ABSTRACT
In this study, low-temperature plasma was used as an efficient pretreatment method of corn kernels (XY335), which developed and optimized a process of enzyme activity. The effects of plasma pretreatment power (300, 400, 500 W), plasma pretreatment time (30, 40, 50 s) and air flow rate (100, 150, 200 mL min−1) on enzyme activity during this process were investigated. Regression equations were established through a three-factor and three-level Box-Behnken design and used to evaluate the optimal conditions for the process. The optimal pretreatment conditions of POD enzyme activity were plasma pretreatment power of 500 W, plasma pretreatment time of 30 s and air flow rate of 150 mL min−1. The optimal pretreatment conditions of CAT enzyme activity were plasma pretreatment power of 350 W, plasma pretreatment time of 30 s and air flow rate of 150 mL min−1. The optimal pretreatment conditions of SOD enzyme activity were plasma pretreatment power of 400 W, plasma pretreatment time of 30 s and air flow rate of 200 mL min−1. Results showed that low-temperature plasma can be used effectively to increase the enzyme activity of corn kernels.
Introduction
Corn is one of the main food crops in China, its yield and quality are closely related to China’s growing grain demand, and it plays an important position in China’s agricultural production and national economy .[Citation1] Due to the high internal water of the newly harvested corn, it is easy to mold and deteriorate in the process of storage and transportation, which seriously affects the quality and brings great economic losses.[Citation2] Numerous studies have shown that the process of plant senescence is a process of dysmetabolism of their own reactive oxygen species. Peroxidase (POD), catalase (CAT) and superoxide dismutase (SOD) are the most important protective enzyme systems in plants, and also the most important reactive oxygen species clearance system.[Citation3–7] POD, CAT and SOD enzyme activities indirectly reflect the vitality of corn kernels. Improving the enzyme activity can improve the vitality of corn kernels and prolong the preservation period of corn kernels.
Low-temperature plasma is a non-thermal treatment technology.[Citation8] It has the advantages of high treatment efficiency, low energy consumption, wide application range and environmental protection,[Citation9,Citation10] and it is widely used in biomedicine, food science, agricultural production and other fields.[Citation11–15] The surface wetting properties of beans, lentils and wheat seeds were greatly changed after air plasma treatment. Air plasma treatment led to the dramatic decrease in the apparent contact angle. Moreover, the speed of germination and yield (germination rate) of seeds can be modified by preliminary plasma treatment.[Citation16,Citation17] The germination and growth of soybean seeds after RF plasma treatment were studied. It was found that plasma treatment had positive effects on seed germination and seedling growth. The seed germination rate, growth characteristics, soluble sugar content and protein content were all higher than the control group. Treatment of 80 W had the highest stimulatory effect.[Citation18] Zahoranova et al. studied the effects of a cold atmospheric pressure plasma treatment on the germination, production of biomass, vigor of seedlings, and uptake of water by wheat seeds. The study indicated that the germination rate, dry weight and vigor of seedlings significantly increased after plasma treatment from 20 to 50 s. The growth inhibition effect of CAPP on the surface microflora of wheat seeds increased with the increase in the treatment time .[Citation19]
This study showed that low plasma treatment could effectively improve the quality of peppers and shorten the drying time, and the antioxidant activity of peppers after plasma treatment at 30 s was the highest, and the content of red pigment was the highest.[Citation20] It was indicated that low plasma treatment under moderate conditions was effective for prolonging cherry storage and decreasing the decay rate, but no compromise on the cherry quality or only a slightly noticeable influence.[Citation21] Li Shuai et al. indicated that low-temperature plasma can effectively improve the corn kernels’ drying efficiency and greatly shorten the drying time.[Citation22,Citation23] The influence of low-temperature plasma pretreatment on the drying process of waxy vegetables was investigated. It was shown that low-temperature plasma pretreatment can effectively improve the drying efficiency of peppers, beans and peas and shorten the drying time. The best process parameters of these were different.[Citation13]
The main objective of this paper was to optimize the effect of process parameters, such as the plasma pretreatment power, plasma pretreatment time, and air flow rate. Response models were obtained from the experimental results in order to evaluate the optimum operational conditions for corn kernels enzyme activity.
Materials and methods
Materials
The corn kernels selected in this experiment were XY335, which were produced in Jilin Academy of Agricultural Sciences, China. It has high yield, stable yield, lodging resistance, moderate ripening period, and wide adaptability. Therefore, it has become the main corn variety in Jilin Province.
Equipment
The low-temperature plasma system (SY-DT02S, Ops Technology Co., Ltd., Su Zhou, China) was used in this study.[Citation2,Citation22] The samples were kept on the tray in the vacuum room (260 × 260 mm2). The vacuum room was degassed to a vacuum degree of 80 Pa using the vacuum pump. Samples were treated under a precursor gas flow of 150 mL min−1 and a frequency of 40 kHz.
Methods
At stable air temperature, 300 g sample, which was selected randomly from the laboratory samples and impurities were removed. After low plasma treatment, samples were crushed to powder by using a microplant grinder and packed in polypropylene bags and stored at 4°C until further use. On the basis of reference,[Citation24] according to the POD, CAT, and SOD kits provided by Nanjing Jiancheng Biology Company, each sample was tested three times in parallel, the average value was taken as the result.
Response surface modeling was used to optimize and establish the pretreatment conditions on corn kernels. Three independent variables, including low plasma pretreatment power (X1), low plasma pretreatment time (X2), and air flow rate (X3) were studied by Box-Behnken design. The responses were POD enzyme activity (Y1) and CAT enzyme activity (Y2) and SOD enzyme activity (Y3). The ranges and levels of the three independent variables are shown in .
Table 1. Levels of response surface design.
The results were statistically analyzed by “Design-Expert (V10.07),” a professional test program design and data processing software. After each group of tests were carried out according to the test scheme, the corresponding regression model and ANOVA were obtained. From the ANOVA, the significance of the regression equation, the significance of each factor, and the inaccuracy of the equation were obtained.
Results and discussion
Effect of variables on enzyme activity
The statistical results of POD, CAT and SOD enzyme activity are presented in .
Table 2. Response experimental results of enzyme activity.
The respective regression models are shown in .
Table 3. Regression models and ANOVA results.
Regression models and ANOVA
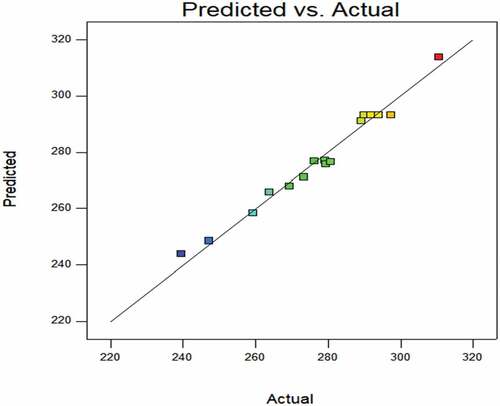
POD enzyme activity: According to , the P-value less than 0.0001 indicates the model is most significant. The regression model has a coefficient of determination (R2) of 0.9795 and RMSE of 2.5701. The good statistical indexes (R2 and RMSE) suggest the experimental data conform to the model.[Citation25,Citation26] The model has adjusted R2 of 0.9531 and Pred R2 of 0.7547, the difference is less than 0.2, indicating that the model can analyze and predict the change of the response index Y1 with different test conditions in a certain range.[Citation27] The significant terms in the model are X1 (P < .0001), X32 (P < .0001), X2 (P < .05), X1X2 (P < .05) and X12 (P < .05).
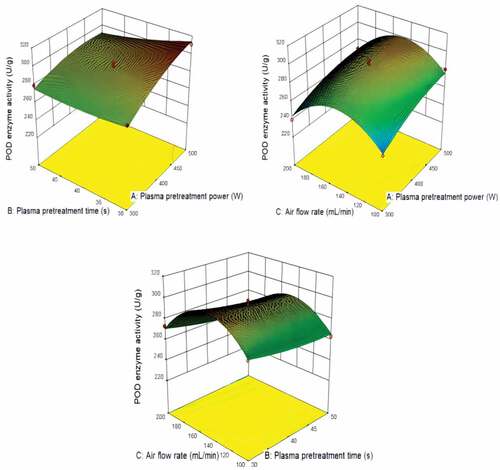
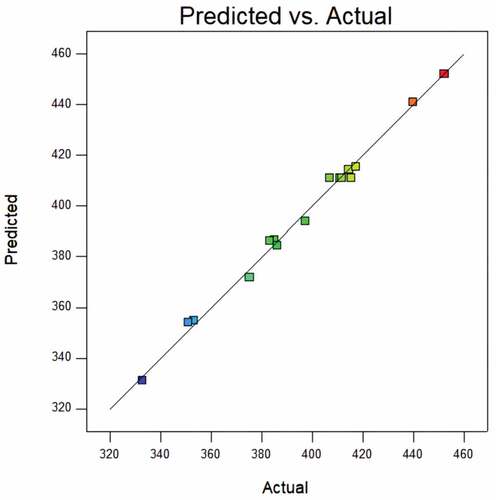
shows the predicted value from the model fit well with the experimental data, suggesting that the model could successfully predict the response value.[Citation28] The 3D interaction between plasma pretreatment power, plasma pretreatment time, and air flow rate is plotted in . In general, the plasma pretreatment power and time significantly affect POD enzyme activity, owing to the more severe etching on the corn surfaces after low plasma pretreatment .[Citation23] The air flow rate has less effect on POD enzyme activity.
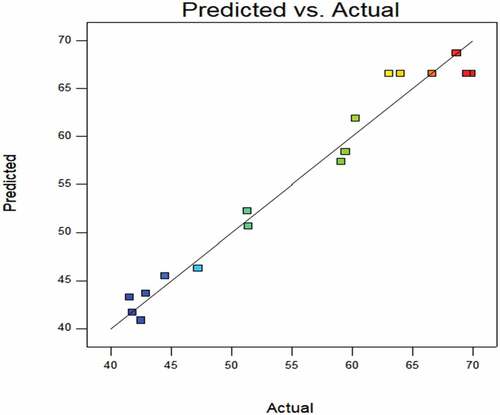
CAT enzyme activity: According to , the model is significant. The regression model has a coefficient of determination (R2) of 0.9697, and RMSE of 1.7962. The model has adjusted R2 of 0.9307 and Pred R2 of 0.8203, the difference is less than 0.2, indicating that the model can analyze and predict the change of the response index Y2 with different test conditions in a certain range.[Citation27] The significant terms in the model are X2 (P < .0001), X1 (P < .05), X1X2 (P < .05), X12 (P < .05), X22 (P < .05) and X32 (P < .05).
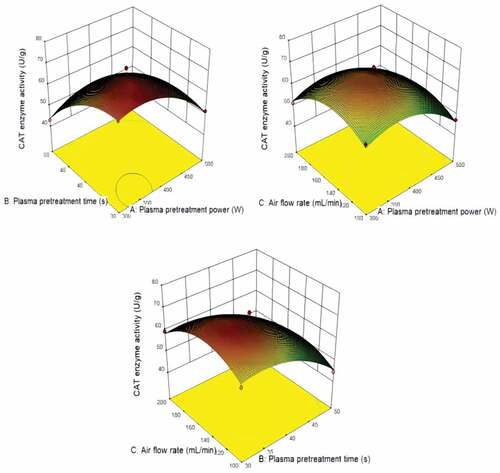
shows that the predicted value from the model fits well with the experimental data, suggesting that the model could successfully predict the response value.[Citation28] The 3D interaction between plasma pretreatment power, plasma pretreatment time, and air flow rate is plotted in . The plasma pretreatment time significantly affects CAT enzyme activity, the plasma pretreatment power also impacts. The air flow rate has less effect on CAT enzyme activity.
SOD enzyme activity: According to , the P-value less than 0.0001 indicates the model is most significant. The regression model has a coefficient of determination (R2) of 0.9940 and RMSE of 2.3587. The model has adjusted R2 of 0.9862 and Pred R2 of 0.9377, the difference is less than 0.2, indicating that the model can analyze and predict the change of the response index Y3 with different test conditions in a certain range.[Citation27] The significant terms in the model are X2(P < .0001), X1X2(P < .0001), X12(P < .0001), X32(P < .0001) and X1(P < .05).
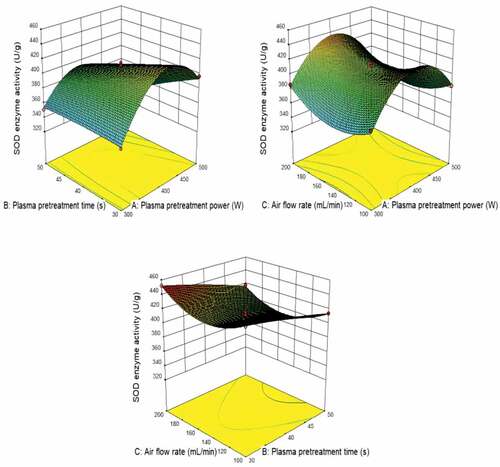
shows the predicted value from the model fit well with the experimental data, suggesting that the model could successfully predict the response value .[Citation28] The 3D interaction between plasma pretreatment power, plasma pretreatment time, and air flow rate is plotted in . The plasma pretreatment power significantly affects SOD enzyme activity, the plasma pretreatment time also impacts. The air flow rate has less effect on SOD enzyme activity.
Parameter optimization
To obtain high enzyme activity values within the experiment range, we performed a response surface optimization assay. The parameters were optimized by Design-Expert (V10.07) software to obtain the desirable experimental conditions. Regression coefficient (R2), adjusted R2 and RMSE related to these models are reported in . Based on the analysis results of the response surface, the optimal conditions of enzyme activity are presented in .
Table 4. Optimal combination verification test of POD enzyme activity.
Table 5. Optimal combination verification test of CAT enzyme activity.
Table 6. Optimal combination verification test of SOD enzyme activity.
shows the optimal pretreatment conditions of POD enzyme activity were plasma pretreatment power of 500 W, plasma pretreatment time of 30 s and air flow rate of 150 mL min−1. shows the optimal pretreatment conditions of CAT enzyme activity were plasma pretreatment power of 350 W, plasma pretreatment time of 30 s and air flow rate of 150 mL min−1. shows the optimal pretreatment conditions of SOD enzyme activity were plasma pretreatment power of 400 W, plasma pretreatment time of 30 s and air flow rate of 200 mL min−1. Under the optimal conditions, POD, CAT and SOD enzyme activity were 313.900 U/g, 71.061 U/g and 452.172 U/g, respectively.
Conclusion
Low-plasma pretreatment technology was employed to corn kernels, it was found that plasma pretreatment can effectively increase the enzyme activity of corn kernels. The effects of parameters were optimized using Design-Expert (V10.07) software. Results of response surface regression illustrated that low plasma pretreatment power, plasma pretreatment time and air flow rate were significantly correlated with enzyme activity. No significant difference was found between actual and predicted values. The optimal conditions of POD enzyme activity were plasma pretreatment power of 500 W, plasma pretreatment time of 30 s and air flow rate of 150 mL min−1. The optimal conditions of CAT enzyme activity were plasma pretreatment power of 350 W, plasma pretreatment time of 30 s and air flow rate of 150 mL min−1. The optimal conditions of SOD enzyme activity were plasma pretreatment power of 400 W, plasma pretreatment time of 30 s and air flow rate of 200 mL min−1.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Niu, L. K.;. Research on Integrated On-line Detector for Temperature Humidity and Grain Moisture Content of Granary Based on Differential Method; Jilin University: China, 2019.
- Li, S.;. Effects of Low-Temperature Plasma Pretreatment on Drying Kinetics and Storage Characteristics of Corn Kernels; Jilin University: China, 2020.
- Hodges, D. M.; Andrews, C. J.; Johnson, D. A.; Hamilton, R. I. Antioxidant Enzyme Responses to Chilling Stress in Differentially Sensitive Inbred Maize Lines. J. Exp. Bot. 1997, 48(310), 1105–1113. DOI: 10.1093/jxb/48.5.1105.
- Hu, T. T.; Yuan, L. N.; Wang, J. F.; Kang, S. Z.; Li, F. F. Antioxidation Responses of Maize Roots and Leaves to Partial root-zone Irrigation. Agric. Water Manage. 2010, 98(1), 164–171. DOI: 10.1016/j.agwat.2010.06.019.
- Apak, R.; Calokerinos, A.; Gorinstein, S.; Segundo, M. A.; Hibbert, D. B.; Gülçin, İ.; Demirci Çekiç, S.; Güçlü, K.; Özyürek, M.; Çelik, S. E., et al. Methods to Evaluate the Scavenging Activity of Antioxidants toward Reactive Oxygen and Nitrogen Species. Pure Appl. Chem. 2022, 94(1), 87–144.
- Köksal, Z.; Kalın, R.; Gülçin, İ.; Özdemir, H.; Atasever, A. Impact of Some Avermectins on Lactoperoxidase in Bovine Milk. Int. J. Food Prop. 2016, 19(6), 1207–1216. DOI: 10.1080/10942912.2015.1076457.
- Koksal, E.; Bursal, E.; Aggul, A. G.; Gulcin, I. Purification and Characterization of Peroxidase from Sweet Gourd (Cucurbita moschata Lam. Poiret). Int. J. Food Prop. 2012, 15(5), 1110–1119. DOI: 10.1080/10942912.2010.513216.
- Hamza, B. Effects of Hot Air, Vacuum Infrared, and Vacuum Microwave Dryers on the Drying Kinetics and Quality Characteristics of Orange Slices. J. Food Process Eng. 2020, 43(10), e13485.
- Lin, H. F.;. Study of Effect of Film Deposition by Low Temperature Plasma on Surface Electrical Properties of Epoxy Resin; North China Electric Power University: China, 2018.
- Qiu, J. X.; He, X.; Peng, F.; Dai, X.; Qiao, R. P. Research Progress in Plasma Technology in the Field of Environmental Protection. China Envr Protection Ind. 2020, 10, 63–67.
- Ehlbeck, J.; Schnabel, U.; Andrasch, M.; Stachowiak, J.; Stolz, N.; Frohling, A.; Schluter, O.; Weltmann, K. D. Plasma Treatment of Food. Contrib Plasma Phys. 2015, 55(10), 753–757. DOI: 10.1002/ctpp.201510013.
- Shen, X. J.; Li, J. R.; Liang, X.; Gu, J. L.; Zhou, W. Treatment of Formaldehyde- Containing Exhaust with Back Corona Enhanced non-thermal Plasma Technology. J Shenyang University of Technology 2020, 42(5), 515–519.
- Wu, W. F.; Jin, X.; Liu, Z.; Xu, Y.; Zhu, F. W.; Zhang, J. S. Effect of low Temperature Plasma Pretreatment on Drying Process of Vegetables with Waxy Layer. J. Food Process Eng. 2021, 44(12), 1–8. DOI: 10.1111/jfpe.13911.
- Nestler, K.; Böttger-Hiller, F.; Adamitzki, W.; Glowa, G.; Zeidler, H.; Schubert, A. Plasma Electrolytic Polishing-An Overview of Applied Technologies and Current Challenges to Extend the Polishable Material Range. Procedia CIRP. 2016, 42, 503–507. DOI: 10.1016/j.procir.2016.02.240.
- Han, G.; Chen, Q.; Kong, B. H. Recent Advances in Application of Cold Plasma Technology in Meat Preservation and Processing. Food Sci. 2019, 40(3), 286–292.
- Bormashenko, E.; Grynyov, R.; Bormashenko, Y.; Drori, E. Cold Radiofrequency Plasma Treatment Modifies Wettability and Germination Speed of Plant Seeds. Sci. Rep. 2012, 2(1), 741.
- Bormashenko, E.; Shapira, Y.; Grynyov, R.; Whyman, G.; Bormashenko, Y.; Drori, E. Interaction of Cold Radiofrequency Plasma with Seeds of Beans (Phaseolus Vulgaris). J. Exp. Bot. 2015, 66(13), 4013–4021.
- Li, L.; Jiang, J. F.; Li, J. G.; Shen, M. C.; He, X.; Shao, H. L.; Dong, Y. H. Effects of Cold Plasma Treatment on Seed Germination and Seedling Growth of Soybean. Sci. Rep. 2014, 4, 5859. DOI: 10.1038/srep05859.
- Zahoranová, A.; Henselová, M.; Hudecová, D.; Kaliňáková, B.; Kováčik, D.; Medvecká, V.; Černák, M. Effect of Cold Atmospheric Pressure Plasma on the Wheat Seedlings Vigor and on the Inactivation of Microorganisms on the Seeds Surface. Plasma Chem. Plasma Process. 2015, 36(2), 397–414.
- Zhang, X. L.; Zhong, C. S.; Mujumdar, A. S.; Yang, X. H.; Deng, L. Z.; Wang, J.; Xiao, H. W. Cold Plasma Pretreatment Enhances Drying Kinetics and Quality Attributes of Chili Pepper (Capsicum Annuum L.). J. Food Eng. 2019, 241, 51–57. DOI: 10.1016/j.jfoodeng.2018.08.002.
- Wu, X. R.; Zhao, W. Q.; Zeng, X. Y.; Zhang, Q. G.; Gao, G. T.; Song, S. J. Effects of Cold Plasma Treatment on Cherry Quality during Storage. Food Sci. Technol. Int. 2021, 27(5), 441–455. DOI: 10.1177/1082013220957134.
- Li, S.; Chen, S. S.; Liang, Q.; Ma, Z. S.; Han, F.; Xu, Y.; Jin, Y.; Wu, W. F. Low Temperature Plasma Pretreatment Enhances hot-air Drying Kinetics of Corn Kernels. J. Food Process Eng. 2019, 42(6). DOI: 10.1111/jfpe.13195.
- Li, S.; Chen, S. S.; Han, F.; Xu, Y.; Sun, H. M.; Ma, Z. S.; Chen, J. Y.; Wu, W. F. Development and Optimization of Cold Plasma Pretreatment for Drying on Corn Kernels. J. Food Sci. 2019, 84(8), 2181–2189. DOI: 10.1111/1750-3841.14708.
- Gu, Y.;. Effect of Application of Ferrihydrite on Growth and Antioxidative Enzyme Activities of Maize and Rice; Nanjing Agricultural University: China, 2018.
- Abasi, S.; Minaei, S.; Khoshtaghaza, M. H. Effect of Desiccant System on Thin Layer Drying Kinetics of Corn. J. Food Sci. Technol. 2017, 54(13), 4397–4404. DOI: 10.1007/s13197-017-2914-z.
- Khoshtaghaza, M. H.; Khojastehnazhand, M.; Mojaradi, B.; Goodarzi, M.; Saeys, W. Texture Quality Analysis of Rainbow Trout Using Hyperspectral Imaging Method. Int. J. Food Prop. 2015, 19(5), 974–983. DOI: 10.1080/10942912.2015.1042111.
- Sun, H. T.; Shao, X. R.; Jiang, R. P., Wang, S., Zhang, D. J., Ma, Z. S. Preparation and Physical Properties of Corn Distarch Phosphate/Corn Straw Cellulose Edible Film. Food Sci. 2016, 37(24), 21–28.
- Yuan, T. T.; Li, X. Q.; Chen, H. R. Process Optimization of Hot Air Drying Quality of Pepper Based on Geometrical Weighting Method. Acad. Period. Farm Produ. Proc. 2018, (9), 22–28.