ABSTRACT
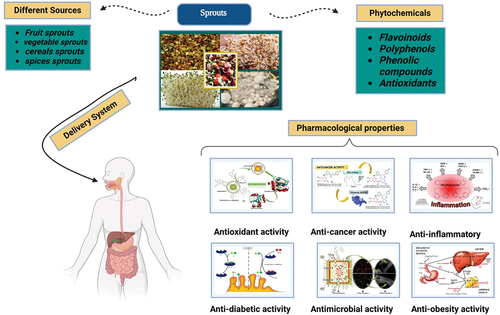
In this review, we discussed different types of sprouts, its antioxidant properties, extraction techniques, functional food application and health benefits. Sprouts are regarded as “functional foods.” It has additional health-regulating or disease prevention qualities in addition to their basic nutritional contents. There are different sources of sprouts including fruits (apricots and almonds), vegetables (broccoli, radish, and others), cereals (buckwheat, soybean, and barley), spices (fenugreek, ginger, turmeric and garlic). It is good way to improve the nutritional value of several foods that are frequently consumed worldwide. Sprouts is utilized in the production of food products. In addition to their nutritional worth, seeds and sprouts have health-promoting properties. Different type of phytochemicals is present in sprouts including a-tocopherol, isothiocyanates, indoles, b-carotene and vitamins. Conventional and Novel methods are used for the extraction of phytochemicals from different sprouts. Novel technologies are gaining more attraction due to its less extraction time and decreased consumption costs. Sprouts have pharmacological properties due to its rich source of bioactive compounds. These are helpful in GIT, anti-inflammatory, anti-hypertension, cardio-protective, neuro-protective, and anti-cancer properties.
Introduction
Sprouts are obtained from different types of plants parts including seeds, root, and shoot. Sprouts are enriched in bioactive compounds including polyphenols, antioxidants, and vitamins. Consumption of sprout is common in Asia which has recently gained favor in Western nations (especially in USA) These are frequently seen as a component of a healthy diet. [Citation1] Sprouts have gained a lot of interest in a society that is increasingly seeking optimal nutrition especially among consumers who are concerned about their health. Sprouts can be eaten raw or even cooked. The sprout can be preserved because microbial degradation limits the length of time. [Citation2,Citation3]
When compared to their corresponding seed, these sprouts are believed to be more nutritious and easier to digest. [Citation3] Sprouts are of interest as a food due to their quick and simple cultivation such as high sustainable production, customer perceptions of prices (health & freshness)and high concentration of bioactive substances, [Citation4] Sprouts are considered to be high phytochemicals as compared to their mature counterparts that help to promote health. In particular, sprouts of cruciferous plants (broccoli & radish) & legumes (alfalfa) are famous for the health-regulating phytochemicals including antioxidant qualities. [Citation5] It is believed that edible sprouts are full of phytochemicals that promote health and lower the risk of several chronic and degenerative diseases. [Citation6] Young sprouts had high levels of total phenolics & similarly increases antioxidant capacity but as plants grew older, both of these values rapidly decreased. [Citation7]
Sprouts are beneficial for health because it is rich source of phytochemicals, vitamins, minerals, and amino acids. [Citation8–10] It was anticipated that the total phenolic contents, anthocyanidin levels, antioxidant properties & total flavonoids of all colored wheat genotypes’ sprouts were higher than its grains. [Citation11] It has been demonstrated that sprouts of the radish plant contains a significant amount of pelargonidin. [Citation12] The recent study suggests that the sprouts of Triticum species may be useful for the development of functional foods because it contained high phenolic acid and antioxidants. [Citation13,Citation14] Despite the fact that sprouts are widely regarded as a health food. It is countable about the phytochemical composition of sprouts in contribution to their health-regulating features or about the variables that can impact their phytochemical composition. [Citation15,Citation16] The sprouts exposed to strong light or chilled have greater total phenolic content and antioxidant capacity as compared to untreated controls. [Citation7]
Citrus sprouts don’t seem to be suited for home production intended for direct consumption due to their delayed germination and harsh flavor, but these may have potential for extraction of food additives, cosmetics, and pharmaceuticals. [Citation17] Sprouting is a new trend in nutritious foods and nutraceuticals because it produces edible seedlings with higher phytochemical contents and lower antinutrient contents than seeds. [Citation18] Seeds like pomegranate and olive have lower total phenolic content as compared to sprouts. [Citation19,Citation20] Phytochemicals identification and rapid isolation is done by using hyphenated chromatographic and accelerated solvent extraction methods. [Citation21] Although traditional Soxhlet extraction takes a long time and uses a lot of solvent. The method is simple and cost-effective for extracts containing larger amounts of phytochemicals. [Citation22] The microwave-assisted acceleration (MAE) has been employed as an alternative to traditional procedures for the extraction of antioxidants because it can cut down on time and extraction solvent volume, [Citation23] The ultrasound-assisted extraction (UAE) has been used in a variety of food-processing applications to obtain bioactive compounds from plant materials, [Citation24]
Compared to the edible organs of adult plants, cruciferous sprouts (such as broccoli and red radish) are a rich source of phytochemicals that promote health. [Citation25] Significant amounts of phytochemicals present in fruits and vegetables which may offer protection against free radical damage. [Citation26] Scientists typically utilized a dried powder of plants to remove the interference of water and to extract bioactive compounds at the same time while extracting phytochemicals using a variety of solvents. [Citation27] The purpose of this review was to explore methods for extracting bioactive components from different types of sprouts to make functional foods and their role against certain various disorders.
Different sources of sprouts
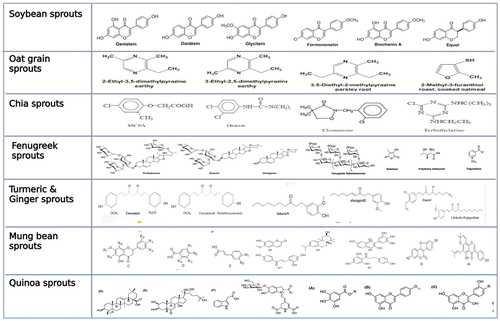
Sprouting is one of the methods used in the food industry to improve the nutritional qualities of cereal, oil seed, and vegetables seeds after they have germinated. [Citation28,Citation29] Additionally, it increases the digestibility and sensory qualities of sprouts, and decreases the levels of anti-nutritional components. According to epidemiological studies, regular intake of sprouts can lower the risk of developing chronic illnesses such as inflammatory bowel disease, arthritis, ischemic stroke, certain cancers, cardiovascular disease, and neurological diseases. [Citation30] Rice, mung beans, wheat, radish, cabbage, broccoli, soybean, sunflower and others are common sprouting seeds. [Citation31] Due to their increased concentrations of nitrogen-sulfur compounds (glucosinolates (GLSs), their derivatives, isothiocyanates (ITCs), and indoles), phenolic compounds (mainly flavonols, anthocyanins, and phenolic acids) and micronutrients, cruciferous types of sprouts and germinates (Brassicaceae, radish, broccoli, mustards, radishes, wasabi, and kale) stand out among the current. [Citation32] Different types of sprout as shown in .
Table 1. Phytochemical in different type of sprout sources.
Fruit sprouts
One of the most vital components of the human diet is fruit which is also a better source to stay healthy. A significant fruit crop grown worldwide is the apricot (Prunus armeniaca L.) which is a member of the Rosaceae family and contains between 2830–3100 species. Natural acids, carbohydrates, vitamins, phenolic compounds, and minerals are present in abundant quantity in fruit. [Citation52] The apricot kernel oil can also be used in the production of cosmetic products due to its high vitamin E and fatty acid content.The abrasive skin-cleansing material has developed by using apricot kernels. [Citation53]
Almonds are among the most widely produced and consumed nuts in the world., The almond demand has been increased for its consumption in recent years due to its physical, nutritional, chemical, and sensory attributes. Almonds are eaten as nuts or snacks (raw product) in the form of whole or sliced, peeled (without the skin) or not (with the skin). These nuts are still commonly used as ingredients in the preparation of bakery and confectionery items. They can also be eaten cooked or dry-roasted, powdered (such as flour), or as nonalcoholic beverages (such as almond “milk”). [Citation41]
Vegetable sprout
Today, various types of sprouts and sprouting seeds are available on the market including mung bean, pea, broccoli, radish, alfalfa, peanut, buck wheat, kale, soybean, and cabbage. [Citation54,Citation55] Mung bean and soybean sprouts are frequently utilized as a source of vitamins and protein. [Citation56,Citation57] Vitamin C, kaempferol, and total phenolics are present in abundant level in cabbage sprouts. [Citation33] The natural plant products have currently received a lot of attention in the food sector due to their potential to boost the immune system and their capacity to prevent most of the common diseases, [Citation58] In order to regulate the quality and nutritional content of foods, edible sprouts may be used in functional food products. The broccoli sprouts (Brassica oleracea L. var. italica) have been known as nutraceutical foods due to their abundant quantities of bioactive substances such as glucosinolates, polyphenols, carotenoids, minerals, and vitamins, [Citation59] These secondary metabolites are involved in the defense biological abilities including anti-diabetic, anti-carcinogenic, anti-inflammatory, and antioxidant characteristics. [Citation60]
Quinoa has been grown for a very long time and has special nutritional benefits. People can also consume quinoa sprouts as leafy vegetables. Quinoa (Chenopodium quinoa Willd.) is a beneficial dicotyledonous herbaceous plant that is edible and medicinal. Quinoa has a significant nutritional value due to its well-balanced mix of minerals, proteins, fibers, amino acids and trace elements (vitamins). [Citation57,Citation61] Sprouts of quinoa are quite valuable in terms of nutrition and functional value including high levels of total flavonoids and total phenolics. [Citation62] The alfalfa seed is also known as Lucerne. It is high value ingredient that use as animal feed. It is good source of proteins, vitamins, polyphenols, and many other nutritional elements. [Citation63] The radish is nutritional crop because its containhigh amount of proteins, flavonoids, and vitamins. [Citation32] The radish sprouts are also good source of bioactive compounds. Anthocyanins are primarily highly glycosylated and acylated forms of cyanidin that are abundant in radish, cabbages, kale and broccoli sprouts. [Citation64,Citation65] Anthocyanins have attracted more attention recently due to their potential to improve brain function and their role in control of disorders including diabetes and obesity. [Citation66]
Cereal sprouts
Cereals provide macronutrients(proteins, fats, and carbohydrates) micronutrients(vitamins and minerals) and non-nutrient food components (dietary fiber, bioactive substances, and phytochemicals) which are essential in the prevention and control of chronic diseases. [Citation67] Indeed, epidemiological research has shown that consuming whole grains regularly lowers the chance of developing type 2 diabetes and chronic cardiovascular diseases. [Citation68] The American States of Cereal Chemists (AACC) and the United States Department of Agriculture (USDA) have agreed on the following definition of “sprouted grains”: “Malt or sprouted grains including all of the original germ, endosperm, and bran should be considered whole grains as long as sprout growth does not exceed kernel length and nutrient values have not been diminished. These grains need to be labeled as whole grains that have been sprouted or malted. [Citation18] The Poaceae family of whole grains includes Oryzeae (rice variants), Aveneae (oats), Andropogoneae (maize and sorghum), minor grains like millet, as well as Triticeae (rye, wheat, barley, and triticale). Due to their nutritional resemblance to the Poaceae family, pseudocereals like amaranth, buckwheat, and quinoa are also classified as whole grains. [Citation18,Citation69]
Sprout consumption is very common worldwide. Common buckwheat sprouts (Fagopyrum esculentum Moench) is the one of the most popular edible buckwheat sprouts that have drawn a lot of interest due to their high concentrations of flavonoids, particularly orientin, vitexin, and rutin and their isomers. [Citation42,Citation43] Buckwheat sprout-rich diets have reportedly been shown to prevent and/or lessen ROS-related illnesses like inflammation and neurological problems. [Citation70] The buckwheat sprouts is composed of flavonoids, antioxidants and other bioactive compounds [Citation71] The content of various water-soluble vitamins including vitamins B1 and B6 are high in buckwheat sprouts whereas vitamin C are high in chickpeas, lupines, mung beans and soybean. The folate is high in green bean seeds and soybean sprouts. [Citation72] When it comes to the fat-soluble vitamins, germination can change the content of vitamin E in edible seeds like sprouted soybeans but this effect was not seen in other seeds. [Citation73] Sprouts can be used as a culinary element in a variety of food products including bakery products. The majority of researches have examined the effects of adding sprouted grain flour to various types of bread. The amount of flour added had a direct impact on the acceptability of the product. Although wheat grain sprout has received the greatest attention as compared to other grains sprouts including mung beans, quinoa, brown rice, and lentils. [Citation74] The multipurpose cereal barley (Hordeum vulgare L.) has a nutty aroma and a consistency similar to pasta. It has been observed that barely contains soluble dietary fiber β-glucans. The concentrations of β-glucans in barley and wheat flour is 1.75 and 5.12% respectively. The β-glucans is improved LDL cholesterol levels as well as lower blood sugar levels. The management of blood sugar levels and prevention of diabetes are two additional benefits of β-glucans. The barely is a better source of protein than wheat due more lysine (essential amino acid) contents. [Citation75] Additionally, sprouted grains are easier to digest than mature grains and have more readily available nutrients. The use of barley sprouts as an enhancing agent in certain baked goods has a longer history. The technological, nutritional, and sensory qualities of baked goods are improved when sprouted barley flour is combined with refined wheat flour. [Citation76] Oat grain (OG) contains unsaturated linoleic acids and oleic acids. This makes OG an interesting source of macronutrients. Oat proteins also contain important amino acids and dietary fiber particularly β-glucans (2–8.5%). [Citation48] Oats is good source of bioactive compounds including tocotrienols, flavonoids and tocopherols. Moreover, it is a particularly rich source of phenolic amides and avenanthramides with hydroxycinnamic acid moieties and anthranilic acid that have anti-oxidant, anti-inflammatory, and anti-proliferative properties. [Citation77,Citation78]
Spices sprouts
Aromatic herbs and spices have always played a significant role in human nutrition. These are used as a flavoring or coloring agents. These are rich source of bioactive compounds. They were utilized in ancient times not just in the food industry (taste and aromatized meals) but also in the medical industry. These have significant source of bioactive substances and also provide health benefits. [Citation79] In South Asia, fenugreek (Trigonella foenum-graecum) is a popular spice and vegetable crop. The fenugreek has produced in dry and semi-arid areas of developing nations. Additionally, it is frequently forage crop that utilized for medical purposes. [Citation80] Fenugreek is used only as a food additive to enhance seasoning and color. It has also been proposed as a dietary advantage due to its medicinal properties. The investigations revealed that it has antidiuretic or anticarcinogenic properties and is used in various medicinal applications. Additionally, it might have hypocholesterolemic, hypoglycemic, antibacterial, cell-reinforcing, gastric energizer, hepatoprotective, and antianorexic effects. [Citation81] Similarly, it is used as a food stabilizer, cement, and emulsifying specialist in modern food innovation due to its fiber, protein, and gum composition, [Citation82] Consumption of turmeric (Curcuma longa L.) and ginger (Zingiber officinale Roscoe) is increasing in the United States because of a rise in consumer interest in their therapeutic benefits. [Citation83] These are both referred to as “superfoods due to the significant concentration of bioactive substances and phytochemicals in both crops’,” There are more than 400 compounds in ginger that provide health benefits such as paradols, terpenoids, shogaols, and gingerols etc. [Citation50] Different compounds have been found in turmeric including phenolics and terpenoids (curcuminoids) with antiviral, antibacterial, antidiabetic, antioxidant, and anti-inflammatory activities. These rhizomes are also appealing supplements for food and beverage usage because of their distinctive flavor, color, and preservation properties. Typically, turmeric and ginger are multiplied via tissue-cultured transplants that yield little during their first crop cycle or by cuttings (10–100 g) of vegetative rhizomes. For commercial production, seed rhizomes have a few drawbacks such as uneven and sluggish sprouting which can have an impact on transplant growth and occasionally the final crop yield of plants in the ground or in containers. [Citation51]
Allium ursinum L. also known as ramson or wild garlic. It is a member of the Alliaceae family, which includes A. cepa L. as its most notable member. It is a popular food spice and a potent plant in traditional medicine. Ramson sprouts can be a significant functional food with a great potential for nutraceuticals due to all the evaluated quality and antioxidant indicators. Although ramson sprouts are less well-known in traditional medicine than the leaves, stems, and roots. They have intriguing qualities that make them a particularly healthy food spice. [Citation84,Citation85] This spices contains biologically active substances including thiosulphinates, alliins, polyphenols, flavonoids, steroid glycosides, lectins, polysaccharides, and fatty- and amino acids, come from this source. [Citation86]
Phytochemicals in different type of sprouts
Phytochemicals are constitutive metabolites that are helpful for humans and plants. They promote growth by inhibiting seed germination until a particular fertilization, time, control pollination, rhizosphere environment and provide protection against microorganisms [Citation87] The intake of sprouts is increasing worldwide because of their high nutrients contents and wide spread availability. [Citation88] Sprouts from particular Brassicaceae plants have been researched including antioxidant capacity [Citation7,Citation89–91] bioactive phytochemicals, [Citation92–95] and sensory quality related to consumers acceptance. [Citation96] One of the main antioxidant components of Brassica plants are phenolic compounds. [Citation90,Citation97] The broccoli is rich source of minerals and vitamins and also a nutritious vegetable. Its contained essential phytochemicals such as a-tocopherol, isothiocyanates, indoles and Beta-carotene. Besides, it is good source of flavonoids and other polyphenol. [Citation98] It is also good source of potassium, folic acid, iron, riboflavin (vitamin B2), vitamin A and C. [Citation99,Citation100] A research shows that sprout is beneficial for health due to presence of more phytochemicals like flavonoids, glucosinolate, isothiocyanates, minerals and vitamins [Citation93] Moreover, sprouts are good sources of pectin’s, sugars & amino acids. The consequences of this study shows that sprouts have high antioxidant level due to increase contents of L-ascorbic acid and polyphenols. [Citation101] Different structure of phytochemical of sprout as shown in .
Extraction of phytochemicals from sprouts
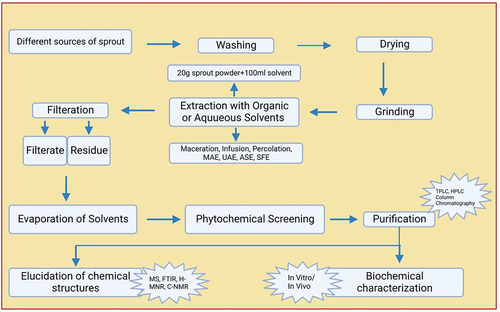
Plants are usually contain natural bioactive components. Hence, appropriate extraction methods are required for the extraction of the active compounds from plants. [Citation102] Different conventional methods (solvent extraction, maceration, soxhlet extraction, and hydro-distillation) and novel technologies (ultrasound-assisted extraction, enzyme associated extraction, microwave-assisted extraction, pulsed electric field extraction, emulsion liquid membrane extraction and supercritical fluid extraction) are used to extract bioactive compounds. The novel extraction technologies are better than traditional methods due to some control conditions including increased selectivity, increased yields, decreased solvent consumption, better energy efficiency, shorter processing time, and potential to control organic solvents. These are generally manufacture to be highly resistible, greener, and environment friendly. [Citation102] Various extraction methods may have various properties and compositions that used to obtain extracts. [Citation103,Citation104] Different extraction method of sprout extraction as shown .
Conventional methods
The maceration, distillation and soxhlet are conventional methods of extraction. [Citation102,Citation105] Most of them depend on use of agitation, temperature, extraction power of solvent, & type of solvent used. Fraiz Ritter Von Soxhlet discover the technology of Soxhlet extraction to extract fat/lipids. [Citation106] One of the oldest extraction methods (Soxhlet extraction) is used to obtain compounds from natural sources. Soxhlet technique is used for the separation of compounds which have medium to low volatility and thermally stable. [Citation107,Citation108] Another technique (Maceration) is used to extract plant materials by soaking plant materials in solvent at room temperature. [Citation102] The most accurate and efficient method than maceration is percolation as it is a continues technique in which the saturated solvent is continuously reestablished by freshly produced solvent. [Citation109] In the steam extraction process & hydro-distillation, volatile substances are transferred by water vapors. To obtain volatile compounds from sample, the water vapors penetrates in herbal cells. [Citation107,Citation110] To achieve extraction in industrial and semi-industrial perform, steam distillation up till now is an efficient process due to ease of economical instrumentation, great selectivity and methodology. [Citation111]
Novel technologies
Limitations linked with conventional technologies make their implementation mostly unprofitable and unsustainable. This is due to various circumstances such as long extraction intervals, decreased extraction yield, decomposition of heat-sensitive compounds, utilization of expensive solvents, polluting nature of solvents, lower purity of extracts, and rising uncertainty regarding their human safety. [Citation112,Citation113] Due to their ability efficiently restore value added materials as well as decreased energy power intake. However, novel techniques are attracting progressive interest by several food processing industries. Low temperature processing methods known as cold extraction are also commonly used. [Citation109] Nonionizing electromagnetic radiation such as microwave containing a frequency of 300 MHz – 300 GHz & wavelength 1 mm – 1 m. [Citation114] These rays are transferred through the system as waves and act thoroughly on the polar molecules including water, ionic conduction & dipole circulation to produce energy. MAE is used for extraction of several antioxidants & nutritional compounds from food matrix and waste. [Citation115] UAE green technology is employed for the extraction of biologically active components. This technology has shown an influence on several processes in chemical industries and food. The UAE may be beneficial due to short time, decreased solvent consumption, increased volume to the end product, improved work-up and operation, removed post-extraction treated waste water and higher extraction rates. [Citation112] The study shows that atmosphere-friendly separation technologies increase due to increased requirements for foods fortified with bioactive components. The best alternative to traditional methods are these novel techniques with less extraction time & decreased consumption costs. [Citation116]
Uses of sprouts in food applications
Sprouts are phytonutrient-rich vegetable foods that are a good source of proteins, minerals, vitamins, glucosinolates polyphenols, flavonoids, and isothiocyanates. A good way to improve the nutritional value of several foods that are frequently consumed around the world. Its sprouts utilized in the production of food products. [Citation74] Due to increased awareness of the nutritional and health benefits of sprouts, bakers, chefs, athletes, food manufacturers, and others are all looking for novel methods to include sprouts in common foods. [Citation117] Sprouts are regarded as “functional foods,” which are defined as foods with additional health-regulating or disease prevention qualities [Citation4] Although the process of germination of grains is old and well-known. It has currently drawn interest because of the rise in the nutritional and bioactive content of grains and the enhancement of their flavor. [Citation118] Sprouts can be used as a culinary component to improve the nutritional content of food products while also lowering the risk of contracting foodborne illnesses. However, it is important to understand the characteristics of these foods, their acceptance, and how the production process affects their nutritional value. Sprouts can supply beneficial bioactive compounds if they are regularly included in the diet. [Citation119]
Sprouts as an ingredient in food products is an intriguing way to improve the nutritional value of food. It is also a safer way to consume sprouts. Consumers have already shown a positive attitude toward both the germination procedure and the goods made from the germinated grains or their flours. [Citation120,Citation121] It should be mentioned that following sprouting, the functional and physicochemical characteristics of legume and cereal flour changed. [Citation122] Due to the increased demand for sprouts as a growing source of nutrients and beneficial secondary metabolites, the fresh product is gaining popularity not only in the gourmet culinary or specialized nutrition fields but also in the food sector. [Citation119] Study has been conducted to determine whether sprouted grains utilized in cooking or whether their flours can be used as alternatives to components with poor nutritional value, including wheat flour, which is commonly used in bakery goods. [Citation123] Sprouted wheat flour was used to replace the wheat flour in bread. The addition of sprouted flour slightly enhanced the overall protein content. Compared to control bread, bread that had been enhanced with germinated wheat flour had more resistant starch but less total starch. The result concluded that the investigated bread is recommended for particular consumer groups (obese and diabetic). [Citation124] Beaulieu et al. [Citation125] created a green process of free flow to soften, wet, mill, gelatinize, germinate, and subsequently saccharify brown rice in beverages. There were no fortifications, additives, or additions of oils, or salts in the developed “green” sprouted grain brown rice drink. The results conclude that brown rice beverages are similar possess qualities to the commercial samples. [Citation125] Extruded and sprouted amaranth and chia flour mixtures used to make functional beverages had a high protein and dietary fiber content as well as a high level of sensory acceptability. The functional beverages also had a strong anti-inflammatory and anti-hypertensive potential. [Citation126] Additionally, radish is well-known for its high nutritional value including antioxidants, polyphenols, and vitamins, as well as for its widely consumed sprouts. [Citation32]
The mung bean (Vigna radiata) seeds and sprouts are rich in nutrients that have biological functions. The mung bean is one of the most significant summer-growing, short-season legume that is widely cultivated in tropical and subtropical areas. Mung beans have numerous uses in the agricultural, health food, pharmaceutical, and cosmetics industries [Citation127] Quinoa sprouts have significant functional and nutritional benefits because its contain high levels of total flavonoids and total phenolic . Some people have discovered that quinoa leaves and sprouts can be used as functional food [Citation62] ().
Table 2. Food applications.
Pharmacological properties of sprouts
In the various countries several medicinal plants are being used for the treatment of various disorders. [Citation132,Citation133] Antioxidant compounds are obtained from plants or functional food plants extract that’s utilized anti-inflammatory, anti-tumor, anthelminthic, antimalarial, and antioxidant . [Citation134] Moreover, epidemiological research have identified that the intake of food enriched in bioactive compounds is linked with the inhibition of various psychopathic conditions such as cancer. [Citation135] Polyphenols and carotenoids are considered natural antioxidants that reduce the oxidation of proteins, nucleic acids and lipids The chances and development of colon cancer can be prevented with the help of bioactive compounds recognizes as an essential dietary supplement by scavenging unstable molecules that could develops carcinogenesis. [Citation136] Currently, cruciferous vegetables have gained significant attention as they represent remarkable source of health-maintaining phytochemicals (flavonoids, glucosinolates and phenolics) and nutrients (lipida, proteins, vitamins, minerals and carbohydrates) accountable for the cure and treatment of numerous disorders via various biological characteristics including antioxidant, anticancer, anti-inflammatory, antimicrobial, anti-diabetic and anti-obesity characteristics. [Citation137–140] Pharmacological effect of sprout against different diseases as shown in .
GIT related diseases
The human gut microbiota is a complicated and dynamic community comprising fungi, viruses and bacteria. Thus, regulating a healthy gut and maintaining the host defense system is necessary. Furthermore, any disturbance in microbial intake is associated with several metabolic diseases including obesity, colorectal cancer, diabetes mellitus and other disorders. [Citation141] Cruciferous plants contain isothiocyanates known as sulforaphane that is alsopresent in broccoli (Brassica oleracea var. italica) sprouts . [Citation142,Citation143] SFN exhibits pharmacological effects such as antioxidant, anti-inflammatory, anti-hypertension, cardio-protective, neuro-protective and anti-cancer properties. [Citation144–147] Brassica species is major source of antioxidant compounds including ascorbic acid, carotenoids (lutein and β-carotene), α-tocopherol, phenolic acids (caffeic acid, sinapic acid, ferulic acid, 3,4-di-hydroxybenzoic acid, and gallic acid), and flavonoids (quercetin, kaempferol and rutin) which can maintain the immune system by neutralizing free radicals. [Citation148–151] The medicines utilized for cancer treatment are dangerous & affect both normal cells & cancer cells. Therefore, it is important to utilized compounds obtained from natural sources to lowers cancer chances in numerous cancer types such as lung cancer, stomach cancer, chest cancer and colorectal cancer. The particular inhibited and anti-proliferative properties of Brassica vegetables seeds on tumor cells are most importantly for lung and colon cancers. .[Citation152,Citation153] Because their antioxidant capacity, flavonoids and phenolic have been shown to exhibit tumor action. [Citation154–156] Because, Brassica seeds shows a particular opportunity for the occurrence of drugs & natural antioxidant products. [Citation157] Mung bean seeds are grown by the flowering plant species Vigna radiata, recognized as green gram. [Citation158] Mung bean seeds are famous food legumes in India, Korea, Japan, China and other parts of Southeast Asian countries. It is famous for its capability to restore mental sharpness and minimize heat stroke, maintain gastrointestinal disturbance and detoxification. [Citation127] Isothiocyanates including indole-3-carbinol and sulforaphane are linked with increased detoxifying enzymes and work as antioxidants that lower the oxidative stress, [Citation92,Citation159,Citation160] Thus, it is identified that chronic inflammatory diseases cause an enhanced incidence of carcinogenesis. [Citation160,Citation161]
CVD related diseases
Current research conducted by Tong et al. [Citation162] who demonstrated that wheat bran arabinoxylans decrease the LDL-cholesterol & overall-plasma ratios in hypercholestremic hamsters supplied with increased-cholesterol diet including 0.5% arabinoxylans. Furthermore, results shown that arabinoxylans imparted to cholesterol adsorption reduction in the intestines also include augmentation of bile acid excretion. This experiment also shows that high consumption of arabinoxylan is one of the best reason to reduce cardiovascular disorders. Phytosterols is one of the important method by which cholesterol lowers in the body due to lowering adsorption of cholesterol. Cholesterol and plant sterols both of them are immiscible in water. It is necessary to homogenize in the occurrence of bile acids to develop micelles that can be inhibited by small intestinal cells. [Citation163] Atherosclerotic cardiovascular disorder (ASCVD) establishment is a complicated process that involves vascular remodeling and chronic inflammation linked with the breakdown of the structural and functional properties of the endothelium. Typical conditions linked to atherosclerosis including diabetes, hyperlipidemia, smoking and hypertension that also enhanced or premature aging. [Citation164–166] Cardio metabolic and diabetes disorders including obesity and hypertension are main risk factors for the occurrence of cardiovascular disorders. [Citation167] Obesity significantly raises the chance of hypertension, cardiovascular and cerebrovascular disorders, diabetes, osteoarthritis and cancer. [Citation168] Hence, obesity can be effectively controlled by enhancing lipolysis and reducing fat formation. [Citation169]
Other diseases
Diabetes is one of the biggest reason for mortality & morbidity worldwide. Various experimental studies have found the potential hypoglycemic properties of seeds of the Brassica genus. Brassica seed extracts have been studies in animals. [Citation170] The intake of padding fortified with soluble dietary fiber (DF) from mustard seed mucilages dramatically reduce the blood glucose at particular post-meal durations in adults. [Citation171] Obesity, T2D, dyslipidemia and hypertension are general metabolic syndrome disease common all around the globe. [Citation172] The moderating actions of Brassica seeds and their derivatives on metabolic syndrome have been demonstrated in several researches. Neurodegenerative and psychological diseases such as anxiety, autism spectrum disease (Alzheimer’s, Huntington’s, schizophrenia and depression) which expresses mitochondrial dysfunction, inflammation, neural damage and oxidative stress. [Citation173] Recent study has demonstrated the neuroprotective effects of Brassica seeds and their derivatives in humans. [Citation174] It has been found that wheat bran powder is frequently use in beauty products [Citation175] analyzed melisma linked with hyperpigmentation disease. Moreover, wheat sprout extract also has a particular function in treatment of atopic dermatitis. [Citation176]
Conclusion and future perspectives
It is concluded that different types of sprout are contained various bioactive compounds such as vitamins, minerals, polyphenols & flavonoids. These bioactive compounds are extruded from sprout by using the extraction methods including (conventional & novel). The benefits of novel technologies that give more and sustainable production. These extract in the form of powder & extract added in different food products to increase its function. However, sprouts are valuable in different diseases including GIT and CVD related, also helpful in skin problems. Sprouts’ potential involvement in the prevention and treatment of chronic disorders has to be investigated further. The functional profile of sprouts, which is high in antioxidants, may provide extra anti-oxidant properties. Sprouts, being a fiber-rich food, is likely to provide a feeling of fullness. It’s also worth remembering that in order for functional foods to offer their possibly subtle advantages, they must be consumed on a regular basis. To prove that sprout foods have potentially beneficial effects on chronic lifestyle-related disorders or risk factors, rigorous scientific investigation in human studies is required.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Mart´ınez-Villaluenga, C.; Fr´ıas, J.; Gulewicz, P.; Gulewicz, K.; VidalValverde, C. Food Safety Evaluation of Broccoli and Radish Sprouts. Food Chem. Toxicol. 2008, 46, 1635–1644.
- Aloo, S. O.; Ofosu, F. K.; Oh, D. H. Effect of Germination on Alfalfa and Buckwheat: Phytochemical Profiling by UHPLC-ESI-QTOFMS/MS, Bioactive Compounds, and in-Vitro Studies of Their Diabetes and Obesity-Related Functions. Antioxidants. 2021, 10, 1613. DOI: 10.3390/antiox10101613.
- Penãs, E.; Martínez-Villaluenga, C. Advances in Production, Properties and Applications of Sprouted Seeds. Foods. 2020, 9(6), 790. DOI: 10.3390/foods9060790.
- Reed, E.; Ferreira, C. M.; Bell, R.; Brown, E. W.; Zheng, J.; Schaffner, D. W. Plant-microbe and Abiotic Factors Influencing Salmonella Survival and Growth on Alfalfa Sprouts and Swiss Chard Microgreens. Appl. Environ. Microbiol. 2018, 84(9), 1–11. DOI: 10.1128/AEM.02814-17.
- Hesterman, O. O. B.; Teuber, L. R.; Livingston, A. L. Effect of Environment and Genotype on Alfalfa Sprout Production 1. Crop Sci. 1981, 21(5), 720–726. DOI: 10.2135/cropsci1981.0011183X002100050023x.
- Zielinski, H.; Piskuta, M. K.; Michalska, A. Kozlowska H, Antioxidant ´ Capacity and Its Components of Cruciferous Sprouts. Pol. J. Food Nutr. Sci. 2007, 57, 315–322.
- Oh, M. M.; Rajashekar, C. B. Antioxidant Content of Edible Sprouts: Effects of Environmental Shocks. J. Sci. Food Agric. 2009, 89(13), 2221–2227. DOI: 10.1002/jsfa.3711.
- Finley, J. W.;. Proposed Criteria for Assessing the Efficacy of Cancer Reduction by Plant Foods Enriched in Carotenoids, Glucosinolates, Polyphenols and Selenocompounds. Ann. Bot 2005, 95, 1075–1096. DOI: 10.1093/aob/mci123.
- Schenker, S.;. Facts behind the Headlines, Broccoli, British Nutrition Foundation. Nutr. Bull. 2002, 159–160.
- Webb, G. P.;. Dietary Supplements and Functional Foods; Blackwell Publishing Ltd: Oxford, 2006; pp 1–120.
- Sytar, O.; Bośko, P.; Živčák, M.; Brestic, M.; Smetanska, I. Bioactive Phytochemicals and Antioxidant Properties of the Grains and Sprouts of Colored Wheat Genotypes. Molecules. 2018, 23(9), 2282. DOI: 10.3390/molecules23092282.
- Hanlon, P. R.; Barnes, D. M. Phytochemical Composition and Biological Activity of 8 Varieties of Radish (Raphanus Sativus L.) Sprouts and Mature Taproots. Food Sci. 2011, 76, 185–192. DOI: 10.1111/j.1750-3841.2010.01972.x.
- Cevallos-Casals, B. A.; Cisneros-Zevallos, L. Impact of Germination on Phenolic Content and Antioxidant Activity of 13 Edible Seed Species. Food Chem. 2010, 119, 1485–1490. DOI: 10.1016/j.foodchem.2009.09.030.
- Benincasa, P.; Galieni, A.; Manetta, A. C.; Pace, R.; Guiducci, M.; Pisante, M.; Stagnari, F. Phenolic Compounds in Grains, Sprouts and Wheatgrass of Hulled and non-hulled Wheat Species. J. Sci. Food Agric. 2015, 95, 1795–1803.
- Dixon, R. A.; Paiva, N. L. Stress-induced Phenylpropanoid Metabolism. Plant Cell. 1995, 7, 1085–1097. DOI: 10.2307/3870059.
- Zobayed, S. M. A.; Afreen, F.; Kozai, T. Phytochemical and Physiological Changes in the Leaves of St John’s Wort Plants under a Water Stress Condition. Environ. Exp. Bot. 2007, 59(2), 109–116. DOI: 10.1016/j.envexpbot.2005.10.002.
- Falcinelli, B.; Famiani, F.; Paoletti, A.; D’Egidio, S.; Stagnari, F.; Galieni, A.; Benincasa, P. Phenolic Compounds and Antioxidant Activity of Sprouts from Seeds of Citrus Species. Agriculture. 2020, 10(2), 33. DOI: 10.3390/agriculture10020033.
- Benincasa, P.; Falcinelli, B.; Lutts, S.; Stagnari, F.; Galieni, A. Sprouted Grains: A Comprehensive Review. Nutrients. 2019, 11, 421. DOI: 10.3390/nu11020421.
- Falcinelli, B.; Marconi, O.; Maranghi, S.; Lutts, S.; Rosati, A.; Famiani, F.; Benincasa, P. Effect of Genotype on the Sprouting of Pomegranate (Punicagranatum L.) Seeds as a Source of Phenolic Compounds from Juice Industry by-products. Plant. Food Hum. Nutr. 2017, 72, 432–438. DOI: 10.1007/s11130-017-0645-y.
- Falcinelli, B.; Maranghi, S.; Paoletti, A.; Marconi, O.; Rosati, A.; Famiani, F.; Benincasa, P. Sprouting Olive (Oleaeuropaea, L.) Seeds as a Source of Antioxidants from Residual Whole Stones. Sci. Hortic. 2018, 240, 558–560. DOI: 10.1016/j.scienta.2018.06.066.
- Pellegrini, N.; Chiavaro, E.; Gardana, C.; Mazzeo, T.; Contino, D.; Gallo, M.; Riso, P.; Fogliano, V.; Porrini, M. Effect of Different Cooking Methods on Color, Phytochemical Concentration, and Antioxidant Capacity of Raw and Frozen Brassica Vegetables. J. Agric. Food Chem. 2010, 58, 4310–4321. DOI: 10.1021/jf904306r.
- Koubaa, M.; Roselló-Soto, E.; Šic Žlabur, J.; Režek Jambrak, A.; Brnčić, M.; Grimi, N.; Boussetta, N.; Barba, F. J. Current and New Insights in the Sustainable and Green Recovery of Nutritionally Valuable Compounds from Stevia Rebaudiana Bertoni. J. Agric. Food Chem. 2015, 63, 6835–6846. DOI: 10.1021/acs.jafc.5b01994.
- Suzara, S.; Costa, D. A.; Gariepyb, Y.; Rochaa, S. C. S.; Raghavanb, V. Spilanthol Extraction Using Microwave: Calibration Curve for Gas Chromatography. Chem. Eng. Trans. 2013, 32, 1783–1788.
- Williams, O. J.; Raghavan, G. S. V.; Orsat, V.; Dai, J. Microwave-assisted Extraction of Capsaicinoids from Capsicum Fruit. J. Food Biochem. 2004, 28, 113–122. DOI: 10.1111/j.1745-4514.2004.tb00059.x.
- Wagner, A. E.; Terschluesen, A. M.; Rimbach, G. Health Promoting Effects of Brassica-derived Phytochemicals: From Chemopreventive and Antiinflammatory Activities to Epigenetic Regulation. Oxid. Med. Cell Longev. 2013, 2013, 1–12. 12 Article ID 964539 DOI: 10.1155/2013/964539.
- Suffredini, I. B.; Sader, H. S.; Gonçalves, A. G.; Reis, A. O.; Gales, A. C.; Varella, A. D.; Younes, R. N. Screening of Antibacterial Extracts from Plants Native to the Brazilian Amazon Rain Forest and Atlantic Forest. Braz. J. Med. Biol. Res. 2004, 37(3), 379–384. DOI: 10.1590/S0100-879X2004000300015.
- Koffi, E.; Sea, T.; Dodehe, Y.; Soro, S. Effect of Solvent Type on Extraction of Polyphenols from Twenty Three Ivorian Plants. J. Anim. Plant Sci. 2010, 5, 550–558.
- Zhang, C.; Zhao, Z.; Yang, G.; Shi, Y.; Zhang, Y.; Xia, X.; Shi, C. Effect of Slightly Acidic Electrolyzed Water on Natural Salmonella Reduction and Seed Germination in the Production of Alfalfa Sprouts. Food Microbiol. 2020.
- Šamec, D.; Pavlović, I.; Redovniković, I. R.; Salopek-Sondi, B. Comparative Analysis of Phytochemicals and Activity of Endogenous Enzymes Associated with Their Stability, Bioavailability and Food Quality in Five Brassicaceae Sprouts. Food Chem. 2018, 269, 96–102. DOI: 10.1016/j.foodchem.2018.06.133.
- Mir, S. A.; Farooq, S.; Shah, M. A.; Sofi, S. A.; Dar, B. N.; Hamdani, A. M.; Khaneghah, A. M. An Overview of Sprouts Nutritional Properties, Pathogens and Decontamination Technologies. LWT. 2021, 141, 110900. DOI: 10.1016/j.lwt.2021.110900.
- De la Fuente, B.; López-García, G.; Mañez, V.; Alegría, A.; Barberá, R.; Cilla, A. Evaluation of the Bioaccessibility of Antioxidant Bioactive Compounds and Minerals of Four Genotypes of Brassicaceae Microgreens. Foods. 2019, 8, 250. DOI: 10.3390/foods8070250.
- Baenas, N.; Gómez-Jodar, I.; Moreno, D. A.; García-Viguera, C.; Periago, P. M. Broccoli and Radish Sprouts are Safe and Rich in Bioactive Phytochemicals. Postharvest Boil. Technol. 2017, 127, 60–67. DOI: 10.1016/j.postharvbio.2017.01.010.
- Sola, I.; Vujci´c Bok, V.; Pinteri´c, M.; Auer, S.; Ludwig-Müller, J.; Rusak, G. Improving the Phytochemical Profile and Bioactivity of Chinese Cabbage Sprouts by Interspecific Transfer of Metabolites. Food Res. Int. 2020, 137, 109726. DOI: 10.1016/j.foodres.2020.109726.
- Di Bella, M. C.; Niklas, A.; Toscano, S.; Picchi, V.; Romano, D.; Lo Scalzo, R.; Branca, F. Morphometric Characteristics, Polyphenols and Ascorbic Acid Variation in Brassica Oleracea L. Novel Foods: Sprouts, Microgreens and Baby Leaves. Agronomy. 2020, 10(6), 782. DOI: 10.3390/agronomy10060782.
- Neugart, S.; Baldermann, S.; Hanschen, F. S.; Klopsch, R.; Wiesner-Reinhold, M.; Schreiner, M. The Intrinsic Quality of Brassicaceous Vegetables: How Secondary Plant Metabolites are Affected by Genetic, Environmental, and Agronomic Factors. Sci. Hortic. 2018, 233, 460–478. DOI: 10.1016/j.scienta.2017.12.038.
- Lenzi, A.; Orlandini, A.; Bulgari, R.; Ferrante, A.; Bruschi, P. Antioxidant and Mineral Composition of Three Wild Leafy Species: A Comparison between Microgreens and Baby Greens. Foods. 2019, 8(10), 487. DOI: 10.3390/foods8100487.
- Bantis, F.;. Light Spectrum Differentially Affects the Yield and Phytochemical Content of Microgreen Vegetables in a Plant Factory. Plants. 2021, 10(10), 2182. DOI: 10.3390/plants10102182.
- Hasib, A.; Jaouad, A.; Mahrouz, M.; Khouili, M. Hplc Determination of Organic Acids in Moroccan Apricot Determinación Por Hplc de Ácidos Orgánicos En Albaricoque Marroquí Determinación Por Hplc de Ácidos Orgánicos En Albaricoque Marroquí. CYTA- J. Food Sci. 2002, 3(4), 207–211.
- De La Lastra, C. A.; Villegas, I. Resveratrol as an anti-inflammatory and anti-aging Agent: Mechanisms and Clinical Implications. Mol. Nutr. Food Res. 2005, 49(5), 405–430. DOI: 10.1002/mnfr.200500022.
- Wang, K. H.; Lai, Y. H.; Chang, J. C.; Ko, T. F.; Shyu, S. L.; Chiou, R. Y. Y. Germination of Peanut Kernels to Enhance Resveratrol Biosynthesis and Prepare Sprouts as a Functional Vegetable. J. Agric. Food Chem. 2005, 53(2), 242–246. DOI: 10.1021/jf048804b.
- Prgomet, I.; Gonçalves, B.; Domínguez-Perles, R.; Pascual-Seva, N.; Barros, A. Valorization Challenges to Almond Residues: Phytochemical Composition and Functional Application. Molecules. 2017, 22, 1774. DOI: 10.3390/molecules22101774.
- Kim, S. J.; Zaidul, I. S. M.; Suzuki, T.; Mukasa, Y.; Hashimoto, N.; Takigawa, S.; Noda, T.; Matsuura-Endo, C.; Yamauchi, H. Comparison of Phenolic Compositions between Common and Tartary Buckwheat (Fagopyrum) Sprouts. Food Chem. 2008, 110, 814–820. DOI: 10.1016/j.foodchem.2008.02.050.
- Nam, T. G.; Kim, D. O.; Eom, S. H. Effects of Light Sources on Major Flavonoids and Antioxidant Activity in Common Buckwheat Sprouts. Food Sci. Biotechnol. 2018, 27(1), 169–176. DOI: 10.1007/s10068-017-0204-1.
- Kim, E. H.; Kim, S. H.; Chung, J. I.; Chi, H. Y.; Kim, J. A.; Chung, I. M. Analysis of Phenolic Compounds and Isoflavones in Soybean Seeds (Glycine Max (L.) Merill) and Sprouts Grown under Different Conditions. Eur. Food Res. Technol. 2006, 222(1), 201–208. DOI: 10.1007/s00217-005-0153-4.
- Prokudina, E. A.; Havlíček, L.; Al-Maharik, N.; Lapčík, O.; Strnad, M.; Gruz, J. Rapid UPLC–ESI–MS/MS Method for the Analysis of Isoflavonoids and Other Phenylpropanoids. J. Food Compos. Anal. 2012, 26(1–2), 36–42. DOI: 10.1016/j.jfca.2011.12.001.
- Wang, M. L.; Gillaspie, A. G.; Morris, J. B.; Pittman, R. N.; Davis, J.; Pederson, G. A. Flavonoid Content in Different Legume Germplasm Seeds Quantified by HPLC. Plant Genet. Res. 2008, 6(1), 62–69. DOI: 10.1017/S1479262108923807.
- Paśko, P.; Bartoń, H.; Zagrodzki, P.; Gorinstein, S.; Fołta, M.; Zachwieja, Z. Anthocyanins, Total Polyphenols and Antioxidant Activity in Amaranth and Quinoa Seeds and Sprouts during Their Growth. Food Chem. 2009, 115(3), 994–998. DOI: 10.1016/j.foodchem.2009.01.037.
- Perrelli, A.; Goitre, L.; Salzano, A. M.; Moglia, A.; Scaloni, A.; Retta, S. F. Biological Activities, Health Benefits, and Therapeutic Properties of Avenanthramides: From Skin Protection to Prevention and Treatment of Cerebrovascular Diseases. Oxid. Med. Cell. Longev. 2018, 6015351, Oxidative Medicine and Cellular Longevity, 2018 10.1155/2018/6015351
- Billaud, C.;. Composition, Nutritional Value and Physiological Properties. Adrian J. Fenugreek Sci-des-ailment. 2001, 21, 3–26.
- Ma, R. H.; Ni, Z. J.; Zhu, Y. Y.; Thakur, K.; Zhang, F.; Zhang, Y. Y.; Hu, F.; Zhang, J. G.; Wei, Z. J. A Recent Update on the Multifaceted Health Benefits Associated with Ginger and Its Bioactive Components. Food Funct. 2021, 12(2), 519–542. DOI: 10.1039/D0FO02834G.
- Retana-Cordero, M.; Fisher, P. R.; Gómez, C. Modeling the Effect of Temperature on Ginger and Turmeric Rhizome Sprouting. Agronomy. 2021, 11(10), 1931. DOI: 10.3390/agronomy11101931.
- Saini, D.; Rawat, N.; Negi, T.; Barthwal, R.; Sharma, S. K. Utilization, Valorization and Functional Properties of Wild Apricot Kernels. J. Pharmacogn. Phytochem. 2021, 10(4), 119–126.
- Guenter, D.; Friebel, M. “Method of Producing a Cosmetic Abrasive.” 2010; U.S. Patent Application No. 20080248144
- Aziz, A.; Noreen, S.; Khalid, W.; Mubarik, F.; Niazi, M. K.; Koraqi, H.; AL-Farga, A.; Lima, C. M. G.; Alansari, W. S.; Eskandrani, A. A. Extraction of Bioactive Compounds from Different Vegetable Sprouts and Their Potential Role in the Formulation of Functional Foods against Various Disorders: A Literature-Based Review. Molecules. 2022, 27(21), 7320. DOI: 10.3390/molecules27217320.
- Renna, M.; Gioia, F. D.; Leoni, B.; Santamaria, P. Due espressioni dell’agrobiodiversita ` in orticoltura: Germogli e microortaggi. Italus Hortus 2016, 23(1), 31–44.
- Chon, S.-U.; Kim, D.-K.; Kim, Y.-M. Phenolics Content and Antioxidant Activity of Sprouts in Several Legume Crops. Korean J. Plant Res. 2013, 26(2), 159–168. DOI: 10.7732/kjpr.2013.26.2.159.
- Le, L.; Gong, X.; An, Q.; Xiang, D.; Zou, L.; Peng, L.; Wan, Y.; Tan, M.; Nie, Z.; Wu, Q. Quinoa Sprouts as Potential Vegetable Source: Nutrient Composition and Functional Contents of Different Quinoa Sprout Varieties. Food Chem. 2021, 357, 129752. DOI: 10.1016/j.foodchem.2021.129752.
- Chaudhary, A.; Choudhary, S.; Sharma, U.; Vig, A. P.; Singh, B.; Arora, S. Purple Head Broccoli (Brassica Oleracea L. Var. Italica Plenck), a Functional Food Crop for Antioxidant and Anticancer Potential. J. Food Sci. Technol. 2018, 55, 1806–1815. DOI: 10.1007/s13197-018-3095-0.
- Fahey, J. W.; Wade, K. L.; Stephenson, K. K.; Panjwani, A. A.; Liu, H.; Cornblatt, G.; Cornblatt, B. S.; Ownby, S. L.; Fuchs, E.; Holtzclaw, W. D. Bioavailability of Sulforaphane following Ingestion of Glucoraphanin-Rich Broccoli Sprout and Seed Extracts with Active Myrosinase: A Pilot Study of the Effects of Proton Pump Inhibitor Administration. Nutrients. 2019, 11(7), 1489. DOI: 10.3390/nu11071489.
- Moreira-Rodríguez, M.; Nair, V.; Benavides, J.; Cisneros-Zevallos, L.; Jacobo-Velázquez, D. UVA, UVB Light, and Methyl Jasmonate, Alone or Combined, Redirect the Biosynthesis of Glucosinolates, Phenolics, Carotenoids, and Chlorophylls in Broccoli Sprouts. Int. J. Mol. Sci. 2017, 18(11), 2330. DOI: 10.3390/ijms18112330.
- Vidueiros, S. M.; Curti, R. N.; Dyner, L.; Binaghi, M. J.; Peterson, G.; Bertero, H. D.; Pallaro, A. Diversity and Interrelationships in Nutritional Traits in Cultivated Quinoa (Chenopodium Quinoa Willd.) from Northwest Argentina. J. Cereal. Sci. 2015, 62, 87–93. DOI: 10.1016/j.jcs.2015.01.001.
- Lim, J. G.; Park, H.-M.; Yoon, K. S. Analysis of Saponin Composition and Comparison of the Antioxidant Activity of Various Parts of the Quinoa Plant (Chenopodium Quinoa Willd.). Food Sci. Nutr. 2020, 8(1), 694–702. DOI: 10.1002/fsn3.1358.
- Mattioli, S.; Dal Bosco, A.; Castellini, C.; Falcinelli, B.; Sileoni, V.; Marconi, O.; Mancinelli, A. C.; Cotozzolo, E.; Benincasa, P. Effect of Heat- and Freeze-Drying Treatments on Phytochemical Content and Fatty Acid Profile of Alfalfa and Flax Sprouts. J. Sci. Food Agric. 2019, 99, 4029–4035. DOI: 10.1002/jsfa.9630.
- Baenas, N.; Ferreres, F.; García-Viguera, C.; Moreno, D. A. Radish sprouts—Characterization and Elicitation of Novel Varieties Rich in Anthocyanins. Food Res. Int. 2015, 69, 305–312. DOI: 10.1016/j.foodres.2015.01.009.
- Qian, H.; Liu, T.; Deng, M.; Miao, H.; Cai, C.; Shen, W.; Wang, Q. Effects of Light Quality on Main health-promoting Compounds and Antioxidant Capacity of Chinese Kale Sprouts. Food Chem. 2016, 196, 1232–1238. DOI: 10.1016/j.foodchem.2015.10.055.
- Sandoval-Ramírez, B. A.; Catalán, Ú.; Fernández-Castillejo, S.; Rubió, L.; Macià, A.; Solà, R. Anthocyanin Tissue Bioavailability in Animals: Possible Implications for Human Health. A Systematic Review. J. Agric. Food Chem. 2018, 66, 11531–11543. DOI: 10.1021/acs.jafc.8b04014.
- Poole, N.; Donovan, J.; Erenstein, O. Agri-nutrition Research: Revisiting the Contribution of Maize and Wheat to Human Nutrition and Health. Food Policy. 2021, 100, 101976. DOI: 10.1016/j.foodpol.2020.101976.
- Brouns, F.; van Rooy, G.; Shewry, P.; Rustgi, S.; Jonkers, D. Adverse Reactions to Wheat or Wheat Components. Compr. Rev. Food Sci. Food Saf. 2019, 18, 1437–1452. DOI: 10.1111/1541-4337.12475.
- Nelson, K.; Stojanovska, L.; Vasiljevic, T.; Mathai, M. Germinated Grains: A Superior Whole Grain Functional Food. J. Physiol. Pharmacol. 2013, 91, 429–441. DOI: 10.1139/cjpp-2012-0351.
- Gulpinar, A. R.; Orhan, I. E.; Kan, A.; Senol, F. S.; Celik, S. A.; Kartal, M. Estimation of in Vitro Neuroprotective Properties and Quantification of Rutin and Fatty Acids in Buckwheat (Fagopyrum Esculentum Moench) Cultivated in Turkey. Food Res. Int. 2012, 46, 536–543. DOI: 10.1016/j.foodres.2011.08.011.
- Xiao, J.; Capanoglu, E.; Jassbi, A. R.; Miron, A. Advance on the Flavonoid C-glycosides and Health Benefits. Crit. Rev. Food Sci. Nutr. 2016, 56, S29–S45. DOI: 10.1080/10408398.2015.1067595.
- Gan, R. Y.; Wang, M. F.; Lui, W. Y.; Wu, K.; Corke, H. Dynamic Changes in Phytochemical Composition and Antioxidant Capacity in Green and Black Mung Bean (Vigna Radiata) Sprouts. Int. J. Food Sci. Technol. 2016, 51, 2090–2098. DOI: 10.1111/ijfs.13185.
- Gan, R. Y.; Lui, W. Y.; Wu, K.; Chan, C. L.; Dai, S. H.; Sui, Z. Q.; Corke, H. Bioactive Compounds and Bioactivities of Germinated Edible Seeds and Sprouts: An Updated Review. Trends Food Sci. Technol. 2017, 59, 1–14. DOI: 10.1016/j.tifs.2016.11.010.
- Miyahira, R. F.; Lopes, J. D. O.; Antunes, A. E. C. The Use of Sprouts to Improve the Nutritional Value of Food Products: A Brief Review. Plant Foods Hum. Nutr. 2021, 76(2), 143–152. DOI: 10.1007/s11130-021-00888-6.
- Gupta, O. P.; Sharma, P.; Gupta, R. K.; Sharma, I. MicroRNA Mediated Regulation of Metal Toxicity in Plants: Present Status and Future Perspectives. Plant Mol. Biol. 2014, 84(1), 1–18. DOI: 10.1007/s11103-013-0120-6.
- Honcu, I.; Krejcirova, L.; Prihoda, J.; Slukova, M. The Effect of Addition of Malt Flour on the Dough, Volume and Sensory Properties of Bread. Indian J. Sci. 2015, 4(9), 152–155.
- Sur, R.; Nigam, A.; Grote, D.; Liebel, F.; Southall, M. D. Avenanthramides, Polyphenols from Oats, Exhibit anti-inflammatory and anti-itch Activity. Arch. Derm. Res. 2008, 300, 569–574. DOI: 10.1007/s00403-008-0858-x.
- Jiménez-Pulido, I. J.; Rico, D.; Martinez-Villaluenga, C.; Pérez-Jiménez, J.; Luis, D. D.; Martín-Diana, A. B. Sprouting and Hydrolysis as Biotechnological Tools for Development of Nutraceutical Ingredients from Oat Grain and Hull. Foods. 2022, 11(18), 2769. DOI: 10.3390/foods11182769.
- Cicero, N.; Gervasi, T.; Durazzo, A.; Lucarini, M.; Macrì, A.; Nava, V.; Santini, A.; Tardugno, R.; Vadalà, R.; Santini, A. Mineral and Microbiological Analysis of Spices and Aromatic Herbs. Foods. 2022, 11(4), 548. DOI: 10.3390/foods11040548.
- Narayana, P. K.; Bueno, E.; Baur, A.; Ahmed, S.; von Wettberg, E. J. Fenugreek, A Legume Spice and Multiuse Crop Adapted to A Changing Climate. In Developing Climate Resilient Grain and Forage Legumes, 2022; pp 105–123.
- El-Gebaly, A. A.; Sadek, E. S.; Taha, N. M.; Abou Hadid, A. F. Effect of Salinity on Seed Germination, Growth and Amino Acid Content in Fenugreek (Trigonella foenum-graecum L) Sprouts. Arab Univ J Agric Sci 2022, 30(2), 1–9.
- Meghwal, M.; Goswami, T. K. A Review on the Functional Properties, Nutritional Content, Medicinal Utilization and Potential Application of Fenugreek. Journal of Food Processing and Technology. 2012, 3(9).
- Nguyen, L.; Duong, L. T.; Mentreddy, R. S.; The, U. S. Import Demand for Spices and Herbs by Differentiated Sources. J. Appl. Res. Med. Aromat. Plants. 2019, 12, 13–20.
- Amagova, Z.; Golubkina, N.; Matsadze, V.; Elmurzaeva, F.; Muligova, R.; Caruso, G. Biochemical Characteristics of Allium Ursinum L. Sprouts as Affected by the Growing Location in Chechen Republic. Italus Hortus. 2020, 27(2), 66–81. DOI: 10.26353/j.itahort/2020.2.6681.
- Sekara, A.; Pokluda, R.; Del Vacchio, L.; Somma, S.; Caruso, G. Interactions among Genotype, Environment and Agronomic Practices on Production and Quality of Storage Onion (Allium Cepa L.). Review. Hortic. Sci. 2017, 44(1), 21–42. DOI: 10.17221/92/2015-HORTSCI.
- Sobolewska, D.; Podolak, I.; Makowska-Was, J. Allium Ursinum: Botanical, Phytochemical and Pharmacological Overview. Phytochem Rev. 2015, 14, 81–97. DOI: 10.1007/s11101-013-9334-0.
- Molyneux, R. J.; Lee, S. T.; Gardner, D. R.; Panter, K. E.; James, L. F. Phytochemicals: The Good, the Bad and the Ugly? Phytochem. 2007, 68(22–24), 2973–2985. DOI: 10.1016/j.phytochem.2007.09.004.
- Yang, Y.; Meier, F.; Ann, L.; Yuan, J.; Lee Pei, W.; Sze, V.; Chung, H. J.; Yuk, H. G. Overview of Recent Events in the Microbiological Safety of Sprouts and New 597 Intervention Technologies. Compr. Rev. Food Sci. Food Saf. 2013, 598 12(3), 265–280. DOI: 10.1111/1541-4337.12010.
- Martinez-Villaluenga, C.; Peñas, E.; Ciska, E.; Piskula, M. K.; Kozlowska, H.; Vidal Valverde, C.; Frias, J. Time Dependence of Bioactive Compounds and 524 Antioxidant Capacity during Germination of Different Cultivars of Broccoli and Radish 525 Seeds. Food Chem. 2010, 120(3), 710–716. DOI: 10.1016/j.foodchem.2009.10.067.
- Podsędek, A.;. Natural Antioxidants and Antioxidant Capacity of Brassica Vegetables: A Review. LWT. Food Sci. Technol. 2007, 40(1), 1–11.
- Vale, A. P.; Cidade, H.; Pinto, M.; Oliveira, M. B. P. P. Effect of Sprouting 584 and Light Cycle on Antioxidant Activity of Brassica Oleracea Varieties. Food Chem. 2014, 585 165, 379–387. DOI: 10.1016/j.foodchem.2014.05.122.
- Fahey, J. W.; Zhang, Y.; Talalay, P. Broccoli Sprouts: An Exceptionally Rich Source of Inducers of Enzymes that Protect against Chemical Carcinogens. Proc 479 Nat Acad of Sci USA. 1997, 94(19), 10367–480 10372. DOI: 10.1073/pnas.94.19.10367.
- Moreno, D. A.; Pérez-Balibrea, S.; Ferreres, F.; Gil-Izquierdo, Á.; García-Viguera, C. Acylated Anthocyanins in Broccoli Sprouts. Food Chem. 2010, 123(2), 358–363. 528. DOI: 10.1016/j.foodchem.2010.04.044.
- Pérez-Balibrea, S.; Moreno, D. A.; García-Viguera, C. Influence of Light on health-promoting Phytochemicals of Broccoli Sprouts. J. Sci. Food Agric. 2008, 88(5), 904–910. DOI: 10.1002/jsfa.3169.
- Sousa, C.; Lopes, G.; Pereira, D. M.; Taveira, M.; Valentao, P.; Seabra, R. M.; Pereira, J. A.; Baptista, P.; Ferreres, F.; Andrade, P. B. Screening of Antioxidant Compounds during Sprouting of Brassica Oleracea L. Var. Costata DC. Comb. Chem. High Throughput Screen. 2007, 10(5), 377–386. DOI: 10.2174/138620707781662817.
- Troszyńska, A.; Lamparski, G.; Kozłowska, H. Sensory Quality of Sprouts of Selected Cruciferous Species. Pol. J. Food Nutr. Sci. 2002, 52(SI 1), 582 138–141.
- Cartea, M. E.; Francisco, M.; Soengas, P.; Velasco, P. Phenolic Compounds in Brassica Vegetables. Molecules. 2010, 16(1), 251–280. DOI: 10.3390/molecules16010251.
- Moreno, D. A.; Pérez-Balibrea, S.; García-Viguera, C. Phytochemical Quality and Bioactivity of Edible Sprouts. Nat. Prod. Commun. 2006, 1, 1037–1048.
- Podsedek, A.;. Natural Antioxidants and Antioxidant Capacity of Brassica Vegetables: A Review. LWT. 2007, 40(1), 1–11. DOI: 10.1016/j.lwt.2005.07.023.
- Singh, J.; Rai, M.; Upadhyay, A. K.; Bahadur, A.; Chaurasia, S. N. S.; Singh, K. P. Antioxidant Phytochemicals in Broccoli (Brassica Oleracea L. Var. Italica Plenck) Cultivars. J. Food Sci. Technol. 2006, 43(4), 391–393.
- Wojdyło, A.; Nowicka, P.; Tkacz, K.; Turkiewicz, I. P. Sprouts Vs. Microgreens as Novel Functional Foods: Variation of Nutritional and Phytochemical Profiles and Their in Vitro Bioactive Properties. Molecules. 2020, 12;25(20), 4648. DOI: 10.3390/molecules25204648.
- Chuo, S. C.; Nasir, H. M.; Mohd-Setapar, S. H.; Mohamed, S. F.; Ahmad, A.; Wani, W. A.; Muddassir, M.; Alarifi, A. A. Glimpse into the Extraction Methods of Active Compounds from Plants. Crit. Rev. Anal. Chem. 2022, 52(4), 667–696. DOI: 10.1080/10408347.2020.1820851.
- Abuduwaili, A.; Rozi, P.; Mutailifu, P.; Gao, Y.; Nuerxiati, R.; Aisa, H. A.; Yili, A. Effects of Different Extraction Techniques on Physicochemical Properties and Biological Activities of Polysaccharides from Fritillaria Pallidiflora Schrenk. Process Biochem. 2019, 83, 189–197. DOI: 10.1016/j.procbio.2019.05.
- Ali, A.; Yu, L.; Kousar, S.; Khalid, W.; Maqbool, Z.; Aziz, A.; Arshad, M. S.; Aadil, R. M.; Trif, M.; Riaz, S., et al. Functional Characteristics, Extraction, Food Applications and Efficacy against Brain Related Disorders. Front. Nutr. 2022, 9,1009807.
- Koçak, E.; Pazır, F. Ffect of Extraction Methods on Bioactive Compounds of Plant Origin. Turkish J. Agri.-Food Sci. Tech. 2018, 6(6), 663–675. DOI: 10.24925/turjaf.v6i6.663-675.1527.
- Soxhlet, F. V.;. Die gewichtsanalytische bestimmung des milchfettes. Dingler’s Polytech. J. 1879, 232, 461–465.
- Jafari, S. M.; Tsimidou, M. Z.; Rajabi, H.; Kyriakoudi, A. Bioactive Ingredients of Saffron: Extraction, Analysis, Applications. In Saffron; Woodhead Publishing, 2020; pp 261–290.
- Heydari, S.; Haghayegh, G. H. Extraction and Microextraction Techniques for the Determination of Compounds from Saffron. Can Chem. Trans. 2014, 2, 221–247.
- Rifna, E. J. N. N.; Misra, M. D.; Dwivedi, M. Recent Advances in Extraction Technologies for Recovery of Bioactive Compounds Derived from Fruit and Vegetable Waste Peels: A Review. Crit. Rev. Food Sci. Nutr. 2021, 1–34. DOI: 10.1080/10408398.2021.1952923.
- Azmir, J.; Zaidul, I.; Rahman, M.; Sharif, K.; Mohamed, A.; Sahena, F.; Jahurul, M.; Ghafoor, K.; Norulaini, N.; Omar, A. Techniques for Extraction of Bioactive Compounds from Plant Materials: A Review. J. Food Eng. 2013, 117(4), 426–436. DOI: 10.1016/j.jfoodeng.2013.01.014.
- El Asbahani, A.; Miladi, K.; Badri, W.; Sala, M.; Addi, E. A.; Casabianca, H.; El Mousadik, A.; Hartmann, D.; Jilale, A.; Renaud, F. Essential Oils: From Extraction to Encapsulation. Int. J. Pharm. 2015, 483(1–2), 220–243. DOI: 10.1016/j.ijpharm.2014.12.069.
- Chemat, F. N.; Rombaut,; Meullemiestre, A.; Turk, M.; Perino, S.; Fabiano-Tixier, A. S.; Abert-Vian, M. Review of Green Food Processing Techniques. Preservation, Transformation, and Extraction. Innov. Food Sci. Emerg. Technol. 2017, 41, 357–377. DOI: 10.1016/j.ifset.2017.04.016.
- Cravotto, G.; Binello, A.; Orio, L. Green Extraction Techniques. Agro food Ind. Hi Tech. 2011, 22, 57–59.
- Sadeghi, A.; Hakimzadeh, V.; Karimifar, B. Microwave Assisted Extraction of Bioactive Compounds from Food: A Review. Int. J. Food Sci. Nutr. Eng. 2017, 7, 19–27. DOI: 10.5923/j.food.20170701.03.
- Refaat, A. A.; Sheltawy, E.; Sadek, K. U. Optimum Reaction Time, Performance and Exhaust Emissions of Biodiesel Produced by Microwave Irradiation. Int. J. Environ. Sci. Technol. 2008, 5, 315–322. DOI: 10.1007/BF03326026.
- Sania, Z.; Khan, M. R.; Shabbir, M. A.; Aslam. Maan, A.; Khan, M. K. I.; Nadeem, M.; Khalil, A. A.; Din, A.; Aadil, R. M. An Inclusive Overview of Advanced Thermal and Nonthermal Extraction Techniques for Bioactive Compounds in Food and Food-related Matrices. Food Rev. Int. 2022, 38(6), 1166–1196. DOI: 10.1080/87559129.2020.1772283.
- Awulachew, M. T.;. A Review to Nutritional and Health Aspect of Sprouted Food. Int. J. Food Sci. Nutr. Diet. 2022, 10(7), 564–568. DOI: 10.19070/2326-3350-2200097.
- Santos, C. S.; Silva, B.; Valente, L. M. P.; Gruber, S.; Vasconcelos, M. W. The Effect of Sprouting in Lentil (Lens Culinaris) Nutritional and Microbiological Profile. Foods. 2020, 9(4), 1–11. DOI: 10.3390/foods9040400.
- Abellán, Á.; Domínguez-Perles, R.; Moreno, D. A.; García-Viguera, C. Sorting Out the Value of Cruciferous Sprouts as Sources of Bioactive Compounds for Nutrition and Health. Nutrients. 2019, 11, 1–22. DOI: 10.3390/nu11020429.
- Lemmens, E.; Moroni, A. V.; Pagand, J.; Heirbaut, P.; Ritala, A.; Karlen, Y.; Lê, K. A.; den Broeck, H. C.; Brouns, F. J. P. H.; Brier, N., et al. Impact of Cereal Seed Sprouting on Its Nutritional and Technological Properties: A Critical Review. Compr. Rev. Food Sci. Food Saf. 2019, 18(1), 305–328. DOI: 10.1111/1541-4337.12414.
- Khalid, W.; Arshad, M. S.; Aslam, N.; Mukhtar, S.; Rahim, M. A.; Ranjha, M. M. A. N.; Awuchi, C. G. Food Applications of Sorghum Derived Kafirins Potentially Valuable in Celiac Disease. Int J. Food Propet 2022, 25, 2348–2363. DOI: 10.1080/10942912.2022.2135532.
- Liu, Y.; Xu, M.; Wu, H.; Jing, L.; Gong, B.; Gou, M.; Zhao, K.; Li, W. The Compositional, Physicochemical and Functional Properties of Germinated Mung Bean Flour and Its Addition on Quality of Wheat Flour Noodle. J. Food Sci. Technol. 2018, 55(12), 5142–5552. DOI: 10.1007/s13197-018-3460-z.
- Ojha, P.; Adhikari, R.; Karki, R.; Mishra, A.; Subedi, U.; Karki, T. B. Malting and Fermentation Effects on Antinutritional Components and Functional Characteristics of Sorghum Flour. Food Sci. Nutr. 2018, 6(1), 47–53. DOI: 10.1002/fsn3.525.
- Świeca, M.; Dziki, D.; Gawlik-Dziki, U. Starch and Protein Analysis of Wheat Bread Enriched with phenolics-rich Sprouted Wheat Flour. Food Chem. 2017, 228, 643–648. DOI: 10.1016/j.foodchem.2017.02.052.
- Beaulieu, J. C.; Reed, S. S.; Obando-Ulloa, J. M.; McClung, A. M. Green Processing Protocol for Germinating and Wet Milling Brown Rice for Beverage Formulations: Sprouting, Milling and Gelatinization Effects. Food Sci. Nutr. 2020, 8, 2445–2457. DOI: 10.1002/fsn3.1534.
- Argüelles-López, O. D.; Reyes-Moreno, C.; Gutiérrez-Dorado, R.; Sánchez-Osuna, M. F.; López-Cervantes, J.; Cuevas-Rodríguez, E. O.; Milán-Carrillo, J.; Perales-Sánchez, J. X. K. Functional Beverages Elaborated from Amaranth and Chia Flours Processed by Germination and Extrusion. Biotecnia. 2018, 20(3), 135–145. DOI: 10.18633/biotecnia.v20i3.721.
- Tang, D.; Dong, Y.; Ren, H.; Li, L.; He, C. A Review of Phytochemistry, Metabolite Changes, and Medicinal Uses of the Common Food Mung Bean and Its Sprouts (Vigna Radiata). Chem. Cent. J. 2014, 8(1), 1–9. DOI: 10.1186/1752-153X-8-4.
- Diego, S. E.; Andrea, B.; Stefania, I.; Marengo, M.; Pagani, M. A.; Alessandra, M. Effect of Sprouting on Proteins and Starch in Quinoa (Chenopodium Quinoa Willd.). Plant Foods for Human Nutrition. 2020, 75(4).
- Alvarez-Jubete, L.; Auty, M.; Arendt, E. K.; Gallagher, E. Baking Properties and Microstructure of Pseudocereal Flours in gluten-free Bread Formulations. Eur. Food Res. Technol. 2010, 230(3), 437–445. DOI: 10.1007/s00217-009-1184-z.
- Hernandez-Aguilar, C.; Dominguez-Pacheco, A.; Palma Tenango, M.; Valderrama-Bravo, C.; Soto Hernández, M.; Cruz-Orea, A.; Ordonez-Miranda, J. Lentil Sprouts: A Nutraceutical Alternative for the Elaboration of Bread. J. Food Sci. Technol. 2020, 57(5), 1817–1829. DOI: 10.1007/s13197-019-04215-5.
- HO, C. Y.; LIN, Y. T.; Labbe, R. G.; Shetty, K. Inhibition of Helicobacter Pylori by Phenolic Extracts of Sprouted Peas (Pisum Sativum L.). J. Food Biochem. 2006, 30(1), 21–34. DOI: 10.1111/j.1745-4514.2005.00032.x.
- Khalid, W.; Ali, A.; Arshad, M. S.; Afzal, F.; Akram, R.; Siddeeg, A.; Saeed, A.; Rahim, M. A.; Aziz, A.; Maqbool, Z. Nutrients and Bioactive Compounds of Sorghum Bicolor L. Used to Prepare Functional Foods: A Review on the Efficacy against Different Chronic Disorders. Int J. Food Prop. 2022, 25(1), 1045–1062. DOI: 10.1080/10942912.2022.2071293.
- Konaté, K.; Sanou, A.; Aworet-Samseny, R. R.; Benkhalti, F.; Sytar, O.; Brestic, M.; Dicko, M. H.; Dicko, M. H. Safety Profile, in Vitro anti-inflammatory Activity, and in Vivo Antiulcerogenic Potential of Root Barks from Annona Senegalensis Pers. (Annonaceae). J. Evid. Based Complementary Altern. Med. 2021, 2021, 1–12. DOI: 10.1155/2021/4441375.
- Akoto, C. O.; Acheampong, A.; Boakye, Y. D.; Akwata, D.; Okine, M. In Vitro Anthelminthic, Antimicrobial and Antioxidant Activities and FTIR Analysis of Extracts of Alchornea Cordifolia Leaves. J. Pharmacogn. Phytochem. 2019, 8(4), 2432–2442.
- Gawlik-Dziki, U.; Świeca, M.; Dziki, D.; Sęczyk, Ł.; Złotek, U.; Różyło, R.; Czyż, J.; Ryszawy, D.; Czyż, J. Anticancer and Antioxidant Activity of Bread Enriched with Broccoli Sprouts. BioMed Res. Int. 2014, 2014, 1–14. DOI: 10.1155/2014/608053.
- Macharia, J. M.; Mwangi, R. W.; Rozmann, N.; Zsolt, K.; Varjas, T.; Uchechukwu, P. O.; Wagara, I. N.; Raposa, B. L. Medicinal Plants with anti-colorectal Cancer Bioactive Compounds: Potential game-changers in Colorectal Cancer Management. Biomed. Pharmacother. 2022, 153, 113383. DOI: 10.1016/j.biopha.2022.113383.
- Shankar, S.; Segaran, G.; Sundar, R. D. V.; Settu, S.; Sathiavelu, M. Brassicaceae-A Classical Review on Its Pharmacological Activities. Int. J. Pharm. Sci. Rev. Res. 2019, 55, 107–113.
- Ramirez, D.; Abellán-Victorio, A.; Beretta, V.; Camargo, A.; Moreno, D. A. Functional Ingredients from Brassicaceae Species: Overview and Perspectives [CrossRef]. Int. J. Mol. Sci. 2020, 21(6), 1998. DOI: 10.3390/ijms21061998.
- Peña, M.; Guzmán, A.; Martínez, R.; Mesas, C.; Prados, J.; Porres, J. M.; Melguizo, C. Preventive Effects of Brassicaceae Family for Colon Cancer Prevention: A Focus on in Vitro Studies. Biomed. Pharmacother. 2022, 151, 113145. DOI: 10.1016/j.biopha.2022.113145.
- da Mattosinhos, P. S.; Sarandy, M. M.; Novaes, R. D.; Esposito, D.; Gonçalves, R. V. Anti-Inflammatory, Antioxidant, and Skin Regenerative Potential of Secondary Metabolites from Plants of the Brassicaceae Family: A Systematic Review of in Vitro and in Vivo Preclinical Evidence (Biological Activities Brassicaceae Skin Diseases). Antioxidants. 2022, 11(7), 1346. DOI: 10.3390/antiox11071346.
- Sekirov, I.; Russell, S. L.; Antunes, L. C. M.; Finlay, B. B. Gut Microbiota in Health and Disease. Physiol. Rev. 2010, 90, 859–904. DOI: 10.1152/physrev.00045.2009.
- Yagishita, Y.; Fahey, J. W.; Dinkova-Kostova, A. T.; Kensler, T. W. Broccoli or Sulforaphane: Is It the Source or Dose that Matters. Molecules. 2019, 24, 3593. DOI: 10.3390/molecules24193593.
- Gu, Y.; Guo, Q.; Zhang, L.; Chen, Z.; Han, Y.; Gu, Z. Physiological and Biochemical Metabolism of Germinating Broccoli Seeds and Sprouts. J. Agric. Food Chem. 2012, 60, 209–213. DOI: 10.1021/jf203599v.
- Uddin, M. S.; Mamun, A. A.; Jakaria, M.; Thangapandiyan, S.; Ahmad, J.; Rahman, M. A.; Mathew, B.; Abdel-Daim, M. M.; Aleya, L. Emerging Promise of sulforaphane-mediated Nrf2 Signaling Cascade against Neurological Disorders. Sci. Total Environ. 2020, 707, 135624. DOI: 10.1016/j.scitotenv.2019.135624.
- Ruhee, R. T.; Suzuki, K. The Integrative Role of Sulforaphane in Preventing Inflammation, Oxidative Stress and Fatigue: A Review of A Potential Protective Phytochemical. Antioxidants. 2020, 9, 521. DOI: 10.3390/antiox9060521.
- Kamal, M. M.; Akter, S.; Lin, C. N.; Nazzal, S. Sulforaphane as an Anticancer Molecule: Mechanisms of Action, Synergistic Effects, Enhancement of Drug Safety, and Delivery Systems. Arch. Pharm. Res. 2020, 43, 371–384. DOI: 10.1007/s12272-020-01225-2.
- Kang, Y.; Zhang, G.; Huang, E. C.; Huang, J.; Cai, J.; Cai, L.; Wang, S.; Keller, B. B. Sulforaphane Prevents Right Ventricular Injury and Reduces Pulmonary Vascular Remodeling in Pulmonary Arterial Hypertension. Am. J. Physiol. Heart Circ. Physiol. 2020, 318, H853–H866. DOI: 10.1152/ajpheart.00321.2019.
- Salehi, B.; Quispe, C.; Butnariu, M.; Sarac, I.; Marmouzi, I.; Kamle, M.; Tripathi, V.; Kumar, P.; Bouyahya, A.; Capanoglu, E. Phytotherapy and Food Applications from Brassica Genus. Phytother. Res. 2021, 35, 3590–3609. DOI: 10.1002/ptr.7048.
- Nawaz, H.; Shad, M. A.; Muzaffar, S. Phytochemical Composition and Antioxidant Potential of Brassica. Brassica Germplasm Charact. Breed. Util. 2018, 1, 7–26.
- Mustard, G. A.;. Seeds as a Bioactive Component of Food. Food Rev. Int. 2022, 1–14.
- Dua, A.; Chander, S.; Agrawal, S.; Mahajan, R. Antioxidants from Defatted Indian Mustard (Brassica Juncea) Protect Biomolecules against in Vitro Oxidation. Physiol. Mol. Biol. Plants. 2014, 20, 539–543. DOI: 10.1007/s12298-014-0260-4.
- Kwak, Y.; Lee, J.; Ju, J. Anti-Cancer Activities of Brassica Juncea Leaves in Vitro. EXCLI J. 2016, 15, 699. DOI: 10.17179/excli2016-586.
- Mori, N.; Shimazu, T.; Sasazuki, S.; Nozue, M.; Mutoh, M.; Sawada, N.; Iwasaki, M.; Yamaji, T.; Inoue, M.; Takachi, R. Cruciferous Vegetable Intake Is Inversely Associated with Lung Cancer Risk among Current Nonsmoking Men in the Japan Public Health Center (JPHC) Study. J. Nutr. 2017, 147, 841–849. DOI: 10.3945/jn.117.247494.
- Fang, Y.; Yang, C.; Yu, Z.; Li, X.; Mu, Q.; Liao, G.; Yu, B. Natural Products as LSD1 Inhibitors for Cancer Therapy. Acta Pharm. Sin. B. 2021, 11, 621–631. DOI: 10.1016/j.apsb.2020.06.007.
- Ghanbari-Movahed, M.; Jackson, G.; Farzaei, M. H.; Bishayee, A.; Systematic, A. Review of the Preventive and Therapeutic Effects of Naringin against Human Malignancies. Front. Pharmacol. 2021, 12, 639840. DOI: 10.3389/fphar.2021.639840.
- Afzal, M. F.; Khalid, W.; Akram, S.; Khalid, M. A.; Zubair, M.; Kauser, S.; Anusha Siddiqui, S.; Aziz, A.; Anusha Siddiqui, S. Bioactive Profile and Functional Food Applications of Banana in Food Sectors and Health: A Review. Int. J. Food Prop. 2022, 25(1), 2286–2300. DOI: 10.1080/10942912.2022.2130940.
- Ayadi, J.; Debouba, M.; Rahmani, R.; Bouajila, J. Brassica Genus Seeds: A Review on Phytochemical Screening and Pharmacological Properties. Molecules. 2022, 27(18), 6008. DOI: 10.3390/molecules27186008.
- Ganesan, K.; Xu, B. A Critical Review on Phytochemical Profile and Health Promoting Effects of Mung Bean (Vigna Radiata). Food Sci. Hum. Wellness. 2018, 7(1), 11–33. DOI: 10.1016/j.fshw.2017.11.002.
- Shapiro, K.; Stephenson, W.; Fahey, L.; Wade, Chemoprotective, L. J. C. 1998, 7.
- Tanaka, T.; Kohno, H.; Suzuki, R.; Yamada, Y.; Sugie, S.; Mori, H. A Novel Inflammation‐related Mouse Colon Carcinogenesis Model Induced by Azoxymethane and Dextran Sodium Sulfate. Cancer Sci. 2003, 94(11), 965–973. DOI: 10.1111/j.1349-7006.2003.tb01386.x.
- Tanaka, T.; Shnimizu, M.; Moriwaki, H. Chemoprevention by Carotenoids. Molecules. 2012, 17(3), 3202–3242. DOI: 10.3390/molecules17033202.
- Tong, L. T.; Zhong, K.; Liu, L.; Qiu, J.; Guo, L.; Zhou, X.; Cao, L.; Zhou, S. Effects of Dietary Wheat Bran Arabinoxylans on Cholesterol Metabolism of Hypercholesterolemic Hamsters. Carbohydr. Polym. 2014, 112, 1–5. DOI: 10.1016/j.carbpol.2014.05.061.
- He, W. S.; Zhu, H.; Chen, Z. Y. Plant Sterols: Chemical and Enzymatic Structural Modifications and Effects on Their Cholesterol Lowering Activity. J. Agric. Food Chem. 2018, 66, 3047–3062. DOI: 10.1021/acs.jafc.8b00059.
- Feig, J. E.;. Regression of Atherosclerosis: Insights from Animal and Clinical Studies. Ann. Glob. Health. 2014, 80, 13–23. DOI: 10.1016/j.aogh.2013.12.001.
- Liska, D. J.; Dioum, E.; Chu, Y.; Mah, E. Narrative Review on the Effects of Oat and Sprouted Oat Components on Blood Pressure. Nutrients. 2022, 14, 4772. DOI: 10.3390/nu14224772.
- Maqbool, Z.; Arshad, M. S.; Ali, A.; Aziz, A.; Khalid, W.; Afzal, M. F.; Lorenzo, J. M.; Addi, M.; Hano, C.; Lorenzo, J. M. Potential Role of Phytochemical Extract from Saffron in Development of Functional Foods and Protection of Brain-Related Disorders. Oxid. Med. Cell. Longev. 2022, 2022, 6480590–6480590. DOI: 10.1155/2022/6480590.
- Patel, B.; Mann, G. E.; Chapple, S. J. Concerted Redox Modulation by Sulforaphane Alleviates Diabetes and Cardiometabolic Syndrome, Free Radic. Biol. Med. 2018, 122, 150–160. DOI: 10.1016/j.freeradbiomed.2018.02.004.
- Kowalska, K.; Olejnik, A.; Rychlik, J.; Grajek, W. Cranberries (Oxycoccus Quadripetalus) Inhibit Adipogenesis and Lipogenesis in 3T3-L1 Cells. Food Chem. 2014, 148, 246–252. DOI: 10.1016/j.foodchem.2013.10.032.
- Cariou, B.; Postic, C.; Boudou, P.; Burcelin, R.; Kahn, C. R.; Girard, J.; Burnol, A. F.; Mauvais-Jarvis, F. Cellular and Molecular Mechanisms of Adipose Tissue Plasticity in Muscle Insulin Receptor Knockout Mice. Endocrinology.2004 (145), 1926–1932.
- Grover, J. K.; Yadav, S.; Vats, V. Hypoglycemic and Antihyperglycemic Effect of Brassica Juncea Diet and Their Effect on Hepatic Glycogen Content and the Key Enzymes of Carbohydrate Metabolism. Mol. Cell. Biochem. 2002, 241, 95–101. DOI: 10.1023/A:1020814709118.
- Khalid, W.; Arshad, M. S.; Ranjha, M. M. A. Z.; Różańska, M. B.; Irfan, S.; Shafique, B.; Rahim, M. A.; Khalid, M. Z.; Abdi, G.; Kowalczewski, P. L. Functional Constituents of plant-based Foods Boost Immunity against Acute and Chronic Disorders. Open Life Sci. 2022, 17(1), 1–19. DOI: 10.1515/biol-2022-0104.
- Eckel, R. H.; Grundy, S. M.; Zimmet, P. Z. The Metabolic Syndrome. Lancet. 2005, 365, 1415–1428. DOI: 10.1016/S0140-6736(05)66378-7.
- Bhandari, R.; Kaur, J.; Kaur, S.; Kuhad, A. The Nrf2 Pathway in Psychiatric Disorders: Pathophysiological Role and Potential Targeting. Expert Opin. Ther. Targets 2021, 25, 115–139. DOI: 10.1080/14728222.2021.1887141.
- Jing, W.; Zhao, X.; Liu, A.; Wei, F.; Ma, S. Two New Nitrogenous Compounds from the Seeds of Brassica Napus. Chem. Nat. Compd. 2022, 58, 501–505. DOI: 10.1007/s10600-022-03719-5.
- Bavarsad, N.; Mapar, M. A.; Safaezadeh, M.; Latifi, S. M. A double-blind, placebo-controlled Randomized Trial of skin-lightening Cream Containing Lycopene and Wheat Bran Extract on Melasma. J. Cosmet. Dermatol. 2021, 20, 1795–1800.
- Lee, J. H.; Ki, H. H.; Kim, D. K.; Lee, Y. M. Triticum Aestivum Sprout Extract Attenuates 2, 4-dinitrochlorobenzene-induced Atopic dermatitis-like Skin Lesions in Mice and the Expression of Chemokines in Human Keratinocytes. Mol. Med. Rep. 2018, 18, 3461–3468. DOI: 10.3892/mmr.2018.9339.