?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
This study aimed to evaluate the physicochemical properties of okra residue obtained after pectin extraction from three okra genotypes (Asha, Balabi, and Agbagoma). The okra residue was oven-dried. Proximate analysis and functional properties were determined using standard AOAC methods, whereas mineral content was determined using atomic absorption spectroscopy. Phenolics and antioxidant capacity were determined using the Folin-Ciocalteu and DPPH methods. The okra pectin extraction residues were rich in carbohydrates (70.0–71.7%) and ash (19.55–21.9%), but had relatively low proteins (0.87–3.62%) and moisture (4.71–5.94%) contents. The okra residue samples had high potassium (8.59–9.27 mg/100 g) and sodium (.380–3.93 mg/100 g) contents. The solubility index for the varieties ranged from 18% to 25%, whiles swelling power ranged from 8% (Balabi) to 10% (Asha). The pectin extraction by-products showed high water absorption (546% to 617%) and oil absorption (216% to 318%) capacities. Residues from all okra genotypes demonstrated antioxidant activity (7.13–15.15%) and contained varied amounts of phenolic compounds (13.85–33.58 mg GAE/100 g). The results showed that okra residue obtained after pectin extraction has high nutritive and functional values, and could be exploited for other economic utilization instead of discarded as waste.
Introduction
Okra plant (Abelmoschus esculentus) originates from the Malvaceae family and is cultivated mainly for the pods (fruit). On a global scale, production of okra is around 9.96 million tonnes.[Citation1] Okra is considered as a multipurpose crop because of its various uses (fresh leaves, stems, pods, and seeds).[Citation2] The fibrous pods contain round, white seeds, and about 50 varieties of okra have been identified.[Citation3] The immature okra pod is used fresh, boiled, dried, canned, or frozen in soups and stews.[Citation4] Many food and non-food applications of okra have been reported. In its application in the food sector, flour produced from okra was used in bread production to increase dough resistance.[Citation5] The seed from okra are also considered as high protein oilseed crop and thus, it can be used to complement other sources of protein.[Citation6] Okra plays an essential role in diet because of its minerals and fiber content. The functional and nutritional properties of the fruit have awakened the interest of food and pharmaceutical industries to research on its potential uses.
The thick and slimy nature of okra water-extracts is due to its polysaccharide content.[Citation7] These extracts can be used as natural food-grade emulsifiers[Citation8] or emulsion stabilizers and thickeners.[Citation9] Pectin is a complex polysaccharide found in the primary cell wall of plants. It is made up of a high amount of galacturonic acid. Pectin extracts are employed as emulsifiers, gelling agents, stabilizers, and thickeners in food products, such as juices, milk drinks, ice cream, yogurt, and jam.[Citation10] The source or type of fruit (such as citrus fruits, apple, melon, etc.) and its availability are the main factors considered for the production of pectin. Thus, only sustainable sources can be considered for production of novel pectin. Commercial sources of pectin are from apple pulp (15–18%) and citrus peel (20–30%). Okra pectin yield ranged between 11% and 14% as previously reported.[Citation11]
Okra plant is considered as an economically important and sustainable vegetable that could be used as a potential source of pectin.[Citation3] Okra polysaccharides exhibit both foam stabilizing and emulsion properties. Pectin extracted from okra has been characterized for food and pharmaceutical applications.[Citation11] Okra pectin has been used as emulsifier or milk-fat substitute in chocolate.[Citation12]
However, a constraint in the production of pectin from okra is the volume of residue generated after the pectin extraction process. Unlike other pectin extracted from citrus peels and other plant peels that are considered waste materials, okra pectin is extracted directly from okra pods. As a result, significant amount of okra residue (~70%) is generated after pectin extraction and currently not utilized. Disposal of this residue will constitute food waste and thus contribute to food insecurity. Therefore, there is the need for effective directed utilization okra pectin residue for economic and social benefits. Agricultural wastes have been considered a good source of nutrients for mushroom production,[Citation13] and can be recycled as feed ingredients for fish and other livestock production.[Citation14] Hence, it is imperative to determine the characteristics of the residue obtained from okra after the pectin extraction process for other commercial and value addition purposes. The objective of this study, therefore, was to evaluate the physicochemical properties of okra residue obtained after pectin extraction from three okra genotypes.
Materials and methods
Materials
Three okra genotypes namely: Asha, Balabi, and Agbagoma (soft and ∼3 months matured okra pods of about 5–9 cm) were obtained from local farm at Ho in the Volta region of Ghana. All chemicals used were analytical-grade reagents. Distilled water was used throughout the extraction process.
Pectin extraction and residue preparation
A 5000 ml phosphate buffer solution (0.1 M) was prepared with 11.496 g of K2HPO4 and 59.063 g of KH2PO4 dissolved with a small amount of distilled water in separate beakers. The solutions were then transferred into a 5000 mL round bottom flask and topped up with distilled water to the 5000 ml mark, making sure a pH of 6 was maintained by adjusting the final solution with HCl.
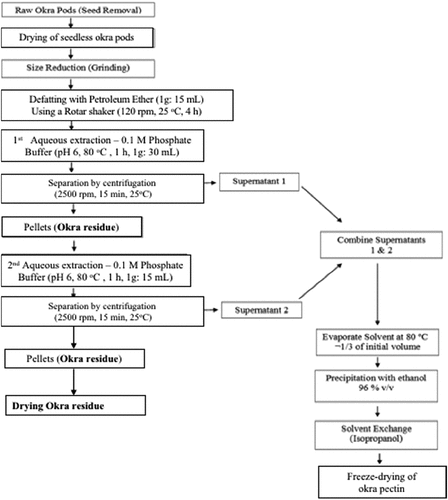
Pectin was isolated from three genotypes, Asha, Agbagoma, and Balabi using protocols as described by Kpodo et al.[Citation11] and illustrated in .
Briefly pectin was extracted from dried okra pods using phosphate buffer at pH 6.0. Following extraction, the pectin was precipitated using alcohol, dialyzed, and freeze-dried.[Citation11]
After the extraction of pectin from the milled okra pods, the pellets or residue obtained after sieving and centrifugation (as shown in ) were set aside and dried in a hot air oven (Gallenkamp, England) at a temperature of 40°C overnight. The dried residue for each okra genotype was stored in a refrigerator until further analysis.
Proximate analysis
Proximate composition (moisture, ash, and protein contents) was determined according to AOAC methods.[Citation15] Crude protein was calculated by multiplying the obtained nitrogen content by 6.25. The carbohydrate content was calculated by a difference, thus subtracting the percentage of crude protein, ash, moisture, and fat from 100. Acid insoluble ash was determined by igniting 2 g of each sample in a muffle furnace at 600 °C for 2 h. The ash was removed and cooled in a desiccator. Dilute HCl (25 mL; 10%) was added and filtered through a weighed fisher’s crucible and washed severally with distilled water. The residue in the crucible was oven dried at 105°C and ignited in a muffle furnace at 600 °C. The crucible was cooled in a desiccator and weighed. Acid insoluble ash was calculated from difference in masses expressed as percentage.
Minerals content
The amount of sodium, calcium, potassium, and magnesium in the okra residue was determined by Atomic Absorption Spectroscopy (Buck 210, Buck Scientific, USA). Five (5) grams of each sample was ignited at 600 °C in a muffle furnace and the resulting ash was dissolved in 10% (v/v) HCl and filtered through a filter paper and made up to the 100 mL mark using distilled water. The wavelength for the respective minerals were used to calculate their concentration[Citation16]
Functional properties
Swelling power and solubility index
The solubility and swelling power were determined as previously reported.[Citation16] One gram of the sample was weighed into a pre-weighed 50 mL centrifuge tube and 40 mL of distilled water was added. The suspension was vortexed and heated in a water bath for 30 min at constant temperature of 85 °C. The suspension was centrifuged using Hettich Zentrifugen D7200 centrifuge (Tuttlingen, Germany) at 2200 rpm for 15 min. The supernatant was decanted and placed in a weighed crucible and dried in a hot air oven to constant weight. The swollen granule was weighed. The swelling power and solubility were calculated using the equations below:
(%) Swelling Power =
(%) Solubility Index =
Water and oil absorption capacities
Water and oil absorption capacities were determined as previously reported.[Citation16] One (1) gram of the sample was weighed into a pre-weighed 15 mL centrifuge tube and 10 mL of distilled water/oil was added and vortex for 5 min. The suspension was centrifuged at 3500 rpm for 30 min using a centrifuge (model: Hettich Zentrifugen D7200, Tuttlingen, Germany). The volume of water/oil remaining was decanted and weighed. Water/Oil absorption capacity was calculated using the equation below:
(%) Water/Oil absorption capacity =
Total phenol and antioxidant determination
Total phenolic content
The Folin-Ciocalteu test was used to determine the total phenolic content of the okra residue using Gallic acid as standard.100 mL of deionized water was used to dissolve 0.5 g of the sample and filtered. 2 mL of the filtrate was pipetted into a test tube. The extract was mixed with Folin-Ciocalteu reagent and sodium carbonate and allowed to stand for 30 mins at room temperature. The absorbance of the mixture was measured using UV-Vis spectrophotometer (model: Mettler-Toledo GmbH Im Langacher 8606) at 760 nm. A calibration curve was constructed using Gallic acid solutions of concentrations 5, 10, 20, 40 and 50 ppm as standard reference. Total phenol content was calculated from calibration curve using Gallic acid equivalents (GAE) in milligrams (mg) per 100 g of the extract.
Antioxidant activity: The antioxidant activity was determined as described by Gemede et al.[Citation17] with some modifications. DPPH was used to determine free radical scavenging activity. 0.5 g of sample was weighed into 15 mL centrifuge tube and suspended with 10 ml of distilled water and centrifuged at 1000 rpm for 15 min. Sample (0.2 ml of the supernatant), 0.2 mL of distilled water, and 6 ml of 0.004% DPPH (1, 1-diphenyl-2-picrylhydrazyl) were obtained in a tube and shaken. The mixture was kept for 30 min at room temperature in the dark. Using a spectrophotometer (model: Lemfield Spectrulab 23A), absorbance of the reaction mixture and blank were read at 517 nm. The ability to scavenge the DDPH was calculated as:
DPPH radical scavenging activity (% inhibition) = [1-(As/Ao)] x 100
As is absorbance of sample. Ao is the absorbance of DPPH solution diluted to same volume of distilled water. Distilled water was used as blank.
Statistical analysis
The data were analyzed using one-way analysis of variance (ANOVA) to compare the means of sample characteristics. Differences between means were considered significant at p < .05. Tukey’s test was used to separate the mean values.
Results and discussion
Proximate composition of okra pectin residue
The proximate composition of the okra residue after pectin extraction is shown in . The moisture, ash, protein, acid insoluble ash, and carbohydrate content were determined. The acid insoluble ash ranged from 1.42 ± 0.52 to 2.36 ± 0.28. Agbagoma recorded the highest and the lowest recorded by Balabi. There was no significant difference (p > .05) between the varieties for acid insoluble ash.
Table 1. Proximate composition of okra residue after pectin extraction.
Proximate analysis is the basic test done on food to determine its nutritional composition. It is an important analysis, which can be used to detect adulterations and quality of a food product. Moisture content ranged from 4.71% to 5.94% and were within acceptable range. The moisture content of Asha residue was statistically the same (p > .05) as residues from Agbagoma, but different (p < .05) from Balabi. The recorded values were slightly higher than that reported[Citation14] for fruit wastes from pineapple (1.16–1.36%), jack fruit (1.62–2.41%), and grated coconut (2.59%) but lower than values obtained sweet orange peel (9.68%).[Citation18] Moisture content is an estimation of water content in a food product. It is an important parameter in powdered food samples because of its direct relation to the shelf life of the product. Samples with lower moisture content are more shelf stable. Food samples with 14% or less moisture content can resist microbial growth.[Citation19] Ash content is a measure of mineral content present in food. Ash content ranged from 19.55% to 21.99%, and increased in the order Asha >Agbagoma > Balabi. The ash content was high in all the three varieties which is an indication of high mineral content. This was different from a study by Gemede et al.,[Citation17] who recorded ash content of dried okra pods to be less than 12 g/100 g. Agbagoma and Asha were not significantly different from each other but Balabi differed significantly (p < .05). The high ash content observed can be as a result of heat treatment of the milled okra pods with phosphate buffer during pectin extraction.
Protein content was low in all the varieties and ranged from 0.87 ± 0.06 to 3.62 ± 0.30%. The protein content recorded was high in Asha and low in Agbagoma. The protein content of Agbagoma was significantly different (p < .05) from Asha and Balabi. Differences in the protein content can be attributed to varietal differences. Plant protein is said to contain a protein content of about 12% of its calorific value.[Citation20] The protein content from the residue was below this range and also lower than values reported for mango peel (6.3%).[Citation21]
The carbohydrate content of the residue was found to be 70.00 ± 0.62 for Asha variety, followed by Agbagoma (71.23 ± 0.35) and Balabi (71.74 ± 0.12) which recorded the highest value. Carbohydrate content was high in all the varieties and higher than values reported for fruit wastes from orange (58.62%), yellow passion (59.01%), and avocado (7.98%).[Citation22] This could be attributed to the fact that after pectin extraction the residue left is mainly fiber (non-starch carbohydrate). Agbagoma and Balabi were not significantly different (p > .05), but Asha was different from the other varieties.
The mineral composition of the three varieties of okra residue is shown in . Mineral composition is an important component in analyzing food quality, nutrition, and microbial viability. It is considered to be essential in human nutrition.[Citation23,Citation24] Sodium content in the study ranged from 3.80 to 3.93 mg/100 g for Asha and Balabi, respectively. There was no significant differences between the three varieties. Calcium is a mineral that aid the development of strong bones and teeth. The concentration of calcium varied from 0.14 to 0.19 mg/100 g with Asha having the highest calcium content and Balabi having the lowest. Potassium concentrations were very high compared to the other minerals. The potassium concentration ranged from 8.59 to 9.27 mg/100 g. This high potassium concentration can be a result of heating the sample with potassium phosphate buffer during the pectin extraction. Magnesium content also ranged from 0.002 to 0.003 mg/100 g. Asha had the highest magnesium content followed by Balabi and Agbagoma having the same magnesium content. There were no significant differences between the various varieties (p < .05). The mineral content of the okra residue, however, is comparatively lower than those of wheat-rain tree (Samanea saman) pod composite flours[Citation25] and palmyra palm (Borassus aethiopum) fruit flour[Citation26] and lima bean flours.[Citation27]
Table 2. Minerals content of okra residue.
The swelling power, solubility index, oil absorption capacity and water absorption capacity of the okra residue samples are presented in . Swelling power indicates the ability of a product to imbibe water and swell. It determines the tendency of a substance to be hydrated and is useful indicator for measuring food quality. The swelling power of Asha (10.82) was the highest and the lowest (8.15) was recorded by Balabi. Asha was significantly different from Balabi and Agbagoma (p < .05). The reported swelling capacity values were higher than values obtained for waste peels from avocado (4.36%), pineapple (7.57%), but lower than waste from yellow passion fruit (16.94%).[Citation22] Solubility index which indicates the degree of granules dispersion after cooking also ranged from 18.2% to 26.84%. The swelling power (765–882%) and solubility index (0.8 − 2.4%) reported for flour/starch samples from two new cassava accessions[Citation16] are comparatively higher and lower, respectively, for the okra residues in the present study.
Table 3. Functional properties of okra residue.
Swelling power and solubility index are mostly inversely related as apparent in the present results (). Components with stronger intermolecular interaction and higher hydrophobicity accounts greatly for greater swelling power and lower solubility.[Citation16] Samples/fours with low solubility indices alongside high swelling powers are suitable for making dough with high elasticity. Conversely, flours with low solubility and corresponding high swelling power could be utilized effectively as functional ingredients for pastry products.[Citation16]
Water absorption capacity (WAC) is the functional property of quantifying the amount of water retained by food.[Citation28] Water absorption capacity for all the three samples were higher than their respective oil absorption capacities. This could be as a result of the high carbohydrate content of the okra residue. Proteins and carbohydrates influence water absorption capacity due to the presence of hydrophilic groups.[Citation29] The WAC ranged between 546.17% and 617.87%, while oil absorption capacity (OAC) ranged from 216.74% to 318.49%. Balabi had the highest water absorption and oil absorption capacity while Agbagoma and Asha respectively recorded the least water absorption and oil absorption capacities. There were no significant differences between the water absorption capacity and oil absorption capacity for all the three varieties. WAC and OAC values of all okra residues in this study were higher than values obtained for peel wastes from avocado (6.33, 3.10%), pineapple (4.30, 1.90%), yellow passion fruit (9.63, 2.85%), and orange (6.33, 2.40%).[Citation22] The okra residues also had higher values in comparison to previous studies on okra seed flour, which had WAC of 504–511%, OAC of 88–159% and solubility index of 14–16%. The swelling power of the okra residues, however, was lower than those of the okra seed flour (10–14%).[Citation30] The difference in the hydrophilic components of the samples account for the variation in WAC and OAC. The higher the carbohydrate and protein contents, which are hydrophilic components, the lower the OAC and vice versa. Flours with lower OAC are known to have higher flavor retention abilities, whereas high OAC suggests that it could be useful in food formulation where oil-holding capacity is needed, such as sausage and bakery products.[Citation16]
The total phenolic content and antioxidant activity of the three different okra residues after pectin extraction are presented in . All the okra residues from the different genotypes demonstrated antioxidant activities and contained phenolic compounds in varied amounts. Antioxidant activity ranged from 7.13% to 15.15% with the least in Agbagoma and the highest in Balabi. The phenolic content was not significantly different between Asha and Balabi, however, the antioxidant activity among the three varieties was significantly different (). Previous studies have shown that higher phenolic content is associated with higher DPPH scavenging activity[Citation31] and thus the phenolic content in okra fruits possess significant antioxidant activity.[Citation32,Citation33] In the present study, total phenolic content was highest in Balabi (33.58 ± 8.98 mg GAE/100 g), followed by Asha (30.78 ± 5.42 ppm) and the lowest was recorded by Agbagoma (13.85 ± 0.76 mg GAE/100 g).
Table 4. Total phenolic content and antioxidant activity of the okra residue.
However, the total phenol content (28–31 mg GAE/100 g) and DPPH scavenging activity (36−74%) of okra seeds from different genotypes were previously reported[Citation34] and those of ackee fruit arils[Citation35] and watermelon seeds[Citation36] were higher than those of okra residue in the present study. It is worth to note that antioxidant activity is not limited to phenolic content but possibly due to other antioxidant compounds, such as vitamins A (beta-carotene, carotenoids), C (ascorbic acid), and E that may be present.
Conclusion
This study revealed that okra residue obtained after pectin extraction for all three genotypes had low moisture values and hence would be shelf stable. The proximate composition and functional properties were appreciable. The okra residues had high carbohydrate content and can thus serve as a source of fiber. Ash content was higher than and attributed to high mineral content specifically potassium that might have come from heat treatment with potassium phosphate buffer. All the okra residues from the different genotypes had water absorption capacity higher than their corresponding oil absorption capacity. The okra residues varied in total phenols content and demonstrated some antioxidant activity. The present findings indicate that okra residue could be used as potential ingredient in food and non-food products, such as animal feed.
Disclosure statement
No potential conflict of interest was reported by the authors.
Correction Statement
This article has been corrected with minor changes. These changes do not impact the academic content of the article.
References
- FAOSTAT (2020). Food and Agricultural Organization Statistics. https://www.fao.org/faostat/en/#data/QCL
- Yonas, M.; Garedew, W.; Debela, A. Multivariate Analysis among Okra (Abelmoschus Esculentus (L.) Moench) Collection in South Western Ethiopia. J. Plant Sci. 2014, 9(2), 43. DOI: 10.3923/jps.2014.43.50.
- Alba, K.; Ritzoulis, C.; Georgiadis, N.; Kontogiorgos, V. Okra Extracts as Emulsifiers for Acidic Emulsions. Food Res. Int. 2013, 54(2), 1730–1737. DOI: 10.1016/j.foodres.2013.09.051.
- Ndunguru, J.; Rajabu, A. C. Effect of Okra Mosaic Virus Disease on the Above- Ground Morphological Yield Components of Okra in Tanzania. Sci. Hortic. 2004, 99(3), 225–235. DOI: 10.1016/S0304-4238(03)00108-0.
- Acquistucci, R.; Francisci, R. Effect of Okra (Hibiscus Esculentus L.) Addition on the Technological Properties of a Wheat Flour. Int. J. Food Sci. Nutr. 2002, 53(5), 375–379. DOI: 10.1080/0963748021000044714.
- Bryant, L. A.; Montecalvo, J.; Morey, K. S.; Loy, B. Processing, Functional, and Nutritional Properties of Okra Seed Products. J. Food Sci. 1988, 53(3), 810–816. DOI: 10.1111/j.1365-2621.1988.tb08960.x.
- BeMiller, J. N.; Whistler, R. L.; Barbalow, D. G. Aloe, Chia, Flexseed, Okra, Psyllium Seed, Quince Seed, and Tamarind Gums. In Industrial Gums: Polysaccharide and Their Derivatives, 3rd ed.; Whistler, R. L., JN, B., Eds.; Academic Press: San Diago, Calif, 1993; pp 235–255.
- Ndjouenkeu, R.; Akingbala, J. O.; Oguntimein, G. B. Emulsifying Properties of Three African Food Hydrocolloids: Okra (Hibiscus Esculentus), Dika Nut (Irvingia Gabonensis), and Khan (Belschmiedia Sp.). Plant Foods Human Nutr. 1997, 51(3), 245–255. DOI: 10.1023/A:1007917608137.
- Georgiadis, N.; Ritzoulis, C.; Sioura, G.; Kornezou, P.; Vasiliadou, C.; Tsioptsias, C. Contribution of Okra Extracts to the Stability and Rheology of oil-in- Water Emulsions. Food Hydrocolloids. 2011, 25(5), 991–999. DOI: 10.1016/j.foodhyd.2010.09.014.
- Laurent, M. A.; Boulenguer, P. Stabilization Mechanism of Acid Dairy Drinks (ADD) Induced by Pectin. Food Hydrocolloids. 2003, 17(4), 445–454. DOI: 10.1016/S0268-005X(03)00028-6.
- Kpodo, F. M.; Agbenorhevi, J. K.; Alba, K.; Bingham, R. J.; Oduro, I. N.; Morris, G. A.; Kontogiorgos, V. Pectin Isolation and Characterization from Six Okra Genotypes. Food Hydrocolliods. 2017, 72, 323–330. DOI: 10.1016/j.foodhyd.2017.06.014.
- Datsomor, D. N.; Agbenorhevi, J. K.; Kpodo, F. M.; Oduro, I. N. Okra Pectin as Lecithin Substitute in Chocolate, Scientific African. 2019, 3, e00070 doi:10.1016/j.sciaf.2019.e00070.
- Wei, Q.; Zhong, X.; Haruna, M. H.; Lui, S.; Zhou, F.; Chen, M. (2022). Evaluation of Different Agricultural Waste for the Production of Polysaccharides from Oudemansiella Raphanipes and Its Antioxidant Properties.
- Sulaiman, M. A.; Yusoff, F. M.; Kamarudin, M. S.; Nurul Amin, S. M.; Kawata, Y. (2022). Fruit Waste Improved the Growth and Health of Hybrid Red Tilapia Oreochromis Sp. And Malaysain Mahseer, Tor tambroides(Bleeker,1854).
- AOAC. Official Methods of Analysis of the Association of Official Analytical Chemists (AOAC) International, 18th ed.; AOAC: Washington D. C., USA, 2005.
- Aidoo, R.; Oduro, I. N.; Agbenorhevi, J. K.; Ellis, W. O.; Pepra-Ameyaw, N. B. Physicochemical and Pasting Properties of Flour and Starch from Two New Cassava Accessions. Int. J. Food Prop. 2022, 25(1), 561–569. DOI: 10.1080/10942912.2022.2052087.
- Gemede, H. F.; Woldegiorgis, A. Z.; Woldegiorgis, A. Z.; Woldegiorgis, A. Z.; Rakshit, S. K. Proximate, Mineral, and Antinutrient Compositions of Indigenous Okra (Abelmoschus Esculentus) Pod Accessions: Implications for Mineral Bioavailability. Food Sci. Nutr. 2016, 4(2), 223–233. DOI: 10.1002/fsn3.282.
- Egbuonu, A. C. C.; Osuji, C. A. Proximate Compositions and Antibacteria Activity of Citrus Sinensis (Sweet Orange) Peel and Seed Extracts. European Journal of Medicinal Plants. 2016, 12(3), 1–7.
- Hayma, J.;. The Storage of Tropical Agricultural Products; Agromisa Foundation: Wageningen, Netherlands, 2003; pp 84.
- Aberoumand, A. A comparative study of nutrients and mineral molar ratios of some plant foods with recommended dietary allowances. Advanced Journal of Food Science and Technology. 2010, 2(2), 104–108.
- Sanchez-Camargo, A. P.; Gutierrez, L. F.; Vargas, S. M.; Martinez-Correa, H. A.; Parada-Alfonso, F.; Narvaez-Cuenca, C. E. 2021. Valorisation of Mango Peel: Proximate Composition, Supercritical Fluid Extraction of Carotenoids and Application as an Antioxidant Additive for an Edible Oil.
- Dias, P. G. I.; Sajiwanie, J. W. A.; Rathnayaka, R. M. U. S. K. (2020). Chemical Composition, Physicochemical and Technological Properties of Selected Fruit Peels as a Potential Food Source. S240–S251
- Ibanga, O. I.; Okon, D. E. Minerals and anti-nutrients in Two Varieties of African Pear (Dacryodes Edulis). J Food Technol. 2009, 7(4), 106–110.
- Valvi, S. R.; Rathod, V. S. Mineral Composition of Some Wild Edible Fruits from Kolhapur District. Int J Appl Biol Pharm. 2011, 2, 112–119.
- Amankwah, N. Y. A.; Agbenorhevi, J. K.; Rockson, M. A. Physicochemical and Functional Properties of wheat-rain Tree (Samanea Saman) Pod Composite Flours. Int. J. Food Prop. 2022, 25(1), 1317–1327. DOI: 10.1080/10942912.2022.2077367.
- Abe-Inge, V.; Arthur, C.; Agbenorhevi, J. K.; Kpodo, F. M. Mineral Composition, Antioxidant Properties, Phytochemical and anti-nutrient Composition of African Palmyra Palm (Borassus Aethiopum) Fruit Flour. Am. J. Food Nutr. 2018, 6(5), 143–152.
- Yellavila, S. B.; Agbenorhevi, J. K.; Asibuo, J. Y.; Sampson, G. O. Proximate Composition, Minerals content and Functional Properties of Five Lima Beans Accession. J Food Secur. 2015, 3(3), 69–74 doi:10.12691/jfs-3-3-1.
- Kohn, C. R.; Fontoura, A. M.; Kempka, A. P.; Demiate, I. M.; Kubota, E. H.; Preestes, R. C. Assessment of Different Methods for Determining the Capacity of Water Absorption of Ingredients and Additives Used in Meat Industry. Int. Food Res. J. 2015, 22, 356–36225.
- Kinsella, J. E. Functional Properties of Soy Protein. J. Am. Oil Chem. Soc. 1979, 56(3), 242–258.
- Ofori, J.; Tortoe, C.; Agbenorhevi, J. K. Physicochemical and Functional Properties of Dried Okra (Abelmoschus Esculentus L.) Seed Flour. Food Sci. Nutr. 2020, (2020)(8), 4291–4296. DOI: 10.1002/fsn3.1725.
- Kumar, S.; Sandhir, R.; Ojha, S. Evaluation of Antioxidant Activity and Total Phenol in Different Varieties of Lantana Camara Leaves. BMC Res. Notes. 2014, 7(1), 1–9. DOI: 10.1186/1756-0500-7-560.
- Petropoulos, S. A.; Fernandes, A.; Barros, L.; Ferreeira, I. C. F. R. Chemical Composition, Nutritional Value and Antioxidant Properties of Mediterranean Okra Genotypes in Relation to Harvest Stage. Food Chem. 2018, 242, 466–474. DOI: 10.1016/j.foodchem.2017.09.082.
- Shen, D. D.; Li, X.; Qin, Y. L.; Li, M. T.; Han, Q. H.; Zhou, J.; Wu, D. T.; Zhao, L.; Zhang, Q.; Qin, W. Physicochemical Properties, Phenolic Profiles, Antioxidant Capacities, and Inhibitory Effects on Digestive Enzymes of Okra (Abelmoschus Esculentus) Fruit at Different Maturation Stages. J. Food Sci. Technol. 2019, 56(3), 1275–1286. DOI: 10.1007/s13197-019-03592-1.
- Graham, J. O.; Agbenorhevi, J. K.; Kpodo, F. M. Total Phenol Content and Antioxidant Activity of Okra Seeds from Different Genotypes. Am. J. Food Nutr. 2017, 5(3), 90–94. DOI: 10.12691/ajfn-5-3-2.
- Dossou, V. M.; Agbenorhevi, J. K.; Combey, S.; Afi-Koryoe, S. Ackee (Blighia Sapida) Fruit Arils: Nutritional, Phytochemicals and Antioxidant Properties. Int. J. Nutr. Food Sci. 2014, 3(6), 534–537. DOI: 10.11648/j.ijnfs.20140306.17.
- Tabiri, B.; Agbenorhevi, J. K.; Wireko-Manu, F. D.; Ompouma, E. I. Watermelon Seeds as Food: Nutrient Composition, Phytochemicals and Antioxidant Activity. Int. J. Nutr. Food Sci. 2016, 5(2), 139–144. DOI: 10.11648/j.ijnfs.20160502.18.