozonation had shown a significant influence on nutritional characteristics of kinnow juice as it increased (p ≤ .05) the acidity, total soluble content, total phenolics content, flavonoids content, and antioxidant potential of kinnow juice and diminished the microbial activity, increasing ozone time from 5 to 15 minutes. OZ-15 (15 minutes of ozonation) showed the maximum value of total soluble content (14.70 ± 0.10 °Brix), titrable acidity (0.179 ± 0.10%), total flavonoid content (244.44 ± 3.70 mg equivalent of catechin/100 mL), and total antioxidant activity (305.95 ± 2.70 µg/g equivalent of ascorbic acid of juice), but it presented the least total mold counts (1.49 ± 0.02 colony forming unit/mL) and plate counts (1.53 ± 0.03 colony forming unit/mL). It was suggested that the ozonation could be used as an alternative to the traditional pasteurization and chemical preservatives on kinnow juice to retain quality characteristics.
Introduction
Fruits have a fresh, high flavor and contained various components, including fiber, vitamins, minerals, a high level of phenol and flavonoid content that provided good resistance against chronic illnesses in the human body, such as inflammation and cancer.[Citation1,Citation2] Fruit juices are among the most popular drinks globally, owing to consumer desire for fresh-tasting and healthy foods. It is easily digested, has no adverse effects on the body, and detoxifies the blood and digestive system. Modern food techniques are a significant concern in transferring important fruit components into juice and producing stable products.[Citation3,Citation4]
Citrus belongs to the family of Rutaceae. It has the top rank in homeland fruits and it has grown in all four provinces of Pakistan where Punjab harvests almost 95% of crops due to favorable growing conditions such as sufficient water. Pakistan has 60% Kinnow and 5% other varieties of mandarin, it has contributed 6.5% to the worldwide production of mandarin.[Citation5] Kinnow (Citrus Reticulata Blanco) is a cross between two varieties of citrus namely king and willow and therefore is termed Kinnow mandarin.[Citation6] Pakistan has the sixth rank mandarin cultivator in the world with an estimated production of 370 thousand tons and an export value of 222 million dollars.[Citation5,Citation7]
Kinnow juice is refreshing, tasty, and high nutritional value. It contains 36–62% juice. From a nutritional point of view, it helps to prevent chronic diseases. It possesses anti-oxidation, anti-bacterial, and anti-blood clotting characteristics. It reduces the chance of cardiovascular diseases. 100 g of Citrus Reticulata Blanco is composed of 88.9, 10.1, 0.5, 0.2, and 0.2 g of water, carbohydrates, protein, dietary fiber, and fat.[Citation8–10] It has phytonutrients such as flavonoids, narirutin, hesperidin, naringin, and phenolic acid compounds.[Citation11] It presents a high amount of total soluble content of 9.5–16%. The minerals present in kinnow are copper, sodium, magnesium, potassium, phosphorus, and calcium.[Citation12,Citation13]
Consumer demands safe, natural, and nutritional products. It has been attained by controlling the process that changed the nutritional and quality attributes. Heat treatment is used to destroy the foodborne microorganism and inactivate the pectin methyl esterase (PME), but loses the quality and nutrients of juice. However, chemical preservatives are another way to lengthen product shelf life, but they also cause a number of health problems in people, including cancer, neurological dysfunction, asthma, hyperactivity and hypersensitivity, dermatitis, allergies, and gastrointestinal and respiratory issues. Nowadays, non-thermal methods are used such as ozone, ultrasonic, high-pressure processing, and plus-electric field. Therefore, these methods can decrease the negative impact of heat on the quality features and nutritional factors of food products.[Citation14,Citation4]
Ozone is made by the three bonding atoms of oxygen and is colorless with a bitter smell. It is significant due to its high oxidizing power, disinfectant, and oxidant that is a promising alternative to reduce microbes such as bacteria, virus, and protozoa in food commodities, thus extending their shelf stability. Ozonation is a quick, effective, cheap, and simple method and thus can be used as an alternative technique in comparison with other thermal preservation and processing techniques. It is a green chemical process and requires GRAS status. FDA approved ozone is used as an antimicrobial agent to preserve food.[Citation15] Ozonation has antimicrobial properties, involving the production of volatile oxygen atoms capable to destroy an extensive range of microorganisms and extending the shelf life of products such as fruits and vegetables. It has been applied to the juice processing industries such as apple juice, apple cider, orange juice, tomato juice, etc. It has not affected the non-enzymatic browning, pH, and acidity of fruit juices[Citation16] [Shah et al., 2019]. During processing, ozone treatment minimized the change in taste and nutritional value of the product. This research was designed to examine the effects of ozonation on the physicochemical properties, bioactive components, and microbiological indices of kinnow juice and to examine the effects of ozonation on the shelf life of kinnow juice at refrigeration (4 ± 2°C).
Materials and methods
Materials and chemicals
Kinnow was bought from the local orchard in Sargodha. Potassium-meta bisulfite (Sigma-Aldrich), sodium hydroxide (Sigma-Aldrich), phenolphthalein, folin ciocalteu reagents (Sigma-Aldrich), sodium carbonate (Sigma-Aldrich), sodium nitrite (Sigma-Aldrich), aluminum chloride (Sigma-Aldrich), ammonium molybdate reagent (Sigma-Aldrich), and plastic bottles were bought from the local market of Sargodha. Potassium-metabisulphite was used as a preservative in the treatment T0+ compared to control and other treatments (Treatment plan is given in ).
Table 1. Treatment plan of kinnow juice.
Preparation of Kinnow juice
Kinnow was sorted based on food quality parameters to separate disease and damage, then washed to eliminate mud and filth. In the next step, the fruits were peeled then processed into fruit juice over the electrical juice extraction unit (Model No. JE-623D, working at power 45 W, frequency of 60 Hz and voltage of 45 V). Then, the juice was filtered through a sieve (mesh size no. 18) to obtain a clear juice. Finally, Kinnow juice was packed in a plastic bottle.
Ozone treatment of Kinnow juice
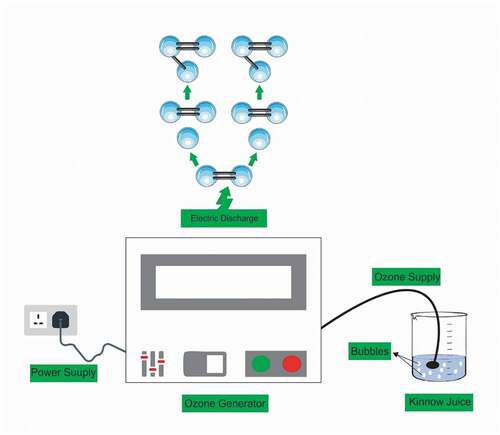
Ozonation was performed by exposing ozone gas with fixed concentration 150 g/ha for 250 mL of kinnow juice in a 500 mL beaker through a pipe connected with a diffuser (Model No: TR-YCA, working at power 15 W, frequency of 50 Hz and voltage of 220 V) which produced bubbles, and uniform gas was distributed (). Kinnow juice was processed by ozone for 5, 10, and 15 minutes. Then, the juice (200 mL) was packed in plastic bottles and kept at refrigerated temperature (4 ± 2°C) for 90 days.
Physicochemical analysis
Total soluble contents were measured by hand held refractometer (Model No. MASTER-53α, Atago, Japan). The standard titration procedure defined by AOAC[Citation17] was used to determine acidity. pH of the juice was calculated using a pH meter [AD 1040 Benchtop meter, Adwa, Hungary].
Total phenolic content
Total phenolic contents of kinnow juice were measured with a spectrophotometer by modified Folin-Ciocalteu reagent method detailed by Saeeduddin et al.[Citation18] For the experiment, a 0.5 mL prepared sample was obtained after dilution. Spectrophotometers were used to measure the absorbance of samples at 760 nm. A calibration curve of standard solution was prepared using Gallic acid. Total phenolic contents were measured in milligrams of gallic acid equivalent [GAE] per 100 mL of juice.
Total flavonoid content
Total flavonoids of juice samples were accessed using the ammonium chloride reagent, as explained by Malik et al.[Citation19] For the experiment, a 0.25 mL prepared sample was obtained after dilution, and absorbance was determined at 510 nm wavelength using a spectrophotometer. Catechin was employed as a standard, and findings were presented as mg of (+) – catechin equivalent (CE) per 100 mL of juice.
Total antioxidant activity
Total antioxidant activity was measured using the method reported by Prieto et al.[Citation20] For the experiment, a 0.4 mL prepared sample was obtained after dilution. 4 mL of reagent solution was taken in a burette and then added to beaker that contained a juice sample. Then, samples in test tubes were held in water bath for 90 minutes at 95°C. The absorbance was calculated using a spectrophotometer at 695 nm. The ascorbic acid standard calibration curve was used, and the values obtained in g ascorbic acid equivalent [AAE/mL juice].
Microbiological analysis
Total mold count
The mold count was determined by the FDA standard procedure of the Bacteriological Analytical Manual, as explained by FDA.[Citation21] 1 mL of juice sample was taken in a test tube. It was added 9 mL saline water and agitated for 1–2 minutes until the cloudy solution. 10 mL dilution was prepared; then, 1 mL was transferred in the previous 9 ml dilution. All dilutions were mixed well. Then, 12 to 15 ml of potato dextrose agar was poured into a petri dish, then added sample dilution and solidified. All potato dextrose agar plates were incubated at 20–25°C. After 2 days of incubation, mold counts were measured in every dish, and the findings were stated as log colony-forming units CFU/mL of juice. All analyses were carried out in duplicate.
Total plate count
The plate count determination was carried out as per FDA’s standard procedure of Bacteriological Analytical Manual described by FDA.[Citation21] 1 ml juice was placed in a test tube and adding a 9 ml saline solution was used for dilution. Diluents were added and shaken for 1–2 minutes at a low speed until the sample was fully cloudy. Dilutions of 10 mL were made by relocating 1 mL of the initial dilution to 9 mL of diluents. All dilutions were thoroughly agitated. Then, 12 to 15 ml of plate count agar was transferred into the petri dish, and sample dilutions were mixed with media, then solidified the media. The plates were inverted over and were taken in an incubator for 48 hours at 32°C. The number of bacterial colonies in the sample was determined and multiplied by the reciprocal, and the findings were stated as log colony-forming units CFU/mL juice.
Statistical analysis
The results were analyzed by utilizing Minitab 16 statistical software by applying the variance analysis method. Mean differences were assessed using the Tucky’s HSD test with a 5% level of significance.[Citation22]
Results and discussions
Impact of ozonation on TSS and pH
Ozone treatment excreted highly significant change on the total soluble content of kinnow juice but a substantial reduction was noticed after 90 days of storage. It observed decrease in the TSS throughout storage was from 13.83 ± 0.15 to 10.20 ± 0.10 °B. The maximum value of total soluble content was observed in OZ-15 (14.70 ± 0.10°B), and the minimum value of total soluble content in OZ-5 (13.83 ± 0.15 °B) at 0 day. The highest TSS value was obtained after 90 days in OZ-15 (12.30 ± 0.10) and the lowest value after 90 days in control (10.20 ± 0.10 °B) as directed in . It was noticed that the ozone processing was a significant alteration in the pH of kinnow juice. The decrease of pH was detected within the treatments. Similarly, the impact of storage time indicated that the pH (3.62 ± 0.02 to 3.92 ± 0.01) enhanced after 90 days of storage. The maximum value of pH was observed in control (3.83 ± 0.01), and the minimum value of pH in OZ-15 (3.62 ± 0.02) at 0 day. The highest pH value was obtained after 90 days in control (3.92 ± 0.01) and the lowest value in OZ-15 (3.76 ± 0.02) as directed in . The current study’s findings on total soluble solids are consistent with prior research findings that stated similar patterns.[Citation23,Citation24] It showed a declining trend of total soluble content in ozonated strawberry and pomegranate fruits during storage, which correlated to the current findings. The total soluble content is reduced due to the fermentation of sugar into ethyl alcohol, carbon dioxide, and water.[Citation25,Citation26] Barboni et al. and Alencar et al. reported an increasing trend of pH in ozonated kiwi juice and banana fruit during storage time with correlated current findings. A similar trend was observed among treatments.[Citation4,Citation27] Fundo et al. and Islam et al. noted the same trend in mixed juices.
Table 2. The mean TSS (°B) values ± SD of kinnow juice during storage.
Table 3. The mean pH values ± SD of kinnow juice during storage.
Titratable acidity
The titrable acidity of treated samples is reduced at the end of storage time (pH results in ) Titrable acidity results are presented in . The maximum value of acidity was observed in OZ-15 (0.179 ±0.01%), and the minimum in T0- (0.130 ± 0.004%) at 0 day. The impact of storage time on acidity indicated that the acidity (0.179 ± 0.01 to 0.092 ± 0.01%) reduced over time. The highest mean acidity value was obtained after 90 days in OZ-15 (0.122 ± 0.01%), and the least value after 90 days in control (0.092 ± 0.004%). Similar trends were reported in ozonated watermelon juice and peach juice among treatments and during storage time. The acid formation through the breakdown of polysaccharides and oxidation of reducing sugar may enhance acidity. They found that ozonation during storage showed significant changes in all the treated samples.[Citation14,Citation28–30] The acidity of kinnow juice blends is reduced during storage conditions. Reduction of acidity might be due to the hydrolysis of acids into sugar and salt by invertase enzyme.[Citation3]
Table 4. The mean titratable acidity (%) values ± SD of kinnow juice during storage.
Total phenolics and flavonoids contents
The impact of ozonation was highly significant on the phenolic contents of kinnow juice (). The increase of total phenolic contents was detected within the treatments at 0–10 minutes of ozone processing and abruptly declined after 10 minutes. The maximum value of total phenolic content was observed in OZ-10 (684 ± 2.0 mg GAE/100 mL juice) and the minimum value of total phenolic content in control (664 ± 2.0 mg GAE/100 mL juice) at 0 day. The reduction of phenolic content of kinnow juice throughout storage was observed from 684 ± 2.0 to 342.67 ± 3.06 mg GAE/100 mL juice. The maximum value of total phenolic content was observed after 90 days in OZ-10 (365 ± 0.58 mg GAE/100 mL juice) and the minimum value after 90 days in control (342.67 ± 3.06 mg GAE/100 mL juice). The alterations in total flavonoid contents exhibited that the treatments were shown as highly significant on the total flavonoid contents of kinnow juice. The maximum value of total flavonoids content was detected in OZ-15 (244.44 ± 3.70 mg CE/100 mL juice) and the minimum value of total flavonoid content in control (170.37 ± 3.70 mg CE/100 mL juice) at start of day. Total flavonoid content during storage reduced from 244.44 ± 3.70 to 96.30 ± 3.70 mg CE/100 mL juice. The maximum value of total flavonoids content was observed after 90 days in OZ-15 (125.93 ± 3.70 mg CE/100 mL juice) and the minimum value after 90 days in control (96.30 ± 3.70 CE/100 mL juice). The impact of storage on the phenolic contents and flavonoid content of kinnow juice indicated that the phenolic contents and flavonoid content were decreased with time (). Shah et al. (2019) showed an increase in phenolic content in orange juice after 0–10 minutes of ozone treatment and quickly declined after 10 minutes with similar current findings. The increase in phenolic content may have resulted from ozone-induced cell wall modification, which liberated some of the conjugated phenolic compounds in the cell wall. The reduction of phenolic content in Roselle fruit juice was noticed at refrigerated storage.[Citation31,Citation32] Mgaya-Kilima et al. and Alothman et al. found the impact of ozonation on the fresh-cut banana and pineapple for 0–30 minutes. Findings indicated that when banana and pineapple were subjected to ozone for 20 minutes, their phenolic and flavonoid content increased significantly. The reduction of flavonoid content in ozone-treated kiwi fruit and tender coconut water was observed during storage time. The phenolics and flavonoid contents might be reduced due to production of free radicals.[Citation33, Citation34]
Table 5. The mean total phenolic contents (mg GAE/100 mL) values ± SD of kinnow juice during storage.
Table 6. The mean total flavonoid contents (mg CE/100 mL) values ± SD of kinnow juice during storage.
Total antioxidant activity
Statistical analysis indicated a highly significant impact of the ozone technique on the total antioxidant activity of kinnow juice during storage (). The increment of total antioxidant activity was observed among treatments. The total antioxidant activity was ranged from 305.95 ±2.70 to 392.42 ± 2.70 µg/g AAE in the treatment. The highest antioxidant activity was recorded in OZ-15 (305.95 ±2.70 µg/g AAE) and lowest total antioxidant activity in control (392.42 ± 2.70 µg/g AAE) at 0 day. TAA may have increased due to ozone processing, which boosted the bond of antioxidant activity such as ascorbic acid, total phenols and flavonoids, resulting in higher total antioxidant activity. Furthermore, ozone treatment reduced polyphenol oxidases, which are involved in enzymatic browning and enhanced TAA. The total antioxidant of treated samples reduced during 90 days. The impact of storage time on total antioxidant activity revealed that the total antioxidant declined by the passage of time. The highest total TAA was found after 90 days in OZ-15 (303.95 ± 2.43 µg/g AAE) and the lowest TAA after 90 days in control (19,514 ± 2.70 µg/g AAE). The total antioxidant of ozone processed fresh piece papaya, banana, and pineapple fruit increased among treatments when fruits were subjected to ozone for 20 minutes. The production of lipoxygenase may be responsible for increasing antioxidant activity after exposure to ozone for 10–20 minutes.[Citation32,Citation35] The reduction of total antioxidant in ozone processed raspberries was observed during storage time.[Citation36]
Table 7. The mean total antioxidant activity (µg/g AAE) values ± SD of kinnow juice during storage.
Mold count
The findings of mold count statistically indicated a significant impact of treatments and storage time on the total mold count of kinnow juice (). The reduction of total mold count was noticed within the treatments. It was observed that the highest total mold count was detected in control (1.61 ± 0.01 log10 CFU/mL), and the lowest mold count was found in OZ-15 (1.49 ± 0.02 log10 CFU/mL) at the start of the day. The impact of storage time on mold count indicated that the total mold count of treated samples increased noticeably during 90 days of storage. The highest value of mold count was acquired after 90 days in control (1.75 ± 0.01 log10 CFU/mL) and the least value of mold count after 90 days in OZ-15 (1.63 ± 0.02 log10 CFU/mL).[Citation28,Citation37] The maximum reduction of mold activity in watermelon juice and sugar cane juice was found by increasing ozone processing time.
Table 8. The mean mold count (log10 cfu/mL) values ± SD of kinnow juice during storage.
Total plate count
The treatments and storage time results were presented as highly significant on the total plate count of kinnow juice (). The reduction of total plate count was noticed within the treatments. It was observed that the highest value total plate count was noticed in control (1.64 ± 0.02 log10 CFU/mL) at 0 day. Meanwhile, the lowest value total plate count was found in OZ-15 (1.53 ± 0.03 log10 CFU/mL). The impact of storage time on the total plate count of treated samples increased noticeably during 90 days of storage. The highest value of total plate count activity was acquired after 90 days in control (1.99 ± 0.01 log10 CFU/mL) and the lowest mean value after 90 days in OZ-15 (1.87 ± 0.01 log10 CFU/mL).[Citation28,Citation37] The maximum reduction of total bacterial activity in watermelon juice and sugar cane juice was found by increasing ozone processing time. Reducing the bacterial population may oxidize the organic substance in bacterial membranes by ozone processing, causing the cell wall to weaken and burst[Citation38] The increasing total bacterial population was observed in ozone-treated sugar cane juice and pummelo juice during storage[Citation39], [Shah et al., 2019].
Table 9. The mean plate count (log10 cfu/mL) values ± SD of kinnow juice during storage.
Conclusion
The research was carried out to determine the impact of ozone treatment on the physicochemical parameters of kinnow juice upto 3 months of storage at refrigerated temperature (4 ± 2°C). According to the findings of this study, ozone treatment produced high-quality kinnow juice with remarkable stability of physicochemical and phytochemical attributes during storage. The ozone treatments slightly increased the acidity, TSS, total phenols, flavonoids, and antioxidant activity of kinnow juice. Ozonation treatment for 15 minutes performed best as it reduced microbial activity within acceptable limits and enhanced physicochemical characteristics, bioactive components and antioxidant capacity. The ozone could be used as an alternative to the traditional pasteurization and chemical preservatives on fruit-based items to maintain quality characteristics.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Gardner, P. T.; White, T. A.; McPhail, D. B.; Duthie, G. G. The Relative Contributions of Vitamin C, Carotenoids and Phenolics to the Antioxidant Potential of Fruit Juices. Food Chem. 2000, 68(4), 471–474. DOI: 10.1016/S0308-8146(99)00225-3.
- Mullen, W.; Marks, S. C.; Crozier, A. Evaluation of Phenolic Compounds in Commercial Fruit Juices and Fruit Drinks. J. Agric. Food Chem. 2007, 55(8), 3148–3157. DOI: 10.1021/jf062970x.
- Bhardwaj, R. L.; Nandal, U. Effect of Storage Temperature on Physico-chemical and Sensory Evaluation of Kinnow Mandarin Juice Blends. J. Food Process. Technol. 2014, 5(8), 1.
- Fundo, J. F.; Miller, F. A.; Tremarin, A.; Garcia, E.; Brandão, T. R.; Silva, C. L. Quality Assessment of Cantaloupe Melon Juice under Ozone Processing. Innovative Food Sci. Emerg. Technol. 2018, 47, 461–466. DOI: 10.1016/j.ifset.2018.04.016.
- Altaf, S.; Khan, M. M.; Jaskani, M. J.; Khan, I. A.; Usman, M.; Sadia, B.; Khan, A. I. Morphogenetic Characterization of Seeded and Seedless Varieties of Kinnow Mandarin (‘citrus reticulata’Blanco). Aust. J. Crop Sci. 2014, 8(11), 1542–1549.
- Jawandha, S. K.; Gill, M. S.; Singh, N. P.; Sangwan, A. Study of Fruit Quality Attributes of Kinnow Mandarin in Relation to Fruit Size. Int J Nat Sci. 2015, 6(1), 103–104.
- Nawaz, R.; Abbasi, N. A.; Hafiz, I. A.; Khalid, A.; Ahmad, T. Economic Analysis of Citrus (Kinnow Mandarin) during on-year and off-year in the Punjab Province. Pakistan J Horticuture. 2018, 5(250), 2354–2376.
- Singh, S. K. Characterization of Kinnow Mandarin Fruit Juice Stored under Incubator. Ann Bio. 2018, 34(2), 126–129.
- USDA. 2019. USDA National Nutrient Database for Standard Reference. United States Department of Agriculture. Data accessed: 07/03/2021. http://www.ars.usda.gov/services/
- Musara, C.; Aladejana, E. B.; Mudyiwa, S. M. Review of the Nutritional Composition, Medicinal, Phytochemical and Pharmacological Properties of Citrus Reticulata Blanco (Rutaceae). F1000Research. 2020, 9(1387), 1387. DOI: 10.12688/f1000research.27208.1.
- Xu, G.; Liu, D.; Chen, J.; Ye, X.; Ma, Y.; Shi, J. Juice Components and Antioxidant Capacity of Citrus Varieties Cultivated in China. Food Chem. 2008, 106(2), 545–551. DOI: 10.1016/j.foodchem.2007.06.046.
- Purewal, S. S.; Sandhu, K. S. Nutritional Profile and Health Benefits of Kinnow: An Updated Review. Int. J. Fruit Sci. 2020, 20(sup3), 1–21. DOI: 10.1080/15538362.2020.1792390.
- Shah, N. N. A. K.; Supian, N. A. M.; Hussein, N. A. Disinfectant of Pummelo (Citrus Grandis L. Osbeck) Fruit Juice Using Gaseous Ozone. J. Food Sci. Technol. 2019, 56(1), 262–272. DOI: 10.1007/s13197-018-3486-2.
- Shah, N. N. A. K.; Sulaiman, A.; Sidek, N. S. M.; Supian, N. A. M. Quality Assessment of ozone-treated Citrus Fruit Juices. Int. Food Res. J. 2019, 26(5), 1405–1415.
- Porto, E.; Alves Filho, E. G.; Silva, L. M. A.; Fonteles, T. V.; Do Nascimento, R. B. R.; Fernandes, F. A.; Rodrigues, S.; Rodrigues, S. Ozone and Plasma Processing Effect on Green Coconut Water. Food Res. Int. 2020, 131, 109000. DOI: 10.1016/j.foodres.2020.109000.
- Karaca, H.; Velioglu, Y. S. Effects of Ozone Treatments on Microbial Quality and Some Chemical Properties of Lettuce, Spinach, and Parsley. Postharvest. Biol. Technol. 2014, 88, 46–53. DOI: 10.1016/j.postharvbio.2013.09.003.
- AOAC. Official Methods of Analysis of AOAC International. In Association of the Official Analytical Chemists, Gaithersburg 20th. 2016, 210-219.
- Saeeduddin, M.; Abid, M.; Jabbar, S.; Hu, B.; Hashim, M. M.; Khan, M. A.; Zeng, X.; Wu, T.; Zeng, X. Physicochemical Parameters, Bioactive Compounds and Microbial Quality of Sonicated Pear Juice. Int. J. Food Sci. Technol. 2016, 51(7), 1552–1559. DOI: 10.1111/ijfs.13124.
- Malik, F.; Nadeem, M.; Ainee, A.; Kanwal, R.; Sultan, M.; Iqbal, A.; Mahmoud, S. F.; Alshehry, G. A.; Al-jumayi, H. A.; Hassan, E., et al. Quality Evaluation of Lemon Cordial Stored at Different Times with Microwave Heating (Pasteurization). Nat. Prod. Res. 2022, 1–12. DOI:10.1080/14786419.2022.2092864.
- FDA. FDA Center for Food Safety and Applied Nutrition. In U.S. Food and Drug Administration Bacteriological Analytical Manual 8th ed.; U.S. Vol. 2. 2001.
- FDA. FDA Center for Food Safety and Applied Nutrition. In U.S. Food and Drug Administration Bacteriological Analytical Manual 8th ed.; U.S. 2001.
- Steel, R. G. D.; Torrie, J. H.; Dicky, D. A. Principles and Procedures of Statistics: A Biometrical Approaches 3rd; McGraw Hill Book Co. Inc: Singapore, 1997; pp 204–227.
- Aday, M. S.; Büyükcan, M. B.; Temizkan, R.; Caner, C. Role of Ozone Concentrations and Exposure Times in Extending Shelf Life of Strawberry. Ozone: Sci. Eng. 2014, 36(1), 43–56. DOI: 10.1080/01919512.2013.833851.
- Buluc, O.; Koyuncu, M. A. Effects of Intermittent Ozone Treatment on Postharvest Quality and Storage Life of Pomegranate. Ozone: Sci. Eng. 2020, 43(5), 1–9.
- Barboni, T.; Cannac, M.; Chiaramonti, N. Effect of Cold Storage and Ozone Treatment on Physicochemical Parameters, Soluble Sugars and Organic Acids in Actinidia Deliciosa. Food Chem. 2010, 121(4), 946–951. DOI: 10.1016/j.foodchem.2010.01.024.
- Alencar, E. R.; Faroni, L. R.; Pinto, M. S.; da Costa, A. R.; Carvalho, A. F. Effectiveness of Ozone on Postharvest Conservation of Pear (Pyrus Communis L.). J. Food Process. Technol. 2014, 5(4). DOI: 10.4172/2157-7110.1000317.
- Islam, M. A.; Ahmad, I.; Ahmed, S.; Sarker, A. Biochemical Composition and Shelf Life Study of Mixed Fruit Juice from Orange & Pineapple. J Environ Sci Nat Res. 2014, 7(1), 227–232.
- Lee, B. J.; Ting, A. S. Y.; Thoo, Y. Y. Impact of Ozone Treatment on the physico-chemical Properties, Bioactive Compounds, Pectin Methylesterase Activity and Microbiological Properties of Watermelon Juice. J. Food Sci. Technol. 2021, 59, 979–989.
- Jaramillo-Sánchez, G. M.; Garcia Loredo, A. B.; Gómez, P. L.; Alzamora, S. M. Ozone Processing of Peach Juice: Impact on Physicochemical Parameters, Color, and Viscosity. Ozone: Sci. Eng. 2018, 40(4), 305–312. DOI: 10.1080/01919512.2017.1417111.
- Arı, B.; Budak, N. H.; Seydim, A. C.; Güzel-Seydim, Z. Effects of Ozonation on Apple Juice Quality. Int. J. Fruit Sci. 2020, 20(sup3), S1570–S1578. DOI: 10.1080/15538362.2020.1822263.
- Mgaya‐Kilima, B.; Remberg, S. F.; Chove, B. E.; Wicklund, T. Influence of Storage Temperature and Time on the Physicochemical and Bioactive Properties of Roselle‐fruit Juice Blends in Plastic Bottle. Food Sci. Nutr. 2014, 2(2), 181–191. DOI: 10.1002/fsn3.97.
- Alothman, M.; Kaur, B.; Fazilah, A.; Bhat, R.; Karim, A. A. Ozone-induced Changes of Antioxidant Capacity of fresh-cut Tropical Fruits. Innovative Food Sci. Emerg. Technol. 2010, 11(4), 666–671. DOI: 10.1016/j.ifset.2010.08.008.
- Goffi, V.; Magri, A.; Botondi, R.; Petriccione, M. Response of Antioxidant System to Postharvest Ozone Treatment in ‘Soreli’kiwifruit. J. Sci. Food Agric. 2020, 100(3), 961–968. DOI: 10.1002/jsfa.10055.
- Rajashri, K.; Roopa, B. S.; Negi, P. S.; Rastogi, N. K. Effect of Ozone and Ultrasound Treatments on Polyphenol Content, Browning Enzyme Activities, and Shelf Life of Tender Coconut Water. J. Food Process. Preserv. 2020, 44(3), e14363. DOI: 10.1111/jfpp.14363.
- Yeoh, W. K.; Ali, A.; Forney, C. F. Effects of Ozone on Major Antioxidants and Microbial Populations of fresh-cut Papaya. Postharvest. Biol. Technol. 2014, 89, 56–58. DOI: 10.1016/j.postharvbio.2013.11.006.
- Piechowiak, T.; Antos, P.; Kosowski, P.; Skrobacz, K.; Józefczyk, R.; Balawejder, M. Impact of Ozonation Process on the Microbiological and Antioxidant Status of Raspberry (Rubus Ideaeus L.) Fruit during Storage at Room Temperature. Agri Food Sci. 2019, 28(1), 35–44. DOI: 10.23986/afsci.70291.
- Panigrahi, C.; Mishra, H. N.; De, S. Effect of Ozonation Parameters on Nutritional and Microbiological Quality of Sugarcane Juice. J. Food Process Eng. 2020, 43(11), e13542. DOI: 10.1111/jfpe.13542.
- Miller, F. A.; Silva, C. L.; Brandão, T. R. A Review on ozone-based Treatments for Fruit and Vegetables Preservation. Food Eng. Rev. 2013, 5(2), 77–106. DOI: 10.1007/s12393-013-9064-5.
- Garud, S. R.; Priyanka, B. S.; Rastogi, N. K.; Prakash, M.; Negi, P. S. Efficacy of Ozone and Lactic Acid as Nonthermal Hurdles for Preservation of Sugarcane Juice. Ozone: Sci. Eng. 2018, 40(3), 198–208. DOI: 10.1080/01919512.2017.1415802.