ABSTRACT
Asiatic acid (AA) is a natural aglycone of pentacyclic triterpenoids and is abundantly present in many edible and medicinal plants, including Centella Asiatica, which is a reputed herb for wound healing and neuropsychiatric disorders in many traditional medicine formulations. Asiatic acid has various pharmacological functions such as antioxidant and anti-inflammatory and controls apoptosis, which in numerous diseases credits to its medicinal impact. In preclinical trials, asiatic acid demonstrated potent antihypertensive, neuroprotective, cardioprotective, antidiabetic, antimicrobial, and antitumor behaviors. It has been shown to influence multiple enzymes, receptors, growth factors, transcription factors, apoptotic proteins, and cell signaling cascades in several in vitro and in vivo experiments. This review aims to reflect the current therapeutic potential reports and the underlying pharmacological and molecular mechanisms of asiatic acid. In various diseases, studies display Asiatic acid’s polypharmacological properties, therapeutic ability, and molecular pathways. With the available research data, asiatic acid appears to be a significant multi-targeted, natural-origin polypharmacological agent for further pharmaceutical production and clinical use. Provided advantageous pharmacokinetics, protection, and effectiveness, along with commonly used modern drugs. Asiatic acid may be a potential agent or adjuvant, with a pharmacological justification for its usage in therapeutics.
Introduction
Centella Asiatica is a promising and prominent source of numerous bioactive moieties especially terpenes are recognized as major compounds. In C. Asiatica, terpenes have occurred in different forms such as monoterpenes and sesquiterpenes including β-element, α-copaene, α-pinene, trans-β-farnesene, β-caryophyllene, β-pinene, bornyl acetate, γ-terpinene, myrcene, germacrene D, and bicycloelemene. Though, pentacyclic triterpenes (glycosides and aglycons) such as asiaticoside, asiatic acid, madecassoside, and madecassic acid have been known to exert several biological activities.[Citation1,Citation2]
There has been an increasing interest in natural triterpenoids over the past several years, concentrating primarily on the theoretical aspects of extraction, separation, structural study, and a broad variety of biological activities. Asiatic acid is the most popular ingredient of Centella saponins and is a naturally occurring triterpenoid pentacyclic. This compound has a wide range of biological activities, including anti-cancer,[Citation3,Citation4] anti-inflammatory and wound healing,[Citation5] antidiabetic,[Citation6] antioxidant and hepatoprotective, anti-hepatitis C virus,[Citation5] antidiabetic.[Citation6–8] Chemically, Asiatic acid (2,3,23-trihydroxy-urs-12-ene-28-oic-acid) is an asiaticoside aglycone shape and is readily produced by hydrolyzing the asiaticoside structure’s sugar moiety under acidic conditions. C30H48O5 is its chemical composition and it has a molecular weight of 488.70 kD.[Citation9–11] This review aims to highlight Asiatic acid underlying pharmacological and molecular mechanisms as well as data regarding its current therapeutic potential. Different studies demonstrated the polypharmacological characteristics, therapeutic potential, and molecular mechanisms of Asiatic acid in an array of disorders were limelighted in this review.
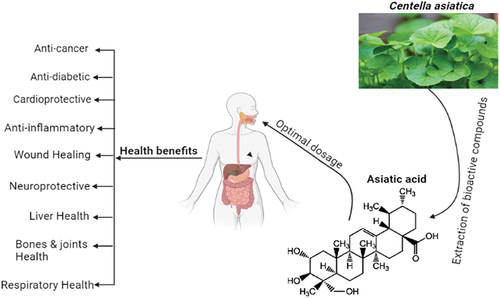
Natural sources of asiatic acids
A monocarboxylic acid called asiatic acid (AA; 2α,23-dihydroxyursolic acid) is produced when ursane is hydrided. Ursane is replaced in the structural formula by hydroxyl groups at C-2, C-3, and C-23 (stereoisomer 2α, 3β), as well as a carboxyl group at C-28. The chemical formula of AA is C30H48O5, and its molecular weight is 488.70 g/mol.[Citation12,Citation13] According to González-Coloma et al.[Citation14] asiatic acid, like other TPs, is a secondary metabolite that defends plants from insect and microbial attack. The leaves of C. Asiatica, also known as gotu kola or kodavan, have particularly high amounts of AA both in its free form and as an aglycone with attached carbohydrate residues (asiaticoside). A total of 30% AA, 40% asiaticoside, and 30% madecassic acid are present in the TPs of C. asiatica. South Africa, Australia, Oceania, and Southeast Asian nations (mostly India and China, but also Japan, Malaysia, and Indonesia) are home to this plant.[Citation15] C. Asiatica has been suggested largely for the treatment of neuropsychiatric disorders as well as wounds, leprosy, and syphilis for over three thousand years. It is one of the principal medicinal substances used in traditional African, Ayurvedic, and Chinese medicine as a panacea. In its countries of origin, this plant is now used prophylactically both in the form of nutraceutical preparations and as a supplement to salads and drinks due to the plant’s general health-promoting effects. Additionally, C. Asiatica is a component of toothpaste, cosmetics, and ointments.[Citation16] Asiatic acid (AA), a pentacyclic triterpenoid that occurs naturally, is mostly present in the traditional herb Centella Asiatica.[Citation17] The main chemical components of C. Asiatica, triterpenoid saponins, are thought to be the cause of its vast medicinal effects.[Citation18] Psidium guajava, Combretum fruticosum, and other organisms with data are known to contain the natural substance asiatic acid.[Citation19] Major source, chemical structure and health benefits of AA were shown in .
Antioxidant potential
The leaves of plant Lagerstroemia speciose were extracted from many terpene acids, which were then investigated against alpha-glucosidase inhibition assay from L. ethyl acetate extract. The six pentacyclic triterpenes speciosa (LSL) were obtained: oleanolic acid, arjunolic acid, asiatic acid, maslinic acid, corosolic acid, and 23-hydroxyursolic acid. Among these, there was substantial inhibition of corosolic acid, maslinic acid, oleanolic acid, and 23-hydroxyursolic acid with IC50 values of 3.53 ± 0.27, 5.52 ± 0.19, 6.29 ± 0.37, and 8.14 ± 0.18 μg/mL. In comparison, arjunolic acid exhibited strong inhibitory ability with an IC50 value of 18.63 ± 0.32 μg/mL, while AA exhibited an IC50 inhibitory activity of 30.03 ± 0.41 μg/mL, respectively.[Citation20]
Asiatic acid is a pentacyclic triterpene that has been documented to have several biological activities, including antioxidant and anti-inflammatory effects, and is typically present in many vegetables and fruits. Researchers stated that their study findings showed that AA treatment attenuated hepatotoxicity caused by LPS/d-GalN and inhibited inflammatory responses and oxidative stress.[Citation21]
Asiatic acid has been found to prevent oxidative stress by maintaining the antioxidant status and mitochondrial activity in the neurons.[Citation22,Citation23] For two human bronchial epithelial cells, 16HBE and BEAS-2B cells, the defensive effects of triterpenic acid, AA (AA), glycyrrhizic acid (GA), or oleanolic acid (OA) were investigated against hydrogen peroxide (H2O2) induced damage. Treatment with H2O2 decreased the activity of Na+ -K+ -ATPase and mitochondrial membrane potential in cells. Mitochondrial membrane potential and Na+ -K+ -ATPase activity were substantially maintained by triterpenic acid pretreatments. In test cells, H2O2 increased reactive oxygen species, interleukin-6, tumor necrosis factor-α, and prostaglandin E2 levels. Three therapies with triterpenic acid dose-dependently reversed these improvements. H2O2 facilitated p47phox, gp91phox, cyclooxygenase-2 (COX-2), mitogen-activated protein kinase, and nuclear factor- α β (NF-aB) expression of the protein. The production of p47phox, COX-2, NF-xB p65, and p-p38 but only decreased gp91phox expression at 8 μmol/L was dose-dependently downregulated by AA, GA, or OA pretreatments. These findings help the defense of bronchial epithelial cells by these triterpenic acids to attenuate apoptotic, oxidative, and inflammatory stress. In conclusion, 4 or 8 μmol/L AA, glycyrrhizic acid, or oleanolic acid preserved human bronchial 16HBE and BEAS-2B cells against oxidative and inflammatory damage triggered by hydrogen peroxide by improving mitochondrial stability, decreasing the development of ROS and PGE2, and suppressing protein expression of NAPDH oxidase, COX-2, NF-2B p65, and p-p38.[Citation24]
Asiatic acid has been shown to produce strong antioxidant and free radical scavenging properties that require different pathways. In a dose-dependent manner, AA prevents hydroxyl radicals and superoxide anions in different animal models of human diseases. AA is an extremely efficient chain-breaking antioxidant that operates against species of reactive oxygen (ROS). Asiatic acid has demonstrated that it attenuates the stimulation of myeloperoxidase and prevents lipid peroxidation by enhancing the concentrations of enzymatic and non-enzymatic antioxidants.[Citation12,Citation25]
Health perspectives of asiatic acid
Cancer incergence
Depending on dosage and time, asiatic acid substantially decreased cell viability, cell proliferation, and cell progression in U87-MG cells by causing cell apoptotic death and improving antiapoptotic proteins.[Citation26] In different human glioblastoma cancer cells lines i.e. U87MG, LN18, and U118MG, AA supplementation (10–100 μM) lowered the cell viability whereas orally supplemented asiatic acid (10–100 μM) to mice markedly decreased tumor volume, orthotopic U87MG xenografts growth, induced apoptotic death by modulating the protein expression of several apoptosis regulators (Bcl2 family members, caspases, and survivin), induced ER stress (decreased Calnexin and IRE1α expression and increased GRP78 and Calpain), damaged cellular organization and increased free intracellular calcium.[Citation27]
Transforming growth factor β1 (TGF-β1) promote tumor growth via a pathway correlated with hyperactive Smad3 and suppressed Smad7 signaling in the tumor microenvironment. The equilibrium between Smad3 and Smad7 AA (AA, a Smad7 inducer) and naringenin (NG, a Smad3 inhibitor) signaling has been documented to effectively inhibit tumor progression in mouse models of invasive melanoma (B16F10) and lung carcinoma (LLC) by encouraging the formation and cytotoxicity of natural killer (NK) cells against cancer. Mechanistically, we find that Smad3 physically binds Id2 and IRF2 to inhibit the development of NK cells and cytotoxicity against cancer induced by NK cells. Treatment with asiatic acid substantially inhibited Smad3 translation and phosphorylation while restoring Smad7 expression and, thus, via Id2/IRF2-associated pathways, largely promoted NK cell differentiation, maturation, and cytotoxicity toward cancer. In comparison, the beneficial effects of AA and NG on NK cell-dependent anti-cancer behaviors were blunted by silencing Id2 or IRF2. Thus, treatment with AA and NG developed an additive impact on the inactivation of TGF-β1/Smad3 signaling and thus suppressed the development of melanoma and lung carcinoma by fostering NK cell cancer tolerance via an Id2 and IRF2-associated process.[Citation28] In another recently described by Guo and their fellows,[Citation29] they investigated the anticancer role of AA against MDA-MB-231 cells of a xenografted tumor in nude mice. They found that supplementation of AA at the rate of (50 mg/kg) showed the following changes such as inhibition of cell viability, p-AKT, p53, p-PI3K, and other proteins levels induction of apoptotic cell death, and enhancement of WAVE3 expressions in the tissue of ductal carcinoma in situ.
In another recent study reported the anticancer role of asiatic acid in a dose and time dependent fashion against cisplatin-resistant human NPC cell lines (cis NPC-039 and cis NPC-BM) by inducing apoptosis via phosphorylating the p38 expressions. Induction of apoptosis is linked with both intrinsic and extrinsic apoptotic pathways (activation of death receptors, alteration in mitochondrial membrane potential, upregulation of caspase 3, 8, and 9 expressions, and enhancement in Bax expression). Moreover, asiatic acid has also promoted the phosphorylation of MAPK pathway proteins.[Citation30] In a previous study conducted by Hsu et al.,[Citation31] they found that asiatic acid has an anticancer role against breast cancer via arresting the cell cycle and mitochondrial apoptotic pathway. Likewise, Cui et al.,[Citation32] found that asiatic acid in lung cancer has been found to suppress the TGF-beta1-induced epithelial-mesenchymal transition.
Nuclear factor kappa B (NF-κB) has been known to regulated multiple transcriptional target genes which are associated with innate and adaptive immune responses, tumor processes, and cellular growth and apoptosis.[Citation33] In different types of cancers, NF-κB activation is primarily associated with tumor cell proliferation, invasion, angiogenesis, and metastasis.[Citation34,Citation35] In vitro study of A549 cells, asiatic acid significantly suppressed the NF-κB activity, cell migration, and induced apoptotic cell death.[Citation36] STAT has exhibited momentous functions in tumor proliferation, cell survival, invasion, and mobility and participated in multiple human cancer cells such as multiple leukemia, myeloma, lymphoma, melanoma, lung cancer, and prostate cancer, respectively.[Citation35] STAT3 undergoes tyrosine phosphorylation, dimerization, DNA binding, and then transcription activation after activation. The phosphorylation mechanism is regulated by the association between Janus-activated Kinases (JAKs) and STAT3. JAK1, JAK2, JAK3, and TYK2, a non-JAK tyrosine kinase, were all involved in STAT3 activation.[Citation37] Cells display intermittent STAT3 phosphorylation under standard physiological circumstances, but STAT3 is constitutively triggered in the vast majority of tumor cells. In tumor development, this robust result includes aberrant STAT3 signaling.[Citation38,Citation39] In this contrast, suppressing STAT expressions are linked with developing anticancer effects.[Citation40] Multiple gene products via STAT3 include survivin, cyclin D1, Bcl-2, Mcl-1, c-Myc, mandatory for growth and survival; (MMP-2, −9 for cell mobility and VEGF for angiogenesis.[Citation41] Importantly, AA has the potential to prevent cancer insurgence by suppressing the activation of STAT3.[Citation42] In a dose-dependent manner, inhibition of proliferation, induction of cell cycle arrest in G0/G1 phase, blockade of G1-S transition, reduction in cyclin-dependent kinase CKD4, cyclin D1, and phosphorylated retinoblastoma protein levels, enhancement in cyclin-dependent kinase inhibitor P15, suppression of migration and invasion stages, and down-regulation of expression of MMP-2 and MMP-9 were reported in human SGC7901 and HGC27 human gastric cancer cells after asiatic acid treatment.[Citation43]
Proliferation and migration periods are synonymous with the activation of the signal transducer, transcription factor, and transcription activator 3 in human cancer cells (STAT3). Furthermore, these behaviors contribute to the improvement or decrease in tumor suppressor genes and, correspondingly, cell survival. Importantly, agents that can inhibit the activation of STAT3 have the ability to be used to avoid and cure different cancers. In this study, asiatic acid (AA) has been reported to dose-dependently suppress STAT3 activation in gastric cancer cells. This inhibition was induced by a Janus-activated kinase 2 blockades. The expression of STAT3-modulated gene products, including cyclin D1, Bax, Bcl-2, c-Myc, and matrix metalloproteinase (MMP)-2 and MMP-99, was further controlled by AA-PMe. Finally, the AA-PMe-induced regulation of STAT3 downstream gene products was reversed by transfection of both a STAT3 mimic and an inhibitor.[Citation44] Asiatic acid has antitumor activity against lung cancer cell lines A549 in which TGF-β1 is used to induce epithelial-mesenchymal transition through enhancing the cell viability, invasion, and migration, lowering the mRNA expressions and protein levels of E-cadherin, enhancing the snail expressions, vimentin, and β-catenin. Administration of asiatic acid to lung cancer cells of experimental subjects reverted these changes.[Citation32] In both in-vivo and in-vitro studies in a dose- and time-dependent manner of mouse lung cancer xenograft model, Asiatic acid exhibits strong anticancer potential against lung cancer cells through multiple pathways such as induction of apoptosis, reduction in p62 expressions, the elevation of microtubule-associated protein 1 light chain 3 (LC3), production of ROS, rupture of mitochondrial membrane potential, suppression of tumor volume and weight and reduction of expression of proliferating cell nuclear antigen.[Citation45]
Antidiabetic
Being a potent antidiabetic agent, asiatic acid has a preventive role against high-fat diet-induced diabetic db/db C57 BL/6J mice through various mechanisms such as mitigating the upregulation of the phosphatidylinositol 3-kinase (PI3K)/protein kinase B (AKT)/glycogen synthase kinase-3β (GSK-3β) signaling pathway, lowering the insulin receptor, and insulin receptor substrate-1 mRNA expression, glucose-6-phosphatase mRNA expression, and GSK-3β levels, accordingly.[Citation46] Similarly, asiatic acid in the pancreatic islets has been found to exhibit reductions in glucose concentrations, regulation of glucose metabolism enzymes, and anti-fibrotic action.[Citation47] Streptozotocin-induced diabetes in rats by enhancing the concentration of blood glucose levels, and insulin resistance, decreasing the antioxidant enzymes, increasing the lipid peroxidation rate, and lowering the insulin sensitivity whereas asiatic acid supplementation reversed these changes.[Citation48,Citation49] Higher levels of total cholesterol and triglycerides are linked with suppression of cholesterol catabolism or enhancement of utilization of free fatty acids from peripheral fat depots. On the other hands, the treatment of asiatic acid significantly suppressed the absorption of cholesterol, lowered the triglycerides concentrations, and modulated lipid metabolism enzymes.[Citation50,Citation51] Another trial conducted by Ramachandran and Saravanan,[Citation52] they found that supplemented asiatic acid to diabetic rats caused significant reductions in glucose levels and production of lipid peroxides, and showed improvement of insulin, restoration of antioxidant enzymes, and augmentation of insulin receptors. Proteins of PI3K, Akt, and IRS-1/2, and glucose transporter 4 (GLUT4). Several experiments have shown that asiatic acid has been shown to be successful in preventing the convergence of numerous studies together, asiatic acid tends to be a promising molecule for type 1 as well as type 2 diabetes and PI3K hyperlipidemia, typically occurring in diabetic subjects via various mechanisms such as glucose homeostasis development, antioxidant protection system enhancement, etc. Improved glucose homeostasis by improved antioxidant protection as well as carbohydrate, GLUT4, protein, and Akt regulation, together with decreases in glucose metabolism involved in skeletal muscles, may be the underlying cause. Via minimizing glycative injury and coagulation elements, asiatic acid at 0.1 or 0.2% concentration fed to diabetic mice preserved the diabetic heart.[Citation53] In diabetic mice, AA decreased plasma glucose, creatine phosphokinase, lactate dehydrogenase, and preserved HbA1c levels/activity levels. Improved amounts in diabetic mice of glutathione, decreased development of ROS, N(ε)-(carboxymethyl)-lysine, pentosidine, methylglyoxal, pro-inflammatory cytokines, and chemokines. Von Willebrand factor, fibrinogen amounts, factor-VII, and circulating antithrombin-III and protein-c activities in the plasma were also decreased by the AA. Asiatic acid has also reduced the function of NADPH oxidase and aldose reductase and glyoxalase 1, NF-3B-p65, NF-3B-p50, and advanced glycation receptor receptors expressions, along with p-p38 and p-ERK1/2 expressions in the diabetic core. In research that evaluated multiple pentacyclic triterpenoids for their effect on rabbit muscle glycogen phosphorylase a (GPa).[Citation54]
Asiatic acid has been reported to have dramatically enhanced the function of main carbohydrate metabolizing enzymes in STZ-induced diabetic rats. There is no reported research yet on the role of AA in oxidative stress in diabetic rats induced by STZ.[Citation48] More effective glycogen phosphorylase inhibitory activity was demonstrated by the asiatic acid with a C2 alpha-OH feature, making it an interesting compound for the treatment of diseases induced by glycogen metabolism disorders, such as diabetes.[Citation55] Asiatic acid can activate the production of proinsulin and the release of insulin, which may help decrease plasma glucose and increase insulin during diabetes.[Citation56]
Cardioprotective role
In myocardial injury, enhanced myocardial infarction size disturbed cardiac functions, increment in the creatinine and lactate dehydrogenase levels, deactivated the Akt/GSK-3β signal pathway, elevation of plasma glucose and lactate concentrations, promotion of the translocation of GLUT4 to the plasma membrane, enhanced glycogen breakdown, the elevation of cardiomycetes apoptosis, enhancement of PPARγ expression (at both mRNA and protein levels) were reported whereas administration of Asiatic acid to the experimental subjects prevented from these changes in myocardial injury.[Citation57] Likewise, in another in vivo trial conducted by Ma and their colleagues, they determined the preventive role of asiatic acid against cardiac hypertrophy in mice subjected to aortic banding. Orally administrated changes such as inhibition of cardiac fibrosis (in vivo), production of collagen (in vitro), suppression of hypertrophic responses induced by pressure overload, inhibition of the mammalian rapamycin target (mTOR) pathway, activation of AMPK alpha and extracellular signal-regulated kinase pathway were triggered by AA at the rate of (10 or 30 mg/kg) for 7 weeks in aortic banding mice and (in vivo and in vitro) AA.[Citation58] A peer group of researchers determined the cardio protective role of AA in hypertensive rats through multiple pathways such as alleviation of cardiovascular remodeling and restoration of eNOS expression which is linked with the direct target of AMPKα.[Citation59] AMPKα has been found to regulate the TGF-β and MAPK signaling pathways in myocytes whereas Asiatic acid reversed these changes.[Citation60,Citation61] Thakur and their colleagues found that AA in vitro and in vivo trials prevented cardiac hypertrophy by promoting the AMPKα activation.[Citation62] AA in hypertrophic heart also has been known to block the mTOR activation, and lower the phosphorylation of P70S6K.[Citation63] It also inhibits the mTOR/P70S6K/S6 pathway and cardiac hypertrophy.[Citation64] Another study reported the in vitro protective role of AA in myocardial ischemia after OGD/R exposure in a dose-dependent fashion using an in vitro rat H9c2 cardiomyocytes model of oxygen-glucose deprivation injury, they determined that 10 μM administration markedly decreased the lactate dehydrogenase level, cell viability, suppressed the apoptotic cell death, suppressed the caspase-3,-9 activities, and reversed Bax/Bcl-2 ratio, lowered the reactive oxygen species accumulation, decreased the intracellular calcium concentration, improved mitochondrial function, enhanced mitochondrial membrane potential, Mediated hypoxia-inducible factor 1 alp expression (HIF-1 alp levels), promoted phosphorylation of Akt and subsequently inactivation of glycogen synthase kinase-3β (GSK-3β) and promoted phosphorylation of Akt phosphorylation and phosphorylation of Akt.[Citation65] Several studies reported by different researchers and scientists determined the cardio protective role of AA in the infarct border zone of the ischemic myocardium. Several pathways are involved such as inhibition of left ventricular remodeling and improved cardiac function. Furthermore, it also blocked the MAPK and ERK1/2 in the infarct border zone of the ischemic myocardium as well as also improved mitochondrial dysfunction, suppressed the matrix metalloproteinase-9 induction and activation, and attenuated the infarct volume.[Citation66–68] In another trial, asiatic acid prevented myocardial infarction in rats via several mechanisms, such as reduced amounts of inflammatory cytokines in mRNA expression, interstitial fibrosis, suppression of cardiac hypertrophy, and blocked phosphorylation of p38 MAPK and ERK1/2/2/2.[Citation66] Si and their fellows investigated the significant impact of AA on the heart by suppressing the pressure overload-induced cardiac hypertrophy by lowering the TGF-β1 and NF-κB levels and blocking p38 MAPK and ERK1/2 phosphorylation.[Citation60] Induction of high glucose diet to the experimental subjects caused cardiomyocytes whilst AA protects against this injury.[Citation61] In C57BL/6 mice of in vivo trial, cardiac hypertrophy was induced through transverse aortic constriction by activating the interleukin (IL)-1β signaling pathway, enhancement of IL-1β-induced hypertrophic response of cardiomyocytes, ANP mRNA expression, surface area of cardiomyocytes, stimulated the NF-κB binding activity, enhanced the IL-1β and ANP levels, and activated the NF-κB in the hypertrophic myocardium whereas AA treatment to experimental subjects reverted these changes.[Citation69] In a recent study, Hu and colleagues determined the in vivo and in vitro cardio protective role of AA against the intraperitoneal DOX (15 mg/kg) induced cardiac complications by applying different doses i.e. (10 mg/kg or 30 mg/kg) for 2 weeks in experimental volunteers. Multiple mechanisms were reported as attenuation of cardiac injury, activation of protein kinase B (AKT) signaling pathway, and improvement in cardiac functions along with suppression of myocardial oxidative damage.[Citation70]
Anti-inflammatory role
Asiatic acid in human dopaminergic neuroblastoma SH-SY5Y cell line, murine microglial BV2 cell line and primary culture of rat embryo mesencephalic neurons protect from methamphetamine-induced TNF receptor (TNFR) expressions (TNFα and IL-6) in a dose-dependent manner. In METH-stimulated cells, AA markedly prevented the pathways of NF-κB/signal transducer translocation, STAT-3 activator, and ERK phosphorylation along with suppressing the proteolytic fragmentation of caspase-3 and PARP. Moreover, it also enhanced the Bcl-xL expressions (anti-apoptotic protein) and lowered the Bax levels (The (pro-apoptotic protein) as well as also protect from mitochondrial membrane damage. AA has been found to have therapeutic potential again various neurological disorders.[Citation71] Improvement in learning and memory in an animal model whereas induction of apoptosis in human neuroblastoma dopaminergic SH-SY5Y cells and attenuation of glutamate-induced cognitive deficits in mice were reported after AA treatment.[Citation72–74] Lipopolysaccharide has been used to induce inflammation levels in human corneal epithelial cells. The concentration of lipopolysaccharides at the rate of 300 ng/mL for 1 h to mice markedly increased the mRNA expression of TNF-α, IL-6, IL-1β, IL-8, and TGF-β, increased the ROS level, lowered the glutathione concentration, and enhanced the p-Akt level whilst AA administrated at the dose of 20 µmol/L reverted these changes.[Citation75] Fulminant hepatic failure (FHF) is predominantly associated with inflammation and oxidative stress. D-galactosamine and lipopolysaccharide have been used to induce fulminant hepatic failure by enhancing the aspartate aminotransferase and alanine transaminase, TNF- α, IL-1β, IL-6, MDA levels, MPO concentrations, lowering the antioxidant enzymes levels, and ROS development i.e. NO, H2O2, and O2− in experimental subjects. Both compounds also elevated the MAPK and NF-κB signaling pathways, and induced programmed cell death. Asiatic acid reverted these changes in d-galactosamine and lipopolysaccharide-induced fulminant hepatic failure in experimental animals. Activation of the nuclear factor-erythroid 2-related factor 2 (Nrf2) via inducing glycogen synthase kinase-3β (GSK3β) phosphorylation and AMP-activated protein kinase (AMPK) which are primarily linked with the induction of glutamate-cysteine ligase catalytic subunit, glutamate-cysteine ligase modifier subunit expressions, NAD (P) H: quinoneoxidoreductase 1, and heme oxygenase-1 induced by AA. Conclusively, AA prevented FHF by suppressing the MAPK and NF-κB activation via the partial induction of PDCD4 and upregulating Nrf2 in an AMPK/GSK3β pathway activation-dependent manner.[Citation21] Among different gynecological diseases, the pelvic inflammatory disorder is more prevailing in sexually active females with upper genital tract infections, including tubal ovarian abscesses, abnormal vaginal discharge, endometritis, adnexal pain, chronic pelvic pain, tubal infertility, ectopic pregnancy, and salpingitis.[Citation76–78]
The pharmacological effects of asiatic acid on the pelvic inflammatory disorder (PID) are, however, still unclear. The goal of this research was to examine the therapeutic effectiveness and possible pathways of AA in rats with regard to PID. The following five classes were randomly classified into a total of 75 female Sprague Dawley rats: the control group; the PID group; the PID + AA 5 mg/kg group; the PID + AA 35 mg/kg group; and the PID + AA 75 mg/kg group. Changes were evaluated in cytokine and chemokine levels, the activity of myeloperoxidase (MPO), nucleotide-binding domain-like receptor protein 3 (NLRP3), inflammasome, and nuclear factor-kB (NF-kB) activation, oxidative stress, and break caspase-3. The excessive development of cytokines and chemokines and the suppression of MPO behavior and the activation of NLRP3 the inflammasome, and caspase-3, as well as oxidative stress, were greatly reduced by AA therapy. These findings indicate that in rats with pathogen-induced PID.[Citation79]
Wound healing
Asiatic acid was used as a medicinal effect for burn wound recovery by speeding the mechanism of nerve degeneration.[Citation80–83] Studies suggest that both asiaticoside and madecassoside can help burn wound healing, probably by dramatically improving the synthesis of collagen I.[Citation84] The wound healing effects of C. asiatica were shown by Somboonwong et al.[Citation82] Extracts of Asiatica with both incision and burn wounds. In addition, thin-layer chromatography examined these extracts and it was seen that the phytoconstituents, specifically β-sitosterol, AA, asiaticoside and madecassocide, were found in various solvent extracts, respectively. Somboonwong et al.[Citation82] reported that the most active component for wound healing is AA in ethyl acetate extract. Bian et al.[Citation85] stated that AA was responsible for keloid management as it inhibited the expression of TGF-1-induced collagen and PAI-1 in keloid fibroblasts by activation of peroxisome proliferator-activated receptors and could be successful in combating the arsenal keloid. Asiatic acid can induce a steady gene Speech reaction in connective tissue disorders such as wound healing and microangiopathy, with its predominant pharmacological impact. Asiatic acid could defend against skin harm via its antiglycative action.[Citation86]
For wound healing, AA has been used and evidence has been increasingly positive of such arguments. Asiatic acid has been described as the primary active ingredient responsible for the medicinal effects of burn wound healing.[Citation80] In fact, asiatic acid is the key worthy component of a wound healing medicine (commercial name Madecassol) and can even accelerate the recovery of the nerves.[Citation83] Studies suggest that both asiaticoside and madecassoside can help burn wound healing, probably by dramatically improving the synthesis of collagen I.[Citation84] The wound healing effects of C were shown by Somboonwong et al.[Citation82] Extracts of Asiatica with both incision and burn wounds. In addition, thin-layer chromatography examined these extracts, and the phytoconstituents, specifically β-sitosterol, AA, asiaticoside, and madecassocide were found in various solvent extracts, respectively.
Asiatic acid has been found to be responsible for keloid control by inhibiting TGF-1-induced collagen and PAI-1 expression through peroxisome proliferator-activated receptor activation in keloid fibroblasts and may be successful in combating keloid in the arsenal. With its dominant pharmacological influence, AA causes a steady gene expression reaction in connective tissue disorders, such as wound healing and microangiopathy. AA could defend against skin harm via its antiglycative action.[Citation13]
Neuroprotective
Asiatic acid has been found to improve memory and cognitive capacity. Asiatic acid, being a neuroprotective factor, offers defense against the harmful effects of the Alzheimer’s mice model caused by amyloid-β (Aβ). Hippocampal neurons from amyloid precursor protein over-expressing Tg2576 mice and their wild-type (WT) littermates were used to investigate the impact of CAW and different compounds present inside the extract on Aβ-induced dendritic simplification and synaptic loss in a mouse model of Alzheimer’s disease, and to enhance learning and memory in stable aged mice as well. In WT neurons, CAW improved arborization and spine densities and avoided the decreased outgrowth of dendrites and spine degradation induced by exposure to Aβ in Tg2576 neurons. Triterpene compounds contained in CAW were found to enhance arborization in a similar way, but they did not influence the density of the spine. Caffeoylquinic acid (CQA) compounds from CAW, on the other hand, were able to modulate each of these endpoints, but there was precision as to which influence was induced by CQAs.[Citation87]
For certain cancer patients, 5-fluorouracil or 5-FU (a chemotherapeutic medication) has been found to cause memory deficits. Asiatic acid is able to inhibit the production of neurons and the memory loss caused by chemotherapy with 5-FU. Male rats of Sprague Dawley were administered AA (30 mg/kg) orally and 5-FU (25 mg/kg) by i.v. 5 times an injection. Some rats were given AA for 20 days prior to and after 15-FU (preventive) therapy, some were given AA for 20 days after 5-FU (recovery) treatment, and some were treated with AA for 40 days during the period of the trial. Important reductions in Notch1, sex defining area Y-box 2 (SOX2), nestin, doublecortin (DCX), and nuclear factor erythroid 2-related factor 2 (Nrf2) levels inside the hippocampus were triggered by 5-FU care. Furthermore, p21 positive cell numbers in the sub-granular zone (SGZ) and malondialdehyde (MDA) amounts in the hippocampus were substantially improved by 5-FU. The administration of both AA and 5-FU was able to stop reductions in Notch1 SOX2, nestin, DCX, and Nrf2 caused by 5-FU. AA therapy also contributed to reductions in p21-positive cells and in hippocampal MDA volumes.[Citation88]
In addition, Xu et al.[Citation72] reported the cognition-enhancing influence in monosodium glutamate-challenged neonatal mice and the underlying cause in human SH-SY5Y neuroblastoma cells. In addition to improved cognition in mice, asiatic acid improved neuronal injury, restored antioxidants and attenuated lipid peroxidation. Patil et al.[Citation89] found that AA modulates various pathophysiological enzymatic pathways correlated with amyloid-β development, including BACE1, ADAM10, IDE, and NEP, in the search for drugs to change the disease mechanism of AD. BACE1 regulates the production of amyloid-β from an amyloid-beta precursor protein (AβPP) as a rate-limiting enzyme, while ADAM10 manages the non-amyloidogenic processing of AβPP in rat-derived cortical neurons and efficiently, degrading Aβ, the enzymes IDE and NEP, are active. By reducing BACE1 and increasing the activities of IDE and ADAM10, AA regulates Aβ development and degradation.
Zhang et al.[Citation90] found that AA’s neuroprotective effects are mediated by ceramide-based lipid signaling pathways, biologically active sphingosine-derived lipids that play an important role in controlling mitochondrial activity and eventual death of neuronal cells.[Citation91] Asiatic acid was a dose-dependent reduction of ceramide-induced cell death and lack of capacity for mitochondrial membranes. In addition, AA also decreased ROS and HtrA2/Omi cytosolic release, increased caspase 3 expressions, Bax, and ERK1/2 dephosphorylation. AA’s neuroprotective function seems to be mediated by the ERK1/2 signaling system, which controls oxidative stress and apoptosis based on mitochondria.[Citation90]
Liver fibrosis
The last widespread mechanism for nearly all chronic liver disorders is liver fibrosis. It is characterized by excessive aggregation of hepatic stellate cells (HSC) and extracellular matrix (ECM) triggered by myofibroblast transformation detected by de novo a-SMA expression.[Citation92] A significant cause of liver disease is liver fibrosis, but therapy remains unsuccessful. The functions and anti-hepatofibrotic behaviors of asiatic acid have been studied in the current research in a rat model of carbon tetrachloride-induced liver fibrosis (CCl4) and in vitro in TGF-beta1-stimulated rat hepatic stellate cell line (HSC-T6). Treatment with AA substantially attenuated CCl4-induced liver fibrosis and dose-dependent functional dysfunction, including blockade of HSC activation as calculated by inhibiting the expression of de novo alpha smooth muscle actin (a-SMA) and collagen matrix, and improved ALT and ASTT output (all p,0.01). Upregulation of hepatic Smad7, an inhibitor of TGF-beta signaling, was correlated with the hepatoprotective effects of AA on fibrosis, thus blocking upregulation of TGFbeta1 and CTGF and activation of TGF-beta/Smad signaling. In HSC-T6, the anti-fibrosis function and functions of asiatic acid have been further identified in vitro. The addition of AA to HSC-T6 cells substantially induced Smad7 expression, thus inhibiting dosage-dependent TGF-beta1-induced Smad2/3 activation, myofibroblast transition, and collagen matrix expression. Smad7 knockdown in HSC-T6 cells, on the other hand, prevented AA-induced inhibition of HSC-T6 cell activation and fibrosis in reaction to TGF-beta1, showing that Smad7 played an important role in AA-induced anti-fibrotic behaviors during in vivo and in vitro liver fibrosis.[Citation93]
Asiatic acid administration effectively prevented CCl4-induced stimulation of HSC and liver fibrosis and significantly increased dosage-dependent liver functional damage in rats. Furthermore, we also observed that AA inclusion was able to inhibit TGF-beta1-induced HSC activation in a rat HSC-T6 cell line, such as a-SMA+ myofibroblast transformation and collagen matrix expression. More specifically, hepatic Smad7 upregulation, thus suppressing the signaling of TGF-beta/Smad, may be the underlying mechanism by which AA attenuates the liver caused by CCl4.[Citation94]
The identification that AA-induced upregulation of hepatic Smad7, thus inhibiting TGF-beta/Smad signaling, was a mechanism by which AA inhibits CCl4 or TGF-beta1-induced HSC activation and in vivo and in vitro liver fibrosis, was a novel and relevant finding in the present research. Indeed, induction of TGFbeta/Smad signaling in both experimental and human chronic liver diseases is a central function of liver fibrosis.[Citation95,Citation96]
Arthritis
In laboratory rats, RANKL has been shown to induce osteoclastogenesis and osteoclast activity, while AA administration substantially suppressed osteoclastogenesis, attenuated NF-dB transcription activity, inhibited osteoclast target genes, and osteoclast creation.[Citation97] The partnership between RANK and RANKL facilitates the degradation rate of IIBA via the release of proteasomes and NF-gesB.[Citation98] NF-egB is important for osteoclast formation and bone resorption via the activation of osteoclast-specific gene transcription.[Citation99]
Asiatic acid has also successfully attenuated the activity of NFAT and the protein level of NFATc1. NFATc1 and its associated factors are a key signal that contributes to osteoclast differentiation.[Citation100] Ca2+ oscillation induced by RANKL contributes to calcineurin activation and auto amplification of NFATc1.[Citation101] It is necessary for osteoclast inhibition to decrease Ca2+ signaling in osteoclasts and the degree of expression of NFATc1 protein after treatment with AA.[Citation102] NFAT signaling is controlled by NF- μB activity, so the observed AA inhibition effect of NFATc1 could be partly due to the attenuation of NF- μB activity.[Citation103] The data showed that NFATc1 activation target genes, such as Atp6v0d2, which were important for osteoclast fusion, were inhibited, suggesting that decreased NFATc1 expression was crucial in the process by which RANKL-induced osteoclastogenesis was inhibited by AA. In the MAPK signaling system involved in osteoclastic formation and survival, which was downstream of tumor necrosis factor receptor-associated factor 6 (TRAF6), AA was also used to inhibit ERK phosphorylation.[Citation104] Recently, Liu and colleagues observed that treatment with various concentrations (0, 5, 10, and 20 μM) of AA in vitro on the ACLT rat OA model substantially suppressed the hypertrophic and fibrotic chondrocyte phenotype, the phosphoinositide-3 kinase/protein kinase B (PI3K/AKT) signaling cascade, and the activated AMP-activated protein kinase (AMPK) phenotype.[Citation30]
Asiatic acid resulted in a dose-dependent inhibition of osteoclastogenesis caused by RANKL. Osteoclastogenesis was greatly decreased by AA at concentrations greater than 5 μM. An MTS cell survival assay was conducted to investigate the cell viability of AA-treated BMMs. At a dosage of 20 μM or below, AA has demonstrated no cytotoxic impact on BMMs. Hydroxyapatite-coated plates were used to classify the possible influence of AA on bone resorption. The results indicated that if osteoclasts were subjected to AA at 10 and 20 μM, the percentage of resorbed area per multinucleated osteoclast was attenuated compared with the usual control group. As per the report, in AA-treated osteoclasts, a slight decrease in osteoclast number was also observed, but this decrease is obviously less than the decrease in resorbing region. Our findings showed that osteoclast restorative feature is suppressed by AA.[Citation104]
Respiratory diseases
Asiatic acid has been successful in an animal model of chronic obstructive pulmonary disease by defending against pulmonary inflammation triggered by tobacco smoke. It reduced the development of ROS, norepinephrine involvement, pro-inflammatory cytokines, and inflammatory cell infiltration in the fluid of bronchoalveolar lavage in mice. In addition to increased development of HO-1 and SOD3 in lung tissues, it also reduced the overproduction of mucus, MCP-1 expression, and MAPKs as well as NF-uesB activation.[Citation7] In rats caused by spinal cord injury, AA was observed to attenuate acute lung injury. AA reduced pulmonary edema and neutrophil penetration, myeloperoxidase activation, pro-inflammatory cytokines and oxidative stress attenuation regulated by Nrf2 enhancement and NLRP3 inflammasome attenuation favorably modulated histologic shifts. In addition, the lung wet-to-dry weight ratio and the index of pulmonary permeability were both attenuated.[Citation105]
LPS-induced acute lung damage was attenuated by AA in another report.[Citation106] By inhibiting the TLR4/NF-μB signaling cascade, AA therapy blocked the activation of TLR4, pro-inflammatory cytokines and NF-kB. As shown by histological data, it prevented pathological changes in the lungs and showed a decline in myeloperoxidase production and neutrophil infiltration in bronchoalveolar lavage fluid.[Citation106] Asiatic acid was recently found to safeguard bleomycin-induced pulmonary fibrosis, a life-threatening disease of bleomycin chemotherapy in humans.[Citation5]
A debilitating, life-threatening illness worldwide is acute lung injury (ALI) triggered by sepsis. However, ALI mortality remains elevated owing to the lack of adequate care. It has been demonstrated that Asiatic acid (AA) has anti-inflammatory effects. In the present research, we observed that by inhibiting TLR4 expression and NF-kB activation in mice, AA attenuated lung damage caused by LPS. Asiatic acid may be a valuable medication to manage ALI caused by lipopolysaccharide (LPS). Gram negative bacteria’s key membrane part, LPS, reaches the lung, causing systemic inflammation and contributes to lung injury. The challenge of LPS contributes to lung edema and invasion by inflammatory cells. Reactive oxygen species, inflammatory cytokines, and hydrolytic proteinase, resulting in lung damage, may be produced by the penetration of neutrophils. Inhibition of the penetration of neutrophils could therefore attenuate LPS-induced lung injury. In this research, we observed that treatment with AA greatly blocked penetration into the lungs of LPS-induced neutrophils. A marker for tissue neutrophil content was used for MPO, an abundant portion of neutrophils.[Citation106]
By guarding against tobacco smoke-induced pulmonary inflammation, asiatic acid has been successful in an animal model of chronic obstructive pulmonary disease.[Citation7] It decreased the production of ROS, norepinephrine function, proinflammatory cytokines, and inflammatory cell infiltration in the fluid of bronchoalveolar lavage in mice. It also decreased mucus overproduction, MCP-1 expression, and MAPKs, as well as NF-2B activation, along with increased HO-1 and SOD3 expression in lung tissues.[Citation7] In rats caused by spinal cord injury, AA was observed to attenuate acute lung injury. Asiatic acid reduced pulmonary edema and neutrophil penetration, myeloperoxidase activation, pro-inflammatory cytokines and oxidative stress attenuation regulated by Nrf2 enhancement and NLRP3 inflammasome attenuation favorably modulated histologic shifts. It also attenuated the ratio of wet-to dry lung weight and pulmonary permeability index.[Citation12Clinical studies published on asiatic acid with findings against different diseases were shown in .
Table 1. Clinical studies published on asiatic acid with findings against different diseases.
Conclusion and future perspectives
This review provides significant evidence of the promising therapeutic applications of Asiatic acid and its derivatives for the treatment of different chronic disorders. Asiatic acid has received a great deal of consideration in molecular biology because of its pharmacological activities, low toxicity, and commercial availability. The results of different experimental data, including in vitro and in vivo studies, have confirmed the possible epidemiological evidence of asiatic acid in different diseases. While investigational results seem quite encouraging, due to its modest therapeutic action and low bioavailability, the clinical experiment of asiatic acid in the treatment of diseases is minimal. Interestingly, in a wide variety of diseases, derivatives of asiatic acid have also been studied and found to be significantly useful for their tremendous medicinal and therapeutic benefits. Studies have shown that the chemical alteration of the asiatic acid backbone has a high impact on the biological function of the backbone and can be a solution for enhancing not only asiatic acid’s antitumor activity, but also its pharmacokinetic properties. To elucidate the molecular mechanisms and pharmacological effects of asiatic acid (analogue and its derivatives), more detailed studies are needed to identify it as an ideal therapeutic agent in a variety of cancer-focused diseases. Because most asiatic acid therapeutic results are focused on in vitro and in vivo models, it is important to develop more detailed and well-controlled human trials in order to reliably evaluate the optimal administration dosage and route of administration with a strong fighting impact.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Alfarra, H. Y.; Omar, M. N. Centella asiatica: From Folk Remedy to the Medicinal Biotechnology - a State Revision. Int. J. Biosci. 2013, 3(6), 49–67.
- Nalinratana, N.; Meksuriyen, D.; Ongpipattanakul, B. Differences in Neuritogenic Activity and Signaling Activation of Madecassoside, Asiaticoside, and Their Aglycones in Neuro-2a Cells. Planta. med. 2018, 84(16), 1165–1173. DOI: 10.1055/a-0619-5710.
- Tang, L.; Yang, G.; Tan, J. Inhibitory Effect of Asiatic Acid on Expression of Collagen I Protein in HSC-T6 Cells. J. Fourth Mil. Med. Univ. 2007, 28, 1178–1180.
- He, Z.; Hu, Y.; Niu, Z.; Zhong, K.; Liu, T.; Yang, M.; Hu, W. A Review of Pharmacokinetic and Pharmacological Properties of Asiaticoside, a Major Active Constituent of Centella Asiatica (L.) Urb. J. Ethnopharmacol. 2022, 302, 115865. DOI: 10.1016/j.jep.2022.115865.
- Dong, S. H.; Liu, Y. W.; Wei, F.; Tan, H. Z.; Han, Z. D. Asiatic Acid Ameliorates Pulmonary Fibrosis Induced by Bleomycin (BLM) via Suppressing Pro-Fibrotic and Inflammatory Signaling Pathways. Biomed. Pharmacother. 2017, 89, 1297–1309. DOI: 10.1016/j.biopha.2017.03.005.
- Mavondo, G.; Tagumirwa, M. Asiatic Acid-Pectin Hydrogel Matrix Patch Transdermal Delivery System Influences Parasitaemia Suppression and Inflammation Reduction in P. Berghei Murine Malaria Infected Sprague–Dawley Rats. Asian. Pac. J. Trop. Med. 2016, 9(12), 1172–1180. DOI: 10.1016/j.apjtm.2016.10.008.
- Lee, J.; Park, H.; Kwon, O.; Jang, Y. G.; Kim, J. Y.; Choi, B. K.; Lee, H. J.; Lee, S.; Paik, J. H.; Oh, S. R., et al. Asiatic Acid Inhibits Pulmonary Inflammation Induced by Cigarette Smoke. Int. Immunopharmacol. 2016, 39, 208–217. DOI: 10.1016/j.intimp.2016.07.010.
- Qi, Z.; Ci, X.; Huang, J.; Liu, Q.; Yu, Q.; Zhou, J.; Deng, X. Asiatic Acid Enhances Nrf2 Signaling to Protect HepG2 Cells from Oxidative Damage Through Akt and ERK Activation. Biomed. Pharmacother. 2017, 88, 252–259. DOI: 10.1016/j.biopha.2017.01.067.
- Lu, Y.; Kan, H.; Wang, Y.; Wang, D.; Wang, X.; Gao, J.; Zhu, L. Asiatic Acid Ameliorates Hepatic Ischemia/Reperfusion Injury in Rats via Mitochondria-Targeted Protective Mechanism. Toxicol. Appl. Pharmacol. 2018, 338, 214–223. DOI: 10.1016/j.taap.2017.11.023.
- Gao, Z.; Gao, R.; Dong, X.; Zou, Z. M.; Wang, Q.; Zhou, D. M.; Sun, D. A. Selective Oxidation-Reduction and Esterification of Asiatic Acid by Pestalotiopsis Microspora and Anti-HCV Activity. Phytochem. 2017, 19, 108–113. DOI: 10.1016/j.phytol.2016.12.014.
- Ternchoocheep, K.; Surangkul, D. The Recovery and Protective Effects of Asiatic Acid on Differentiated Human Neuroblastoma SH-SY5Y Cells Cytotoxic-Induced by Cholesterol. Asian Pac. J. 2017, 7(5), 416–420. DOI: 10.1016/j.apjtb.2017.01.012.
- Nagoor Meeran, M. F.; Goyal, S. N.; Suchal, K.; Sharma, C.; Patil, C. R.; Ojha, S. K. Pharmacological Properties, Molecular Mechanisms, and Pharmaceutical Development of AA: A Pentacyclic Triterpenoid of Therapeutic Promise. Front Pharmacol. 2018, 9, 892. DOI: 10.3389/fphar.2018.00892.
- Lv, J.; Sharma, A.; Zhang, T.; Wu, Y.; Ding, X. Pharmacological Review on AA and Its Derivatives: A Potential Compound. SLAS Technol. 2018, 23(2), 111–127. DOI: 10.1177/2472630317751840.
- González-Coloma, A.; López-Balboa, C.; Santana, O.; Reina, M.; Fraga, B. Triterpene-Based Plant Defenses. Phytochem Rev. 2011, 10(2), 245–260. DOI: 10.1007/s11101-010-9187-8.
- Bylka, W.; Znajdek-Awizeń, P.; Studzińska-Sroka, E.; Dańczak-Pazdrowska, A.; Brzezińska, M. Centella Asiatica in Dermatology: An Overview. Phytother. Res. 2014, 28(8), 1117–1124. DOI: 10.1002/ptr.5110.
- Chandrika, U. G.; Prasad Kumarab, P. A. Gotu Kola (Centella asiatica): Nutritional Properties and Plausible Health Benefits. Adv. Food Nutr. Res. 2015, 76, 125–157.
- Sabaragamuwa, R.; Perera, C. O.; Fedrizzi, B. Ultrasound Assisted Extraction and Quantification of Targeted Bioactive Compounds of Centella Asiatica (Gotu Kola) by UHPLC-MS/MS MRM Tandem Mass Spectroscopy. Food Chem. 2022, 371, 131187. DOI: 10.1016/j.foodchem.2021.131187.
- Mony, T. J.; Elahi, F.; Choi, J. W.; Park, S. J. Neuropharmacological Effects of Terpenoids on Preclinical Animal Models of Psychiatric Disorders: A Review. Antioxid. 2022, 11(9), 1834. DOI: 10.3390/antiox11091834.
- Rutz, A.; Sorokina, M.; Galgonek, J.; Mietchen, D.; Willighagen, E.; Gaudry, A.; Allard, P. M. The LOTUS Initiative for Open Knowledge Management in Natural Products Research. Elife. 2022, 11, e70780. DOI: 10.7554/eLife.70780.
- Abbas, G.; Al Harrasi, A.; Hussain, H.; Hamaed, A.; Supuran, C. T. The Management of Diabetes Mellitus-Imperative Role of Natural Products Against Dipeptidyl Peptidase-4, α-Glucosidase and Sodium-Dependent Glucose Co-Transporter 2 (SGLT2). Bioorg. Chem. 2019, 86, 305–315. DOI: 10.1016/j.bioorg.2019.02.009.
- Lv, H.; Qi, Z.; Wang, S.; Feng, H.; Deng, X.; Ci, X. Asiatic Acid Exhibits Anti-Inflammatory and Antioxidant Activities Against Lipopolysaccharide and D-Galactosamine-Induced Fulminant Hepatic Failure. Front. Immunol. 2017, 8, 785. DOI: 10.3389/fimmu.2017.00785.
- Loganathan, C.; Thayumanavan, P. AA Prevents the Quinolinic Acid-Induced Oxidative Stress and Cognitive Impairment. Metab. Brain Dis. 2018, 33(1), 151–159. DOI: 10.1007/s11011-017-0143-9.
- Zhao, Y.; Shu, P.; Zhang, Y.; Lin, L.; Zhou, H.; Xu, Z.; Suo, D.; Xie, A.; Jin, X. Effect of Centella Asiatica on Oxidative Stress and Lipid Metabolism in Hyperlipidemic Animal Models. OXID. MED. CELL LONGEV. 2014, 2014, 1–7. DOI: 10.1155/2014/154295.
- Tsao, S. M.; Yin, M. C. Antioxidative and Antiinflammatory Activities of AA, Glycyrrhizic Acid, and Oleanolic Acid in Human Bronchial Epithelial Cells. J. Agric. Food. Chem. 2015, 63(12), 3196–3204. DOI: 10.1021/acs.jafc.5b00102.
- Mohapatra, P.; Ray, A.; Jena, S.; Nayak, S.; Mohanty, S. Influence of Various Drying Methods on Physicochemical Characteristics, Antioxidant Activity and Bioactive Compounds in Centella Asiatica L. Leaves: A Comparative Study. BioTechnologia. J. Biotechnol. Comput. Biol. Bionanotechnol. 2022, 103(3), 235–247. DOI: 10.5114/bta.2022.118666.
- Thakor, F. K.; Wan, K.; Welsby, P. J.; Welsby, G. Pharmacological Effects of Asiatic Acid in Glioblastoma Cells Under Hypoxia. Mol. Cell. Biochem. 2017, 430(1–2), 179–190. DOI: 10.1007/s11010-017-2965-5.
- Kavitha, C. V.; Jain, A. K.; Agarwal, C.; Pierce, A.; Keating, A.; Huber, K. M.; Wempe, M. F.; Agarwal, R.; Deep, G. Asiatic Acid Induces Endoplasmic Reticulum Stress and Apoptotic Death in Glioblastoma Multiforme Cells Both in vitro and in vivo. Mol. Carcinog. 2015, 54(11), 1417–1429. DOI: 10.1002/mc.22220.
- Lian, G. Y.; Wang, Q. M.; Tang, P. M. K.; Zhou, S.; Huang, X. R.; Lan, H. Y. Combination of Asiatic Acid and Naringenin Modulates NK Cell Anti-Cancer Immunity by Rebalancing Smad3/Smad7 Signaling. Mol. Ther. 2018, 26(9), 2255–2266. DOI: 10.1016/j.ymthe.2018.06.016.
- Gou, X. J.; Bai, H. H.; Liu, L. W.; Chen, H. Y.; Shi, Q.; Chang, L. S. … Zhang, L. M. Asiatic Acid Interferes with Invasion and Proliferation of Breast Cancer Cells by Inhibiting WAVE3 Activation Through PI3K/AKT Signaling Pathway. Biomed Res. Int. 2020, 2020, 1–12. DOI: 10.1155/2020/1874387.
- Liu, Y. T.; Chuang, Y. C.; Lo, Y. S.; Lin, C. C.; Hsi, Y. T.; Hsieh, M. J.; Chen, M. K. Asiatic Acid, Extracted from Centella Asiatica and Induces Apoptosis Pathway Through the Phosphorylation p38 Mitogen-Activated Protein Kinase in Cisplatin-Resistant Nasopharyngeal Carcinoma Cells. Biomol. 2020, 10(2), 184. DOI: 10.3390/biom10020184.
- Hsu, Y. L.; Kuo, P. L.; Lin, L. T.; Lin, C. C. Asiatic Acid, a Triterpene, Induces Apoptosis and Cell Cycle Arrest Through Activation of Extracellular Signal-Regulated Kinase and p38 Mitogen-Activated Protein Kinase Pathways in Human Breast Cancer Cells. J. Pharmacol. Experiment. Ther. 2005, 313(1), 333–344. DOI: 10.1124/jpet.104.078808.
- Cui, Q.; Ren, J.; Zhou, Q.; Yang, Q.; Li, B. Effect of Asiatic Acid on Epithelial-Mesenchymal Transition of Human Alveolar Epithelium A549 Cells Induced by TGF-Beta1. Oncol. Lett. 2019, 17, 4285–4292. DOI: 10.3892/ol.2019.10140.
- Zhang, Q.; Lenardo, M. J.; Baltimore, D. 30 Years of NF-κB: A Blossoming of Relevance to Human Pathobiology. Cell. 2017, 168(1–2), 37–57. DOI: 10.1016/j.cell.2016.12.012.
- Liu, B.; Sun, L.; Liu, Q.; Gong, C.; Yao, Y.; Lv, X.; Lin, L.; Yao, H.; Su, F.; Li, D., et al. A Cytoplasmic NF-Κb Interacting Long Noncoding RNA Blocks Iκb Phosphorylation and Suppresses Breast Cancer Metastasis. Cancer Cell. 2015, 27(3), 370–381. DOI: 10.1016/j.ccell.2015.02.004.
- Buettner, R.; Mora, L. B.; Jove, R. Activated STAT Signaling in Human Tumors Provides Novel Molecular Targets for Therapeutic Intervention. Clin. Cancer Res. 2002, 8(4), 945–954.
- Huang, R. Z.; Liang, G. B.; Li, M. S.; Fang, Y. L.; Zhao, S. F.; Zhou, M. M.; Wang, H. S. Synthesis and Discovery of Asiatic Acid Based 1, 2, 3-Triazole Derivatives as Antitumor Agents Blocking NF-Κb Activation and Cell Migration. MedChemcomm. 2019, 10(4), 584–597. DOI: 10.1039/C8MD00620B.
- Ren, Z.; Schaefer, T. S. ErbB-2 Activates Stat3 Alpha in a Src- and JAK2-Dependent Manner. J. Biol. Chem. 2002, 277(41), 38486–38493. DOI: 10.1074/jbc.M112438200.
- Abdulghani, J.; Gu, L.; Dagvadorj, A.; Lutz J., Leiby B.; Bonuccelli G.; Lisanti M. P.; Zellweger T., Alanen K.; Mirtti T., et al. Stat3 Promotes Metastatic Progression of Prostate Cancer. Am. J. Pathol. 2008, 172(6), 1717–1728.
- Leeman-Neill, R. J.; Wheeler, S. E.; Singh, S. V.; Thomas S. M.; Seethala R. R.; Neill D. B.; Panahandeh M. C.; Hahm E. R.; Joyce S. C.; Sen M., et al. Guggulsterone Enhances Head and Neck Cancer Therapies via Inhibition of Signal Transducer and Activator of Transcription-3. Carcinogen. 2009, 30(11), 1848–1856.
- Yang, C.; Hornicek, F. J.; Wood, K. B.; Schwab, J. H.; Choy, E.; Mankin, H.; Duan, Z. Blockage of Stat3 with CDDO-Me Inhibits Tumor Cell Growth in Chordoma. Spine (Phila Pa 1976. 2010, 35(18), 1668–1675. DOI: 10.1097/BRS.0b013e3181c2d2b4.
- Fletcher, S.; Turkson, J.; Gunning, P. T. Molecular Approaches Towards the Inhibition of the Signal Transducer and Activator of Transcription 3 (Stat3) Protein. ChemMedchem. 2008, 3(8), 1159–1168. DOI: 10.1002/cmdc.200800123.
- Yu, H.; Jove, R. The STATs of Cancer – New Molecular Targets Come of Age. Nat. Rev. Cancer. 2004, 4(2), 97–105. DOI: 10.1038/nrc1275.
- Jing, Y.; Wang, G.; Ge, Y.; Xu, M.; Tang, S.; Gong, Z. AA-PMe, a Novel Asiatic Acid Derivative, Induces Apoptosis and Suppresses Proliferation, Migration, and Invasion of Gastric Cancer Cells. Oncol. Targets. Ther. 2016, 9, 1605–1621. DOI: 10.2147/OTT.S98849.
- Wang, G.; Jing, Y.; Cao, L.; Gong, C.; Gong, Z.; Cao, X. A Novel Synthetic Asiatic Acid Derivative Induces Apoptosis and Inhibits Proliferation and Mobility of Gastric Cancer Cells by Suppressing STAT3 Signaling Pathway. OncoTargets Therap. 2017, 10, 55. DOI: 10.2147/OTT.S121619.
- Wu, K.; Hu, M.; Chen, Z.; Xiang, F.; Chen, G.; Yan, W.; Peng, Q.; Chen, X. Asiatic Acid Enhances Survival of Human AC16 Cardiomyocytes Under Hypoxia by Upregulating MiR-1290. IUBMB Life. 2017, 69(9), 660–667. DOI: 10.1002/iub.1648.
- Sun, W.; Xu, G.; Guo, X.; Luo, G.; Wu, L.; Hou, Y.; Guo, X.; Zhou, J.; Xu, T.; Qin, L. Protective Effects of Asiatic Acid in a Spontaneous Type 2 Diabetic Mouse Model. Mol. Med. Rep. 2017, 16(2), 1333–1339. DOI: 10.3892/mmr.2017.6684.
- Ramachandran, V.; Saravanan, R.; Senthilraja, P. Antidiabetic and Antihyperlipidemic Activity of Asiatic Acid in Diabetic Rats, Role of HMG CoA: In vivo and in silico Approaches. Phytomed. 2014, 21(3), 225–232. DOI: 10.1016/j.phymed.2013.08.027.
- Ramachandran, V.; Saravanan, R. Efficacy of Asiatic Acid, a Pentacyclic Triterpene on Attenuating the Key Enzymes Activities of Carbohydrate Metabolism in Streptozotocin-Induced Diabetic Rats. Phytomed. 2013, 20(3–4), 230–236. DOI: 10.1016/j.phymed.2012.09.023.
- Ramachandran, V.; Saravanan, R. AA Prevents Lipid Peroxidation and Improves Antioxidant Status in Rats with Streptozotocin-Induced Diabetes. J. Funct. Foods. 2013, 5(3), 1077–1087. DOI: 10.1016/j.jff.2013.03.003.
- Lee, C. H.; Olson, P.; Evans, R. M. Minireview: Lipid Metabolism, Metabolicdiseases, and Peroxisome Proliferatoractivated Receptors. Endocrinol. 2003, 144(6), 2201–2207. DOI: 10.1210/en.2003-0288.
- Azevedo, M. F.; Camsari, C.; Sa, C. M.; Lima, C. F.; Fernandes-Ferreira, M.; Pereira-Wilson, C. Ursolic Acid and Luteolin-7-Glucoside Improve Lipid Profilesand Increase Liver Glycogen Content Through Glycogen Synthase Kinase-3. Phy-Totherapy Res. 2010, 24(S2), S220–224. DOI: 10.1002/ptr.3118.
- Ramachandran, V.; Saravanan, R. Glucose Uptake Through Translocation and Activation of GLUT4 in PI3K/Akt Signaling Pathway by Asiatic Acid in Diabetic Rats. Hum. Exp. Toxicol. 2015, 34(9), 884–893. DOI: 10.1177/0960327114561663.
- Hung, Y. C.; Yang, H. T.; Yin, M. C. Asiatic Acid and Maslinic Acid Protected Heart via Anti-Glycative and Anti-Coagulatory Activities in Diabetic Mice. Food Funct. 2015, 6(9), 2967–2974. DOI: 10.1039/C5FO00549C.
- Wen, X.; Sun, H.; Liu, J.; Cheng, K.; Zhang, P.; Zhang, L.; Ni, P. Naturally Occurring Pentacyclic Triterpenes as Inhibitors of Glycogen Phosphorylase: Synthesis, Structure−activity Relationships, and X-Ray Crystallographic Studies. J. Med. Chem. 2008, 51(12), 3540–3554.
- Zhang, L.; Chen, J.; Gong, Y.; Liu, J.; Zhang, L.; Hua, W.; Sun, H. Synthesis and Biological Evaluation of AA Derivatives as Inhibitors of Glycogen Phosphorylases. Chem. Biodivers. 2009, 6(6), 864–874. DOI: 10.1002/cbdv.200800092.
- Liu, J.; He, T.; Lu, Q.; Shang, J.; Sun, H.; Zhang, L. Asiatic Acid Preserves Beta Cell Mass and Mitigates Hyperglycemia in Streptozocin‐induced Diabetic Rats. Diabetes/metab. res. rev. 2010, 26(6), 448–454. DOI: 10.1002/dmrr.1101.
- Dai, Y.; Wang, Z.; Quan, M.; Lv, Y.; Li, Y.; Xin, H. B.; Qian, Y. Asiatic Acid Protests Against Myocardial Ischemia/Reperfusion Injury via Modulation of Glycometabolism in Rat Cardiomyocyte. Drug Des. Devel. Ther. 2018, 12, 3573. DOI: 10.2147/DDDT.S175116.
- Ma, Z. G.; Dai, J.; Wei, W. Y.; Zhang, W. B.; Xu, S. C.; Liao, H. H.; Tang, Q. Z. Asiatic Acid Protects Against Cardiac Hypertrophy Through Activating AMPKα Signalling Pathway. Int. J. Bio. Sci. 2016, 12(7), 861. DOI: 10.7150/ijbs.14213.
- Chen, Z.; Peng, I. C.; Sun, W.; Su, M. I.; Hsu, P. H.; Fu, Y.; Zhu Y.; DeFea K.; Pan S.; Tsai M. D., et al. AMP-Activated Protein Kinase Functionally Phosphorylates Endothelial Nitric Oxide Synthase Ser633. Circ. Res. 2009, 104(4), 496–505.
- Si, L.; Xu, J.; Yi, C.; Xu, X.; Wang, F.; Gu, W., Zhang Y., Wang X. Asiatic Acid Attenuates Cardiac Hypertrophy by Blocking Transforming Growth Factor-β1-Mediated Hypertrophic Signaling in vitro and in vivo. Int. J. Mol. Med. 2014, 34(2), 499–506.
- Chan, C. Y.; Mong, M. C.; Liu, W. H.; Huang, C. Y.; Yin, M. C. Three Pentacyclic Triterpenes Protect H9c2 Cardiomyoblast Cells Against High-Glucose-Induced Injury. Free Radic. Res. 2014, 48(4), 402–411. DOI: 10.3109/10715762.2014.880113.
- Thakur, S.; Viswanadhapalli, S.; Kopp, J. B.; Shi, Q.; Barnes, J. L.; Block, K.; Gorin, Y.; Abboud, H. E. Activation of AMP-Activated Protein Kinase Prevents TGF-Β1–induced Epithelial-Mesenchymal Transition and Myofibroblast Activation. Am. J. Pathol. 2015, 185(8), 2168–2180. DOI: 10.1016/j.ajpath.2015.04.014.
- Sciarretta, S.; Volpe, M.; Sadoshima, J. Mammalian Target of Rapamycin Signaling in Cardiac Physiology and Disease. Circ. Res. 2014, 114(3), 549–564. DOI: 10.1161/CIRCRESAHA.114.302022.
- Kuwabara, Y.; Horie, T.; Baba, O.; Watanabe, S.; Nishiga, M.; Usami, S.; Izuhara M.; Nakao T.; Nishino T.; Otsu K.; et al. MicroRNA-451 Exacerbates Lipotoxicity in Cardiac Myocytes and High-Fat Diet-Induced Cardiac Hypertrophy in Mice Through Suppression of the LKB1/AMPK Pathway. Circ. Res. 2015, 116(2), 279–288.
- Huang, X.; Zuo, L.; Lv, Y.; Chen, C.; Yang, Y.; Xin, H.; Qian, Y. Asiatic Acid Attenuates Myocardial Ischemia/Reperfusion Injury via Akt/GSK-3β/HIF-1α Signaling in Rat H9c2 Cardiomyocytes. Molecules. 2016, 21(9), 1248. DOI: 10.3390/molecules21091248.
- Huo, L.; Shi, W.; Chong, L.; Wang, J.; Zhang, K.; Li, Y. Asiatic Acid Inhibits Left Ventricular Remodeling and Improves Cardiac Function in a Rat Model of Myocardial Infarction. Exp. Ther. Med. 2016, 11(1), 57–64. DOI: 10.3892/etm.2015.2871.
- Lee, K. Y.; Bae, O. N.; Serfozo, K.; Hejabian, S.; Moussa, A.; Reeves, M.; Rumbeiha, W.; Fitzgerald, S. D.; Stein, G.; Baek, S. H., et al. Asiatic Acid Attenuates Infarct Volume, Mitochondrial Dysfunction, and Matrix Metalloproteinase-9 Induction After Focal Cerebral Ischemia. Stroke. 2012, 43(6), 1632–1638.
- Krishnamurthy, R. G.; Senut, M. C.; Zemke, D.; Min, J.; Frenkel, M. B.; Greenberg, E. J.; Yu, S. W.; Ahn, N.; Goudreau, J.; Kassab, M., et al. Asiatic Acid, a Pentacyclic Triterpene from Centella Asiatica, is Neuroprotective in a Mouse Model of Focal Cerebral Ischemia. J. Neurosci. Res. 2009, 87(11), 2541–2550.
- Xu, X.; Si, L.; Xu, J.; Yi, C.; Wang, F.; Gu, W.; Zhang, Y.; Wang, X. Asiatic Acid Inhibits Cardiac Hypertrophy by Blocking Interleukin-1β-Activated Nuclear Factor-Κb Signaling in vitro and in vivo. J. Thorac. Dis. 2015, 7(10), 1787–1797. DOI: 10.3978/j.issn.2072-1439.2015.10.41.
- Hu, X.; Li, B.; Li, L.; Li, B.; Luo, J.; Shen, B. Asiatic Acid Protects Against Doxorubicin-Induced Cardiotoxicity in Mice. Oxid. Med. Cell. Longev. 2020, 2020, 1–12. DOI: 10.1155/2020/5347204.
- Park, J.; Seo, Y. H.; Jang, J.; Jeong, C.; Lee, S.; Park, B. Asiatic Acid Attenuates Methamphetamine-Induced Neuroinflammation and Neurotoxicity Through Blocking of NF-Kb/STAT3/ERK/STAT3/ERK and Mitochondria-Mediated Apoptosis Pathway. J. Neuroinflammation. 2017, 14(1), 240. DOI: 10.1186/s12974-017-1009-0.
- Xu, M. F.; Xiong, Y. Y.; Liu, J. K.; Qian, J. J.; Zhu, L.; Gao, J. Asiatic Acid, a Pentacyclic Triterpene in C. asiatica, Attenuates Glutamate-Induced Cognitive Deficits in Mice and Apoptosis in SH-SY5Y Cells. Acta. Pharmacol. Sin. 2012, 33(5), 578–587. DOI: 10.1038/aps.2012.3.
- Sirichoat, A.; Chaijaroonkhanarak, W.; Prachaney, P.; Pannangrong, W.; Leksomboon, R.; Chaichun, A.; Wigmore, P.; Welbat, J. U. Effects of Asiatic Acid on Spatial Working Memory and Cell Proliferation in the Adult Rat Hippocampus. Nutrients. 2015, 7(10), 8413–8423. DOI: 10.3390/nu7105401.
- Umka Welbat, J.; Sirichoat, A.; Chaijaroonkhanarak, W.; Prachaney, P.; Pannangrong, W.; Pakdeechote, P.; Sripanidkulchai, B.; Wigmore, P. Asiatic Acid Prevents the Deleterious Effects of Valproic Acid on Cognition and Hippocampal Cell Proliferation and Survival. Nutrients. 2016, 8(5), 303. DOI: 10.3390/nu8050303.
- Chen, H.; Hua, X. M.; Ze, B. C.; Wang, B.; Wei, L. The Anti-Inflammatory Effects of Asiatic Acid in Lipopolysaccharide-Stimulated Human Corneal Epithelial Cells. Int. J. Ophthalmol. 2017, 10(2), 179–185. DOI: 10.18240/ijo.2017.02.01.
- Gradison, M. Pelvic Inflammatory Disease. Am. Fam. Physician. 2012, 85(8), 791–796.
- Ross, J. D. Pelvic Inflammatory Disease. BMJ Clin Evid. 2013, 2013, 1606.
- Bu, X.; Liu, Y.; Lu, Q.; Jin, Z. Effects of “Danzhi Decoction” on Chronic Pelvic Pain, Hemodynamics, and Proinflammatory Factors in the Murine Model of Sequelae of Pelvic Inflammatory Disease. Evid. Based Complement. Alternat. Med. 2015, 2015, 547251. DOI: 10.1155/2015/547251.
- Kong, D.; Fu, P.; Zhang, Q.; Ma, X.; Jiang, P. Protective Effects of Asiatic Acid Against Pelvic Inflammatory Disease in Rats. Exp. Ther. Med. 2019, 17(6), 4687–4692. DOI: 10.3892/etm.2019.7498.
- Wu, F.; Bian, D.; Xia, Y.; Gong, Z.; Tan, Q.; Chen, J.; Dai, Y. Identification of Major Active Ingredients Responsible for Burn Wound Healing of Centella Asiatica Herbs. Evid. Based Complement. Alternat. Med. 2012, 2012, 1–13. DOI: 10.1155/2012/848093.
- Kimura, Y.; Sumiyoshi, M.; Samukawa, K.; Satake N.; Sakanaka M. Facilitating Action of Asiaticoside at Low Doses on Burn Wound Repair and Its Mechanism. Eur. J. Pharmacol. 2008, 584(2–3), 415–423.
- Somboonwong, J.; Kankaisre, M. Wound Healing Activities of Different Extracts of Centella Asiatica in Incision and Burn Wound Models: An Experimental Animal Study. BMC Complement. Altern. Med. 2012, 12(1), 103. DOI: 10.1186/1472-6882-12-103.
- Soumyanath, A.; Zhong, Y.; Yu, X. Centella Asiatica Accelerates Nerve Regeneration Upon Oral Administration and Contains Multiple Active Fractions Increasing Neurite Elongation in-Vitro. J. Pharm. Pharmacol. 2005, 57(9), 122–129. DOI: 10.1211/jpp.57.9.0018.
- Bylka, W.; Znajdek-Awiżeń, P.; Studzińska-Sroka, E.; Brzezińska, M. Centella Asiatica in Cosmetology. Postep. Dermatol. Alergol. 2013, 1, 46–49. DOI: 10.5114/pdia.2013.33378.
- Bian, D.; Zhang, J.; Wu, X.; Dou, Y.; Yang, Y.; Tan, Q.; Xia, Y.; Gong, Z.; Dai, Y. Asiatic Acid Isolated from Centella Asiatica Inhibits TGF-β1-Induced Collagen Expression in Human Keloid Fibroblasts via PPAR-γ Activation. Int. J. Biol. Sci. 2013, 9(10), 1032–1042. DOI: 10.7150/ijbs.7273.
- Wang, Z. Anti-Glycative Effects of Asiatic Acid in Human Keratinocyte Cells. Biomed. 2014, 4(3), 19. DOI: 10.7603/s40681-014-0019-9.
- Gray, N. E.; Zweig, J. A.; Murchison, C.; Caruso, M.; Matthews, D. G.; Kawamoto, C.; Soumyanath, A. Centella Asiatica Attenuates Aβ-Induced Neurodegenerative Spine Loss and Dendritic Simplification. Neurosci. Lett. 2017, 646, 24–29. DOI: 10.1016/j.neulet.2017.02.072.
- Welbat, J. U.; Chaisawang, P.; Pannangrong, W.; Wigmore, P. Neuroprotective Properties of Asiatic Acid Against 5-Fluorouracil Chemotherapy in the Hippocampus in an Adult Rat Model. Nutrients. 2018, 10(8), 1053. DOI: 10.3390/nu10081053.
- Patil, S. P.; Maki, S.; Khedkar, S. A.; Rigby, A. C.; Chan, C. Withanolide a and Asiatic Acid Modulate Multiple Targets Associated with Amyloid-β Precursor Protein Processing and Amyloid-β Protein Clearance. J. Nat. Prod. 2010, 73(7), 1196–1202. DOI: 10.1021/np900633j.
- Zhang, X.; Wu, J.; Dou, Y.; Xia, B.; Rong, W.; Rimbach, G.; Lou, Y. Asiatic Acid Protects Primary Neurons Against C2-Ceramide-Induced Apoptosis. Eur. J. Pharmacol. 2012, 679(1–3), 51–59. DOI: 10.1016/j.ejphar.2012.01.006.
- Arboleda, G.; Morales, L. C.; Benítez, B.; Arboleda, H. Regulation of Ceramide-Induced Neuronal Death: Cell Metabolism Meets Neurodegeneration. Brain Res. Rev. 2009, 59(2), 333–346. DOI: 10.1016/j.brainresrev.2008.10.001.
- Friedman, S. L. Mechanisms of Hepatic Fibrogenesis. Gastroenterol. 2008, 134(6), 1655–1669. DOI: 10.1053/j.gastro.2008.03.003.
- Tang, L. X.; He, R. H.; Yang, G.; Tan, J. J.; Zhou, L., Meng X. M.; Huang X. R.; Lan H. Y. Asiatic Acid Inhibits Liver Fibrosis by Blocking TGF-Beta/Smad Signaling in vivo and in vitro. PLoS One. 2012, 7(2), e31350.
- Dong, M. S.; Jung, S. H.; Kim, H. J.; Kim, J. R.; Zhao, L. X., Lee E. S.; Lee E. J.; Yi J. B.; Lee N.; Cho Y. B., et al. Structure-Related Cytotoxicity and Anti-Hepatofibric Effect of Asiatic Acid Derivatives in Rat Hepatic Stellate Cell-Line, HSC-T6. Arch. Pharm. Res. 2004, 27(5), 512–517.
- Inagaki, Y.; Okazaki, I. Emerging Insights into Transforming Growth Factor Smad Signal in Hepatic Fibrogenesis. Gut. 2007, 56(2), 284–292. DOI: 10.1136/gut.2005.088690.
- Meindl-Beinker, N. M.; Dooley, S. Transforming Growth Factor-β and Hepatocyte Transdifferentiation in Liver Fibrogenesis. J. Gastroenterol. Hepatol. 2008, 23(s1), S122–127. DOI: 10.1111/j.1440-1746.2007.05297.x.
- Xu, J.; Wu, H. F.; Ang, E. S.; Yip, K.; Woloszyn, M.; Zheng, M. H.; Tan, R. X. NF-Κb Modulators in Osteolytic Bone Diseases. Cytokine Growth Factor Rev. 2009, 20(1), 7–17. DOI: 10.1016/j.cytogfr.2008.11.007.
- Brown, K. D.; Claudio, E.; Siebenlist, U. The Roles of the Classical and Alternative Nuclear Factor-kappaB Pathways: Potential Implications for Autoimmunity and Rheumatoid Arthritis. Arthritis Res. Ther. 2008, 10(4), 212. DOI: 10.1186/ar2457.
- Soysa, N. S.; Alles, N. NF-kappaB Functions in Osteoclasts. Biochem. Biophys. Res. Commun. 2009, 378(1), 1–5. DOI: 10.1016/j.bbrc.2008.10.146.
- Hirotani, H.; Tuohy, N. A.; Woo, J. T.; Stern, P. H.; Clipstone, N. A. The Calcineurin/Nuclear Factor of Activated T Cells Signaling Pathway Regulates Osteoclastogenesis in RAW264.7 Cells. J. Biol. Chem. 2004, 279(14), 13984–13992. DOI: 10.1074/jbc.M213067200.
- Kular, J.; Tickner, J.; Chim, S. M.; Xu, J. An Overview of the Regulation of Bone Remodelling at the Cellular Level. Clin. Biochem. 2012, 45(12), 863–873. DOI: 10.1016/j.clinbiochem.2012.03.021.
- Kajiya, H. Calcium Signaling in Osteoclast Differentiation and Bone Resorption. Adv. Exp. Med. Biol. 2012, 740, 917–932. DOI: 10.1007/978-94-007-2888-2_41.
- Takatsuna, H.; Asagiri, M.; Kubota, T.; Oka, K.; Osada, T.; Sugiyama, C.; Saito H.; Aoki K.; Ohya K.; Takayanagi H., et al. Inhibition of RANKL-Induced Osteoclastogenesis by (-)-DHMEQ, a Novel NF-kappaB Inhibitor, Through Downregulation of NFATc1. J. Bone Miner. Res. 2005, 20(4), 653–662.
- Hong, G.; Zhou, L.; Han, X.; Sun, P.; Chen, Z.; He, W.; Xu, J. Asiatic Acid Inhibits OVX-Induced Osteoporosis and Osteoclastogenesis via Regulating RANKL-Mediated NF-Κb and NFATC1 Signaling Pathways. Front. Pharmacol. 2020, 11, 331. DOI: 10.3389/fphar.2020.00331.
- Jiang, W.; Li, M.; He, F.; Yao, W.; Bian, Z.; Wang, X.; Zhu, L. Protective Effects of Asiatic Acid Against Spinal Cord Injury-Induced Acute Lung Injury in Rats. Inflammation. 2016, 39(6), 1853–1861. DOI: 10.1007/s10753-016-0414-3.
- Li, Z.; Xiao, X.; Yang, M. Asiatic Acid Inhibits Lipopolysaccharide-Induced Acute Lung Injury in Mice. Inflammation. 2016, 39(5), 1642–1648. DOI: 10.1007/s10753-016-0398-z.
- Siddique, A. I.; Mani, V.; Arivalagan, S.; Thomas, N. S.; Namasivayam, N. Asiatic Acid Attenuates Pre-Neoplastic Lesions, Oxidative Stress, Biotransforming Enzymes and Histopathological Alterations in 1,2-Dimethylhydrazine-Induced Experimental Rat Colon Carcinogenesis. Toxicol. Mech. Methods. 2017, 27(2), 136–150. DOI: 10.1080/15376516.2016.1273422.
- Siddique, A. I.; Mani, V.; Renganathan, S.; Ayyanar, R.; Nagappan, A.; Namasivayam, N. Asiatic Acid Abridges Pre-Neoplastic Lesions, Inflammation, Cell Proliferation and Induces Apoptosis in a Rat Model of Colon Carcinogenesis. Chem. Biol. Interact. 2017, 278, 197–211. DOI: 10.1016/j.cbi.2017.10.024.
- Hao, Y.; Huang, J.; Ma, Y.; Chen, W.; Fan, Q.; Sun, X.; Shao, M.; Cai, H. Asiatic Acid Inhibits Proliferation, Migration and Induces Apoptosis by Regulating Pdcd4 via the PI3K/Akt/mTOR/p70S6K Signaling Pathway in Human Colon Carcinoma Cells. Oncol. Lett. 2018, 15, 8223–8230. DOI: 10.3892/ol.2018.8417.
- Ren, L.; Cao, Q.; Zhai, F.; Yang, S.; Zhang, H. Asiatic Acid Exerts Anticancer Potential in Human Ovarian Cancer Cells via Suppression of PI3K/Akt/mTOR Signalling. Pharm. Biol. 2016, 54(11), 2377–2382. DOI: 10.3109/13880209.2016.1156709.
- Wu, T.; Geng, J.; Guo, W.; Gao, J.; Zhu, X. Asiatic Acid Inhibits Lung Cancer Cell Growth in vitro and in vivo by Destroying Mitochondria. Acta. Pharmaceutica Sinica B. 2017, 7(1), 65–72. DOI: 10.1016/j.apsb.2016.04.003.
- Xue, W.; Qian, L.; Dong-Sheng, Y.; Yu-Peng, C.; Shang, J.; Zhang, L.; Hong-Bin, S.; Jun, L. Asiatic Acid Mitigates Hyperglycemia and Reduces Islet Fibrosis in Goto-Kakizaki Rat, a Spontaneous Type 2 Diabetic Animal Model. Chin. J. Nat. Med. 2015, 13(7), 529–534. DOI: 10.1016/S1875-5364(15)30047-9.
- Chen, Y. N.; Wu, C. G.; Shi, B. M.; Qian, K.; Ding, Y. The Protective Effect of Asiatic Acid on Podocytes in the Kidney of Diabetic Rats. Am. J. Transl. Res. 2018, 10(11), 3733–3741.
- Liu, J.; Chen, L.; Lu, H. Research Article Asiatic Acid Enhances Antioxidant and Anti-Inflammatory Activity to Suppress Isoproterenol Induced Cardiotoxicity. 2018, 14(7), 1038–1045. DOI: 10.3923/ijp.2018.1038.1045.
- Gao, C.; Wang, F.; Wang, Z.; Zhang, J.; Yang, X. Asiatic Acid Inhibits Lactate-Induced Cardiomyocyte Apoptosis Through the Regulation of the Lactate Signaling Cascade. Int. J. Mol. Med. 2016, 38(6), 1823–1830. DOI: 10.3892/ijmm.2016.2783.
- Si, L.; Xu, J.; Yi, C.; Xu, X.; Ma, C.; Yang, J.; Wang, X. Asiatic Acid Attenuates the Progression of Left Ventricular Hypertrophy and Heart Failure Induced by Pressure Overload by Inhibiting Myocardial Remodeling in Mice. J. Cardiovasc. Pharmacol. 2015, 66(6), 558–568. DOI: 10.1097/FJC.0000000000000304.
- Bunbupha, S.; Prachaney, P.; Kukongviriyapan, U.; Kukongviriyapan, V.; Welbat, J. U.; Pakdeechote, P. Asiatic Acid Alleviates Cardiovascular Remodelling in Rats with L-NAME-Induced Hypertension. Clin. Exp. Pharmacol. Physiol. 2015, 42(11), 1189–1197. DOI: 10.1111/1440-1681.12472.
- Maneesai, P.; Bunbupha, S.; Kukongviriyapan, U.; Prachaney, P.; Tangsucharit, P.; Kukongviriyapan, V.; Pakdeechote, P. Asiatic Acid Attenuates Renin-Angiotensin System Activation and Improves Vascular Function in High-Carbohydrate, High-Fat Diet Fed Rats.
- Fong, L. Y.; Ng, C. T.; Cheok, Z. L.; Moklas, M. A. M.; Hakim, M. N.; Ahmad, Z. Barrier Protective Effect of Asiatic Acid in TNF-α-Induced Activation of Human Aortic Endothelial Cells. Phytomed. 2016, 23(2), 191–199. DOI: 10.1016/j.phymed.2015.11.019.
- Fong, L. Y.; Ng, C. T.; Yong, Y. K.; Hakim, M. N.; Ahmad, Z. Asiatic Acid Stabilizes Cytoskeletal Proteins and Prevents TNF--induced disorganization of cell-cell junctions in human aortic endothelial cells. Vasc. Pharmacol. 2019, 117, 15–26.
- Rather, M. A.; Thenmozhi, A. J.; Manivasagam, T.; Bharathi, M. D.; Essa, M. M.; Guillemin, G. J. Neuroprotective Role of Asiatic Acid in Aluminium Chloride Induced Rat Model of Alzheimer’s Disease. Front. Biosci (Schol. Ed.). 2018, 10(1), 262–275. DOI: 10.2741/s514.
- Rather, M. A.; Justin-Thenmozhi, A.; Manivasagam, T.; Saravanababu, C.; Guillemin, G. J.; Essa, M. M. Asiatic Acid Attenuated Aluminum Chloride-Induced Tau Pathology, Oxidative Stress and Apoptosis via AKT/GSK-3β Signaling Pathway in Wistar Rats. Neurotox. Res. 2019, 35(4), 955–968. DOI: 10.1007/s12640-019-9999-2.
- Cheng, W.; Chen, W.; Wang, P.; Chu, J. Asiatic Acid Protects Differentiated PC12 Cells from a 25–35-Induced Apoptosis and Tauhyperphosphorylation via Regulating PI3K/Akt/GSK-3 Signaling. Life. sci. 2018, 208, 96–101. DOI: 10.1016/j.lfs.2018.07.016.
- Nataraj, J.; Manivasagam, T.; Justin Thenmozhi, A.; Essa, M. M. Neuroprotective Effect of Asiatic Acid on Rotenone-Induced Mitochondrial Dysfunction and Oxidative Stress-Mediated Apoptosis in Differentiated SH-SYS5Y Cells. Nutr. Neurosci. 2017, 20(6), 351–359. DOI: 10.1080/1028415X.2015.1135559.
- Ding, H.; Xiong, Y.; Sun, J.; Chen, C.; Gao, J.; Xu, H. Asiatic Acid Prevents Oxidative Stress and Apoptosis by Inhibiting the Translocation of Synuclein into Mitochondria. Front. Neurosci. 2018, 12, 431. DOI: 10.3389/fnins.2018.00431.
- Nataraj, J.; Manivasagam, T.; Thenmozhi, A. J.; Essa, M. M. Neurotrophic Effect of Asiatic Acid, a Triterpene of Centella Asiatica Against Chronic 1-Methyl 4-Phenyl 1, 2, 3, 6-Tetrahydropyridine Hydrochloride/Probenecid Mouse Model of Parkinson’s Disease: The Role of MAPK, Pi3K-Akt-GSK3β and mTOR Signalling Pathways. Neurochem. Res. 2017, 42(5), 1354–1365. DOI: 10.1007/s11064-017-2183-2.
- Gopi, M.; Janardhanam, V. A. Asiaticoside: Attenuation of Rotenone Induced Oxidative Burden in a Rat Model of Hemiparkinsonism by Maintaining the Phosphoinositide-Mediated Synaptic Integrity. Pharmacol. Biochem. Behav. 2017, 155, 1–15. DOI: 10.1016/j.pbb.2017.02.005.
- Park, J. H.; Seo, Y. H.; Jang, J. H.; Jeong, C. H.; Lee, S.; Park, B. Asiatic Acid Attenuates Methamphetamine-Induced Neuroinflammation and Neurotoxicity Through Blocking of NF-Kb/STAT3/ERK/STAT3/ERK and Mitochondria-Mediated Apoptosis Pathway. J. Neuroinflammation. 2017, 14(1), 1–15. DOI: 10.1186/s12974-017-1009-0.
- Ceremuga, T. E.; Valdivieso, D.; Kenner, C.; Lucia, A.; Lathrop, K.; Stailey, O.; Bailey, H.; Criss, J.; Linton, J.; Fried, J. Evaluation of the Anxiolytic and Antidepressant Effects of Asiatic Acid, a Compound from Gotu Kola or Centella Asiatica, in the Male Sprague Dawley Rat. Aana J. 2015, 83(2), 91–98.
- Wang, Z.; Mong, M.; Yang, Y.; Yin, M. Asiatic Acid and Maslinic Acid Attenuated Kainic Acid-Induced Seizure Through Decreasing Hippocampal Inflammatory and Oxidative Stress. Epilepsy Res. 2018, 139, 28–34. DOI: 10.1016/j.eplepsyres.2017.11.003.
- Wei, L.; Chen, Q.; Guo, A.; Fan, J.; Wang, R.; Zhang, H. Asiatic Acid Attenuates CCl4-Induced Liver Fibrosis in Rats by Regulating the PI3K/AKT/mTOR and Bcl-2/bax Signaling Pathways. Int. Immunopharmacol. 2018, 60, 1–8. DOI: 10.1016/j.intimp.2018.04.016.
- Fan, J.; Chen, Q.; Wei, L.; Zhou, X.; Wang, R.; Zhang, H. Asiatic Acid Ameliorates CCl4-Induced Liver Fibrosis in Rats: Involvement of Nrf2/ARE, NF-kappaB/ikappabalpha, and JAK1/STAT3 Signaling Pathways. Drug Des. Dev. Ther. 2018, 12, 3595–3605. DOI: 10.2147/DDDT.S179876.
- Lu, Y.; Liu, S.; Wang, Y.; Wang, D.; Gao, J.; Zhu, L. Asiatic Acid Uncouples Respiration in Isolated Mouse Liver Mitochondria and Induces HepG2 Cells Death. Eur. J. Pharmacol. 2016, 786, 212–223. DOI: 10.1016/j.ejphar.2016.06.010.
- Xu, Y.; Yao, J.; Zou, C.; Zhang, H.; Zhang, S.; Liu, J.; Ma, G.; Jiang, P.; Zhang, W. Asiatic Acid Protects Against Hepatic Ischemia/Reperfusion Injury by Inactivation of Kupffer Cells via PPARγ/NLRP3 Inflammasome Signaling Pathway. Oncotarget. 2017, 8(49), 86339–86355. DOI: 10.18632/oncotarget.21151.
- Kamble, S. M.; Patil, C. R. Asiatic Acid Ameliorates Doxorubicin-Induced Cardiac and Hepato-Renal Toxicities with Nrf2 Transcriptional Factor Activation in Rats. Cardiovasc. Toxicol. 2018, 18(2), 131–141. DOI: 10.1007/s12012-017-9424-0.