?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
The objective of this study was to investigate the effects of ultrasonically emulsified palm oil (UE-PA), peanut oil (UE-PE), and sunflower oil (UE-SU) on the whiteness, textural, rheological, and microstructural properties of surimi gels from silver carp. Three ultrasonically emulsified oils had different specific fatty acids and oil droplet sizes of 300-400 nm. Compared with the control group, low addition of UE-SU (2.5 g/100 g) showed the highest water-holding capacity (WHC), storage modulus (G’), and hydrogen bond in surimi gels. The low addition of UE-PE (2.5 g/100 g) increased disulfide bond, β-sheet, and β-turn of surimi gel, so had higher hardness and chewiness values, comparing to those with other emulsified oils. As ultrasonically emulsified oil addition increased in surimi gels (2.5–7.5 g/100 g), hardness, chewiness, WHC, G’ were decreased, while whiteness was increased (p < .05). Light microscopy showed the most uniform distribution of oil droplets of UE-SU in surimi gels. Results demonstrated that lower addition of UE-PE and UE-SU (2.5 g/100 g) could potentially improve the whiteness, textural, rheological, and nutritional quality of surimi-based products.
Introduction
Surimi is a high-quality source of protein, because of the unique gel properties of myofibrillar protein. It can be used to produce surimi-base products, due to its excellent texture and high nutritional value. Recently, there is an increase for ready-to-cook surimi-based products in the market, such as fish ball, fish sausage, and fish cake. Silver carp is one of the most popular freshwater fish in China, with its higher nutritional value, shorter production cycle, and lower cost. However, its market value is still low due to its small fishbone and earthy smell.[Citation1] Therefore, it is necessary to improve the economic and edible value by value-added processing, such as surimi processing.
Edible oil plays a major role in the texture, color, and flavor of surimi-based products.[Citation2] However, most of fish fat is removed during the washing. Therefore, appropriate addition of edible oil can not only improve the flavor but also increase the nutritional compositions in surimi-based products. However, the addition of vegetable oil might reduce the protein content and affected the texture,[Citation2] increase the oil oxidation of surimi and reduce the shelflife of surimi-based product.[Citation3] An emulsion is a fine dispersion of the liquid in another immiscible liquid,[Citation4] which requires surfactant to play a stabilizing role in the two liquid phases. Pre-emulsification is a method of forming the emulsion from water and oil by mechanically shearing or high-pressure homogenizing with non-meat protein as the emulsifier.[Citation5] Ultrasound, a powerful method of producing emulsion, can produce more uniformly distributed, smaller particle size emulsion droplets with lower energy consumption and less surfactant.[Citation6] The principle of ultrasonic emulsification is to use the physical effect by bursting the cavitation bubbles to change the characteristics of vegetable oil.[Citation7,Citation8] Ultrasonic treatment improved several indicators of vegetable oil: nanoscale particle size, more concentrated particle size distribution, increased whiteness, and higher emulsion stability. In particular, the high-intensity ultrasound can alter the tertiary structure of the protein, expose more hydrophobic groups to the interface, and form more viscoelastic interface protein film at the oil–water interface, resulting in enhancing the emulsion stability.[Citation9,Citation10] There are two types of ultrasonic applications for the emulsion[Citation11,Citation12]: one is post-emulsification ultrasound, and the other is post-ultrasonic emulsification. The first is the protein as an emulsifier initially homogenized to form coarse emulsion, and then the homogenized and stable emulsion is prepared by the ultrasound. The second is ultrasound modified protein and then emulsified to form the emulsion. High-intensity ultrasound was utilized to improve the particle size distribution, viscosity, and storage stability of diacylglycerol emulsion prepared with sodium caseinate.[Citation13] With the increase of ultrasonic power, the particle size of grape seed oil droplets decreased and the distribution of oil droplets became more concentrated.[Citation14] Therefore, there would be a potential to add ultrasonically emulsified edible oil into surimi to produce the uniform distribution of oil droplets in the surimi system. It might potentially improve the binding ability of fatty acids from edible oil, enhance the physical stability of gel structure, and enrich nutritional values of surimi-based products, such as polyunsaturated fatty acids.
Normally, animal fat and vegetable oil are added into surimi-based product to improve its quality. Animal fat tends to have a strong flavor with the higher content in saturated fatty acids and cholesterol, which can lead to obesity and cardiovascular disease.[Citation15] Vegetable oil has light flavor but higher unsaturated fatty acids than animal fat. From a health perspective, consumers prefer surimi-based product with little or no animal fat.[Citation16] Different vegetable oils have different effects on the quality of surimi-based products due to their compositions of fatty acids.[Citation2] With higher oleic acid, camellia oil could increase the textural and flavor characteristics of surimi gels.[Citation17] With higher lauric acid, coconut oil significantly reduced the water-holding capacity (WHC) and breaking strength of surimi gels.[Citation18] With higher ω-3 fatty acid, vegetable oils could improve the gelling and rheological properties of surimi.[Citation19] There was a lack of research on the application of ultrasonically emulsified vegetable oils with different fatty acid compositions in surimi products. The objective of this research was to compare the effects of palm oil, peanut oil, and sunflower oil emulsified by ultrasound on the physicochemical characteristics of surimi gel from silver carp. It would further understand the interactions of emulsified vegetable oil and fish protein and improve the quality of surimi-based product.
Materials and methods
Materials and chemicals
Frozen silver carp surimi (AA-grade, water content 75.36 ± 0.25%) was obtained from Honghu Jingli Aquatic Food Co., Ltd. (Honghu, Hubei, China) and stored at −20°C. Vegetable oils included peanut oil (PE) (Luhua Group Co., Ltd., Yantai, Shandong, China), palm oil (PA), and sunflower oil (SU) (Yikai Kerry Food Marketing Co., Ltd., Shanghai, China). Sodium caseinate (SC, food grade) was purchased from Yinuo Biotechnology Co., Ltd. (Hangzhou, Zhejiang, China). Analytical grade chemicals were used in the experiment.
Sample preparation
Preparation of emulsified vegetable oils: Ultrasound was used to emulsify palm oil, peanut oil, and sunflower oil.[Citation20] Firstly, sodium caseinate (SC, 6.0 g) was mixed with 64 mL distilled-water and 30 mL vegetable oil (palm oil, peanut oil, and sunflower oil, respectively). Then, coarse emulsion (C) was homogenized at 10,000 r/min for 1 min at 1 min intervals for a total of 5 times using a homogenizer (XHF-DY, Ningbo Xinzhi Biotechnology Co., Ningbo, China). Finally, coarse emulsion was further ultrasonicated by an ultrasonic cell processer (Sonics, Model VC750, Sonica & Materials, Inc., Newtown, CT, USA) to obtain ultrasonic emulsions (U) under a power of 750 W, a constant frequency of 20 kHz ±50 Hz and the amplitude of 40% for 5 min.
Preparation of composite surimi gel: Frozen surimi was thawed in a refrigerator (4°C) for 12 h. Thawed surimi (100 g) was cut into 1 × 1 × 1 cm3 pieces and chopped with 2.5 g NaCl for 3 min. UE-PA, UE-PE, and UE-SU (2.5, 5.0, 7.5 g/100 g surimi) were added into the surimi, respectively, and chopped for 3 min. The water content of surimi was adjusted to 80% with ice water. Finally, surimi paste was filled in the plastic shell with a diameter of 35 mm, drained of air bubbles and sealed with a clasp at both ends. The pastes were set at 40°C for 1 h and then heated at 90°C for 0.5 h in a water bath. All surimi gels were then cooled in crushed ice for 0.5 h and then stored at 4°C.
Emulsified vegetable oil
Fatty acid determination: Fatty acids were determined by the method of Zheng et al.[Citation21] with minor modification. The methyl esterification of fatty acids was analyzed by the gas chromatography (GC) (Agilent 7890A, Agilent Technology, Santa Clara, CA, USA). The volatile compounds were separated with the DB-WAX column (30 m × 0.32 mm I.D.×0.5 μm, Agilent Technology, Santa Clara, CA, USA). The GC was set at 250°C sample injector temperature and 250°C detector temperature. The carrier gas was nitrogen at a constant flow velocity of 3 mL/min. The sample (1 μL) was injected with a split ratio 10:1. Column box heating procedure was initially set at 50°C for 0.5 min, heated at a rate of 30°C/min to 194°C and hold for 3.5 min, and then heated to 240°C at a rate of 5°C/min and hold for 9 min. The relative content of fatty acids was calculated by the method of normalization to the peak areas.
Particle size distribution: As the method of Gani and Benjakul,[Citation20] the particle size distribution (average particle size and polydispersity index) of emulsion droplets was measured by a Zeta potentiometer (Brookhaven Instruments Corporation, Holtsville, NY, USA).
Whiteness
The L*(lightness), a*(redness/greenness), and b*(yellowness/blueness) values of surimi samples were determined by an Ultra Scan XE colorimeter (HunterLab Co., Ltd., Reston, VA, USA). The whiteness was shown by Eq (1), as described in Liu et al.[Citation2]:
Texture profile analysis (TPA)
According to the method of Chang et al.,[Citation22] the TPA of surimi gels were measured by a texture analyzer (TA-XT2i, Stable Micro Systems, Surrey, UK). Surimi gels were equilibrated at room temperature for 2 h and then cut into the cylindrical shape with a length of 20 mm. With a P/36 R cylindrical probe, the sample was compressed at a speed of 5 mm/s (pretest), 1 mm/s (mid-test), 5 mm/s (posttest), and a compression ratio of 40%.
Water holding capacity (WHC)
The WHC of gel samples was determined following the method of Gu et al.[Citation23] with minor modification. The gel sample (W1, g) was placed between two layers of filter paper, wrapped and put in a 50 mL centrifuge tube, then centrifuged (10,000 rpm) for 10 min at 4°C. The sample was removed and weighed for its mass (W2, g). The WHC was calculated by Eq (2):
Dynamic rheological properties
Dynamic rheological measurements were performed on an AR2000ex dynamic rheometer (TA Instrument Ltd., New Castle, DE, USA) referred to the method of Liu et al.[Citation2] with the modification. Surimi paste was placed between the carrier table and the parallel plate (40 mm diameter) at a spacing of 1 mm. The sample was sealed with silicone oil to prevent moisture loss during measurement. Storage modulus (G′) was measured, as the sample was heated from 20 to 90°C at a heating rate of 2°C/min with 1% strain and 1 Hz frequency.
Chemical interactions in surimi gel
The following procedure was described by Gu et al.[Citation23] with minor modification. The chemical interactions of surimi gels were distinguished according to the differences in the dissolution of chemical bonds in the four solutions (SA: 0.05 mol/L NaCl; SB: 0.6 mol/L NaCl; SC: 0.6 mol/L NaCl+1.5 mol/L urea; SD: 0.6 mol/L NaCl+8.0 mol/L urea). The gel samples (2.0 g) were mixed the above five solutions (10 mL), respectively, and homogenized for 1 min. Then, the mixtures were centrifuged (8,000 × g) at 4°C for 20 min. The differences in protein concentration represent the existence of ionic bond (SB – SA), hydrogen bond (SC – SB), hydrophobic interaction (SD – SC), respectively. Protein concentrations were determined by the method of Lowry.[Citation24] Free sulfhydryl (SHF), total sulfhydryl (SHT) and disulfide bond (S-S) were determined by the method of Jia et al.[Citation25] The content of S-S in the sample was calculated by Eq (3) as follows:
where A412 is the absorbance at 412 nm, C is the protein concentration (mg/mL), D is the dilution, and 73.53 is derived from 106/13,600 (13,600 M−1 cm−1 is Ellman’s reagent molar absorptivity).
Fourier-transform infrared spectroscopy (FTIR)
FTIR was used as the method of Zhao et al.[Citation26] with minor modification. Freeze-dried gel sample (1 mg) and dried potassium bromide (100 mg) were placed in an agate mortar for grinding. The mixed powder was placed in a press film (press conditions: pressure<25 kpa, 1 min) and the thin sheet was scanned and detected in a FTIR spectrometer (Nicolet 6700, Thermo Fisher Scientific, Waltham, MA, USA) with 4 cm−1 resolution, 64 scanning times, and 400–4,000 cm−1 scanning range.
Light microscopy
Light microscopy was used to observe the oil droplet distribution in the gel as the method of Zhuang et al.[Citation27] with minor modification. The gel was dehydrated with 30% (w/v) sucrose, then embedded and fixed by the Optimal Cutting Temperature compound (OCT). A gel slice (20 μm) was cut using a frozen sectioning machine (MG-100, Ming Mei Co., Ltd., Guangzhou, China) and adhered to a clean slide. The sample was firstly stained with 0.4 mg/mL bromophenol blue solution (protein dye) for 1 min and then with oil red stain (0.25% (w/v) oil red dissolved in isopropanol solution, diluted 3:2 in distilled water) for 8 min. The excess dye was rinsed in deionized water and the film was sealed with glycerogelatin. The oil droplet distribution of surimi gel was observed by a light microscope at 200 × magnification (MJ30, Guangzhou Mingmei Technology Co., Ltd., Guangzhou, China).
Statistical analysis
The analyses of variances were analyzed with Statistical Package for Social Sciences (SPSS) version 26.0 (SPSS Inc., Chicago, IL, USA). A p < .05 significance level was used to determine the differences between the treatments. All samples’ conditions were replicated in triplicates with three repeat measurements for each replication.
Results and discussion
Properties of emulsified vegetable oils
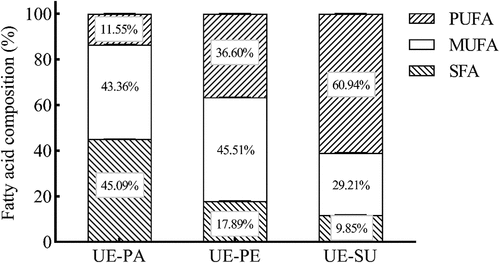
Fat composition analysis: shows fatty acid compositions, while there were significant differences in the fatty acid contents of three ultrasonically emulsified vegetable oils. UE-PA mainly contained palmitic acid (C16:0) and oleic acid (C18:1n9c), while its saturated fatty acid (SFA) content was higher than that of UE-PE and UE-SU. UE-PE mainly contained oleic acid (C18:1n9c), while its monounsaturated fatty acid (MUFA) content was higher than that of UE-PA and UE-SU. UE-SU contained mainly linoleic acid (C18:2n6c) and had a higher polyunsaturated fatty acid (PUFA) content than UE-PA and UE-PE. There were no remarkable change of fatty acid composition in olive oil by ultrasonic treatment.[Citation28] The fatty acid compositions were similar for native sunflower oil and one emulsified with high-frequency ultrasound.[Citation29] In general, each of three ultrasonically emulsified oils had its own specific fatty acids.
Particle size distribution of emulsions: Average particle size indicates a scale of the geometric size of oil droplets. Polydispersity index (PDI) represents the width of the distribution of the droplet size, and shows the deviation from the average particle size.[Citation30] Smaller the PDI indicates the closer oil droplet size of the average particle size.[Citation20] shows the particle size distributions of coarse emulsions and ultrasonic emulsion of three vegetable oils. It ranged from 3,000 to 3,500 nm for the average particle size of coarse emulsions, but it was 300–400 nm for ultrasonically emulsified oils (). There was no obvious difference in PDI values of three coarse emulsions. UE-SU had the lowest PDI value of three ultrasonic emulsions (). The PDI of less than 0.5 indicated a narrow particle size distribution.[Citation20] The particle size of the oil droplets in the ultrasonic emulsion decreased nearly 10 times, compared with the coarse emulsion of three vegetable oils. Mahogany seed oil formed a stable emulsion with a single peak particle size distribution by ultrasonic treatment.[Citation31] Compared with coarse emulsions, the cavitation effect by ultrasound intensified the shearing and destructing of oil droplets in the emulsion.[Citation32]
Figure 2. Average particle size (a) and polydispersity index (b) of coarse emulsions (c) and ultrasonic emulsions (U) of PA, PE and SU.
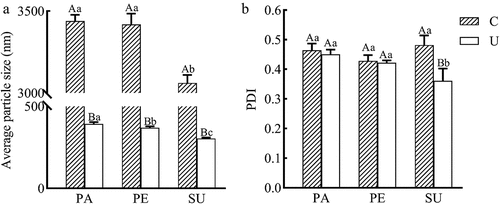
The average particle size of UE-PA was the largest, followed by UE-PE and UE-SU (), probably due to the fatty acid compositions of vegetable oils. The aggregation of oil droplets increased the particle size and flocculation of oil droplets and formed different size particles, resulting in increasing the distribution range of oil droplet size and PDI (polydispersity index) value.[Citation33] In emulsified oil, the interfacial protein film formed around oil droplet particles, leading the reduction of the aggregation and flocculation of oil droplets. The interface protein films formed by fatty acids with different saturation levels. Polyunsaturated fatty acid could form a thick viscoelastic interface film, which hindered the aggregation of oil droplets and reduced particle size of oil droplets.[Citation34] However, it was difficult for saturated and monounsaturated fatty acids to form rigid interfacial protein films, which could not inhibit the aggregation and stratification of emulsion.[Citation21] Results showed that UE-SU with the highest content of polyunsaturated fatty acids appeared the smallest particle size and lowest PDI value.
Whiteness
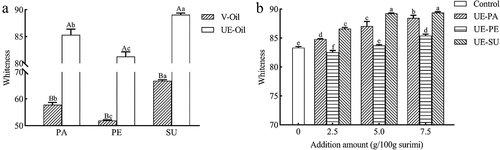
Whiteness is a vital characteristic to evaluate the quality of gel, which directly affects the choice of consumers.[Citation13] shows that the whiteness of sunflower oil is the highest, followed by palm oil and peanut oil. Ultrasonic emulsion significantly increased the whiteness of three vegetable oils. Compared with the control, the whiteness of surimi gels increased clearly with the increase of emulsified vegetable oils, except 2.5 and 5.0 g/100 g UE-PE (). The whiteness was the highest with 7.5 g/100 g emulsified vegetable oils. The type of vegetable oils had a key effect on the whiteness of surimi gel, while the addition of UE-SU had a higher whiteness of surimi gel, followed by UE-PA and UE-PE. When vegetable oil was added directly to surimi, the oil droplets adhered to the surface of the surimi gel, leading to light reflection. Ultrasound generated acoustic cavitation, which could form the emulsion with smaller oil droplets.[Citation32] After ultrasonic emulsification with sodium caseinate, an interfacial protein film formed on the surface of the oil droplets with a milky, white appearance, while its oil droplets were micron-sized in diameter, resulting in forming more uniform distribution in the gel and enhancing the light scattering effect on surimi gel.[Citation20] The whiteness of surimi gel was increased by virgin coconut oil nanoemulsion (5%) and diacylglycerol pre-emulsion (1–7%) by ultrasonic treatment.[Citation13,Citation20] Due to its own color, PE had the lowest whiteness among three vegetable oils and still had the lowest whiteness after ultrasonic emulsification (). Surimi gel with UE-PE also had the lowest whiteness among three emulsified oils (). Results showed that emulsified sunflower oil could significantly enhance the whiteness of surimi gel.
Figure 3. Whiteness of vegetable oil (V-Oil) and ultrasonic emulsified oil (UE-Oil) (a) and the effect of ultrasonic emulsified vegetable oils on whiteness of surimi gel (b).
TPA
The textural properties of surimi gel depend on the formation and stability of the three-dimensional network structure and reflect the ability of the surimi to form a gel during heating.[Citation22] shows the TPA values of surimi gels with different emulsified oils. Compared with the control, hardness, and chewiness of surimi gels changed with the increase of emulsified oils (p < .05). Hardness and chewiness of surimi gel with 2.5 g/100 g UE-PE were higher than those of the control and decreased significantly with more addition of UE-PE. Hardness and chewiness of surimi gel with 2.5 g/100 g UE-SU or UE-PA were not different from those of the control (p > .05), but also decreased with more addition of either UE-SU or UE-PA. UE-PA showed more decreases in the hardness and chewiness of surimi gel, compared to UE-SU. The type of vegetable oils had different effects on the texture of surimi gel, probably due to their fatty acid compositions and contents. Camellia oil with high oleic acid content significantly enhanced the texture of white croaker surimi gel[Citation17]; The emulsified peanut oil, rich in long-chain and unsaturated fatty acids, improved the overall texture of the emulsified surimi sausage[Citation35]; Palm oil, which was rich in medium chain and saturated fatty acids, was difficult to interact with myosin and form a stable emulsion system.[Citation21] UE-PE was rich in a variety of fatty acids, high in polyunsaturated fatty acids and long-chain fatty acids. After ultrasonic emulsification of peanut oil, the oil droplet size decreased to the nanoscale and was fully encapsulated by the protein. Long-chain fatty acids increased the combination of oil droplet and protein hydrophobic point and enhanced the hydrophobic interaction between oil and protein, resulting in a stronger oil-protein binding capacity.[Citation36] The low addition of UE-PE (2.5 g/100 g) improved the texture of surimi gel. However, with more addition of UE-PE, the excessive oil droplets tended to accumulate and become larger, which were difficult to fully be wrapped by protein films and hinder the interaction between protein films, resulting in the reduction of the textural properties of surimi gel.
Table 1. Effects of ultrasonic emulsified vegetable oils on texture of surimi gel.
In addition, three emulsified oils had no significant effect on the springiness, cohesiveness, and resilience of surimi gels, which was consistent with the results of Chang et al.[Citation22] There was no significant change in springiness of croaker surimi gel with virgin coconut oil (0 to 25%).[Citation18] There was no change in resilience and cohesiveness of surimi gel with nanoemulsion virgin coconut, compared to the control.[Citation20] Probably, emulsified oils might not change those textural parameters of surimi gels. Results displayed that lower addition of emulsified oils (2.5 g/100 g) either increased or unchanged TPA values of surimi gels, especially that UE-PE significantly enhanced the texture of surimi gel.
Whc
The WHC is the ability of the protein to bind water, which plays an important role in the gel stability.[Citation35] shows the WHC of surimi gels with different emulsified oils. Compared to the control, UE-PA and UE-SU remarkably increased the WHC of surimi gels, but UE-PE (2.5–7.5 g/100 g) did not change it. When vegetable oil was added into surimi, the large and unstable oil droplets weakened the compactness of the three-dimensional gel network structure and caused the loss of free water trapped in the network structure, resulting in a decrease in the WHC.[Citation37] The control had higher WHC than surimi gels with 2 mL/100 g vegetable oils (peanut, soybean, corn, coconut, olive, safflower seed oils, respectively).[Citation3] However, ultrasonic emulsification of vegetable oils stabilized the oil system and improved the oil-binding capacity, which helped to maintain a stable state in the surimi protein system.[Citation38] As an emulsifier, sodium caseinate had water absorption and could be strongly attracted by the fat water interface. After ultrasonic emulsification, the oil droplets in the emulsion existed as much tiny particles and were wrapped by a thin protein film. The emulsification should be able to increase the binding or absorbing the water of vegetable oils, but the regular vegetable oil is naturally hydrophobic. The protein membrane could also interact with water molecules in surimi gel and bind more water in the gel matrix,[Citation39] resulting in the increase of the WHC (). Ultrasonically emulsified soybean oil significantly improved the water and fat-binding capacities of chicken breast myofibrillar protein composite gels, leading to an increase in the WHC.[Citation40]
Figure 4. Effects of ultrasonic emulsified vegetable oils on WHC of surimi gel.
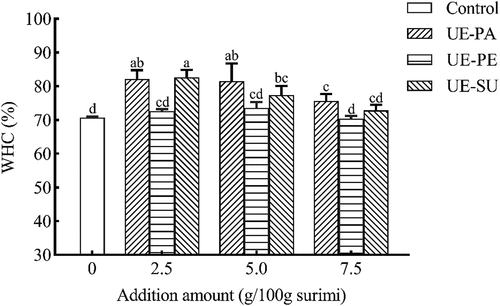
There were differences in the WHC of surimi gels with different emulsified oils, probably due to differently emulsifying characteristics. The ordered gel structure was more likely to hinder the flow of water molecules than the disordered one.[Citation41] UE-SU appeared the higher polyunsaturated fatty acid (), smaller particle size and PDI (). It could form a thick rigid interfacial mask with the emulsification of polyunsaturated fatty acids and proteins in sunflower oil.[Citation21] It reduced the interference of oil on myofibrillar protein crosslinking and formed a more ordered gel network structure to entrap more free water.[Citation34] However, UE-PE with higher oleic acid could not form a stable interfacial mask with protein.[Citation21] Therefore, three emulsified oils (2.5–7.5 g/100 g) either increased or unchanged the WHC of surimi gel, while surimi gels had the higher WHC with 2.5 g/100 g UE-PA or UE-SU.
Dynamic rheological characteristics
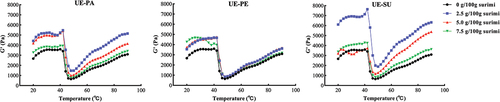
Rheological characteristic is a dynamic change of viscoelastic behavior of the samples from sol to gel state with the steady increase of temperature.[Citation42] shows that the similar trends in G’ of surimi with or without three emulsified oils as the temperature rose from 20°C to 90°C. With the increase of temperature, G’ rose firstly and reached the maximum at about 40°C. It should be attributed to thermally induced expansion of protein and the interaction between actin and myosin, forming a weak gel network structure and enhancing the solid performance of surimi.[Citation43] When the temperature rose over 40°C, G’ decreased rapidly and dropped to the lowest value at about 45°C. It was due to the activation of endogenous cathepsin in surimi, which degraded part of myofibrillar protein, resulting in loose network structure and deterioration of gel.[Citation44] Continuous heating could destroy numerous hydrogen bonds that maintained the protein structure.[Citation45] Further heating (45°C −90°C) resulted in continuous increase of G,’ due to the aggregation of a large number of proteins and the formation of disulfide bonds by the interaction of free sulfhydryl groups. It made myofibrillar protein form a rigid and irreversible gel with a more organized and stable network structure.[Citation46]
The G’ of surimi with three emulsified oils was higher than that of the control during the heating process (). Through ultrasonic emulsification with SC, the fine-grained oil droplets were wrapped in protein film to form the stable oil-water-SC emulsion. More myosin was encapsulated in the emulsified oil droplets due to its lipophilic nature, making it less susceptible to denaturation and more efficient in thermally induced gelation of surimi.[Citation35] With the increase of emulsified oils (2.5–7.5 g/100 g), G’ decreased in surimi with UE-SU and UE-PA, while there was no significant change in the UE-PE. UE-SU caused the highest G’ at an addition level of 2.5 g/100 g, probably due to its specific fatty acid compositions or higher polyunsaturated fatty acids. Peanut oil pre-emulsified by three emulsifiers could retain more myosin in surimi to participate in the formation of the gel structure and enhance the gel performance of surimi sausage.[Citation35] Results indicated that three emulsified oils could maintain or enhance the rheological characteristics of surimi paste, especially UE-SU.
Chemical interactions
In surimi gel system, the interactions between protein molecules (hydrogen bonds, ionic bonds, hydrophobic interactions and disulfide bonds) maintain the gel network structure and affect the gel performance.[Citation47] shows hydrogen bond, ionic bond, hydrophobic interaction, and disulfide bond of surimi gels with different emulsified oils. Hydrogen bond is an important chemical force that maintains the secondary structure of proteins. It can be broken when the protein is denatured by heating, but reformed during the cooling and enhanced the texture of the gel.[Citation48] Compared with the control, there was significant increase in hydrogen bond of surimi gel with 2.5 g/100 g UE-SU, while 2.5 g/100 g UE-PA or UE-PE did not significantly change such bond in surimi gel (). As the amount of emulsified oil increased (5.0–7.5 g/100 g), the hydrogen bond in the UE-SU decreased until there was no significant difference, compared to the control group. The hydrogen bond in the UE-PA did not change significantly, while the UE-PE slowly decreased to the lowest of all samples. The interfacial protein film could wrap a small amount of emulsified oil droplets with small particle size. It increased the specific surface area and enhanced protein–protein interactions, which facilitated the formation of hydrogen bond.[Citation49] Excessive emulsified oil decreased the hydrogen bond content of surimi gel, indicating that the interfacial protein film difficultly wrapped excessive oil droplets, while the aggregation of oil droplets reduced the protein hydration.[Citation50] The highest hydrogen bond content in surimi gels with UE-SU might probably be related to the formation of stable rigid interfacial protein film by the emulsification of polyunsaturated fatty acids and proteins. It was similar to influence of emulsified oils on the WHC of surimi gel (), indicating that hydrogen bond was very important for water stability in surimi gel. Results showed that there was the highest hydrogen bond in surimi gel with 2.5 g/100 g UE-SU, while there was decrease with more addition emulsified oils (>2.5 g/100 g).
Figure 6. Effects of ultrasonic emulsified vegetable oils on hydrogen bond (a), ionic bond (b), hydrophobic interaction (c) and disulfide bond (d) of surimi gel.
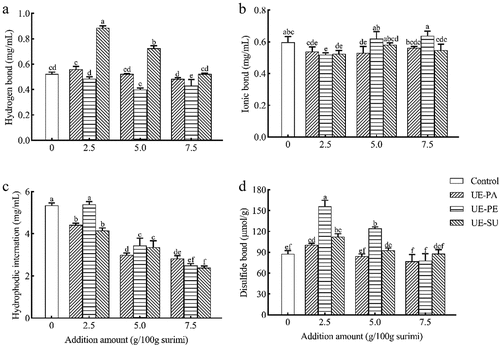
Ionic bond is the electrostatic interaction between amino acid residues with opposite charges.[Citation51] Ionic bond of surimi gel with 2.5 g/100 g UE-PA was not significantly different from the control, but that with UE-PE or UE-SU was lower than the control (). It significantly increased ionic bond of surimi gels with more addition of UE-PE (5.0–7.5 g/100 g), but it did not change with the other two emulsified oils (). This difference might probably be due to different fatty acid compositions. UE-PE with higher oleic acid and stearic acid could improve the surface charge of particles in emulsion and adsorb more proteins at the oil–water interface.[Citation21] Emulsified oils provided hydrophobic environment, exposed amino acid residues in protein molecules, and increased the electrostatic interaction between proteins.[Citation17,Citation51] Results showed that there was the highest ionic bond of surimi gel with more addition of UE-PE (5.0–7.5 g/100 g).
Hydrophobic interaction is produced by the thermal denaturation of protein and the exposure of hydrophobic group when surimi paste is heated to form the gel.[Citation52] Hydrophobic interaction decreased significantly in surimi gels with more addition of emulsified oils (>2.5 g/100 g) (). It was possible that the hydrophobic binding sites of oil droplets were difficult to be exposed due to the easy aggregation and larger particle size of excessive emulsified oils, reducing hydrophobic interactions with proteins. Excessive fish oil hindered protein unfolding and rearrangement, thus decreasing hydrophobic interaction of surimi gel.[Citation50] Compared with the control, extra virgin olive oil blocked the approach of myofibrillar protein hydrophobic groups and reduced hydrophobic interactions of surimi gel.[Citation53] Hydrophobic interaction of surimi gel with 2.5 g/100 g UE-PE was higher than that of other two emulsified oils (), so it was the highest hardness and chewiness () of surimi gel with UE-PE. Since there were the higher long-chain fatty acids in UE-PE, small amounts of oil droplets were more likely to bind to protein hydrophobic sites, forming hydrophobic interaction between lipid and protein.[Citation36] The lower UE-PE (2.5 g/100 g) had the highest hydrophobic interaction in surimi gels, while there were more decreases for hydrophobic interaction in surimi gels with more addition of emulsified oils.
Disulfide bond is important for thermally induced protein gels,[Citation54] which affects the texture and quality of surimi gel. Compared with the control, the content of disulfide bond was the highest in surimi gel with 2.5 g/100 g UE-PE, followed by UE-SU and UE-PA (). It was consistent with the results of hardness, chewiness of surimi gel with three emulsified oils, especially the highest parameters at 2.5 g/100 g UE-PE (). More addition of emulsified oils (>2.5 g/100 g) decreased disulfide bond in surimi gels, but their disulfide bonds were either higher in surimi gels than the control or no significant difference from the control. A small number of evenly distributed small oil droplets, such as UE-PE, probably promoted the interactions of myosin head-head and tail-tail, allowing exposed free sulfhydryl groups to form disulfide bonds during the heating process.[Citation55] And small oil droplets could make the free sulfhydryl groups located far away in space closer and easily to be oxidized into more disulfide bonds.[Citation56] Compared with the control, the addition of extra virgin olive oil remarkably increased disulfide bond of silver carp surimi gel.[Citation53] Hence, three emulsified vegetable oils (2.5–7.5 g/100 g) increased or maintained disulfide bonds in surimi gel, especially that the surimi gel with 2.5 g/100 g UE-PE had the highest disulfide bond content, resulting in a stronger gel network structure.
FTIR
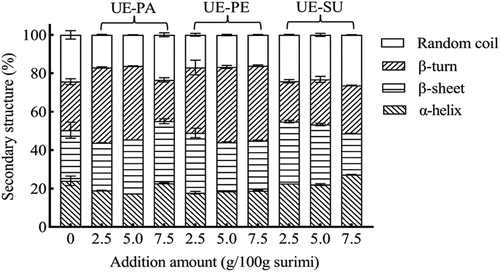
The amide I band (1700–1600 cm−1) of the FTIR spectra most sensitively reflects changes in protein secondary structure,[Citation26] including α-helix (1660–1650 cm−1), β-sheet (1640–1600 cm−1) and β-turn (1695–1660 cm−1), random coil (1650–1640 cm−1). shows the variation in the relative contents of the secondary structures of surimi gels with different emulsified oils. Compared with the control, the addition of UE-PA and UE-PE (2.5–5.0 g/100 g) decreased α-helices and random coil in surimi gels, but increased β-sheets and β-turns. The addition of those emulsified oils induced fish proteins to expose hydrophobic side chains for insertion into the oil droplets and increased protein-lipid and protein–protein interactions, resulting in the change of the secondary structures of fish protein. The increase of β-sheets in meat batter with soybean oil was based on the protein-lipid and protein–protein interactions.[Citation57] With more addition, there was no significant change for those structures in surimi gels with UE-PE. However, there were increases for random coil in surimi gels with 7.5 g/100 g UE-PA and UE-SU. This was consistent with the results that the lower textural properties of surimi gel with 7.5 g/100 g UE-PA and UE-SU (). Surimi protein α-helix tended to decrease with the increase of fish oil, but β-sheet was gradually increased.[Citation50] Excessive emulsified oils might change protein conformation from order to disorder and weaken the network structure of surimi gel.[Citation17] The lower addition of UE-PE (2.5 g/100 g) could significantly decrease α-helix and random coil but increase β-sheets and β-turns, showing the highest textural property of surimi gel ().
Microstructure of emulsified oils in surimi gels
Optical microscope was used to observe dispersion and aggregation of oil droplets in surimi gel. The formation of the microstructure of surimi gels depends on the interaction of myofibrillar protein, reflecting directly its gelling ability under thermal induction.[Citation3] shows the distribution of oil droplets in surimi gels with three emulsified oils. In the control group, there was no oil droplet, but there were small number of oil droplets with small particle size in surimi gels with 2.5 g/100 g emulsified oils. After ultrasonic treatment, acoustic cavitation could boost disruption of emulsion oil droplets and minimize the droplets size of stable O/W emulsion.[Citation32] The small oil droplets of emulsion increased the superficial area, exposed more sodium caseinate to the surface of the oil droplets and facilitated binding to the proteins.[Citation40]
Figure 8. Effects of ultrasonic emulsified vegetable oils on the oil droplet distribution (×200) of surimi gel.
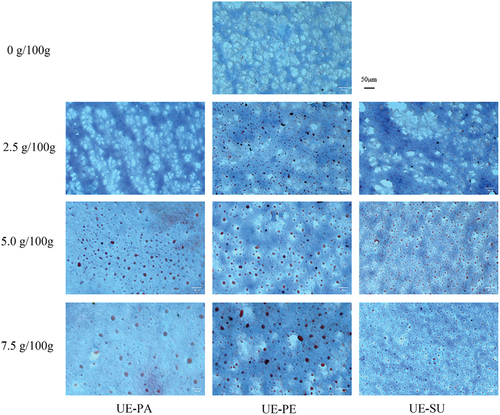
More addition (5.0–7.5 g/100 g) increased the oil droplet size and uneven oil distribution of surimi gels with UE-PA or UE-PE. However, there was not much change in the particle size of surimi gels with UE-SU, which was evenly distributed in the gel. The particle size of UE-SU in surimi gel was smaller than that of UE-PA and UE-PE at the same concentration, which might be related to the fatty acid content and composition of oils. The saturation and length of fatty acids could influence the particle size and distribution of oil droplets in emulsions.[Citation21] Palm oil with higher saturated fatty acids and peanut oil with higher monounsaturated fatty acids could not form rigid interfacial films on the surface of the oil droplets after ultrasonic emulsification, resulting in the aggregation and delamination of emulsified oils.[Citation21] The oil droplets tended to aggregate by excess addition and showed up as large and unevenly dispersed oil droplets in surimi gels. With higher polyunsaturated fatty acids, UE-SU could form a firm viscoelastic interface protein film, which hindered the accumulation of oil droplets, reduced the size of oil droplets and made evenly distributed in surimi gel.[Citation34] It was also consistent with the result that the particle size of UE-SU was smaller than those of UE-PA and UE-PE (). Therefore, with more addition (2.5–7.5 g/100 g), UE-PA and UE-PE aggregated into large particles in surimi gel, but UE-SU is still uniformly distributed with small particles.
Conclusion
In summary, ultrasonic emulsification significantly reduced the particle size of oil droplets in three vegetable oils and enhanced their stability. The whiteness of surimi gels was significantly increased with more addition of three ultrasonically emulsified oils, especially UE-SU. Lower addition of emulsified oils (2.5 g/100 g) either increased or unchanged TPA values of surimi gels, especially that UE-PE significantly enhanced the texture of surimi gel. Three emulsified oils (2.5–7.5 g/100 g) either increased or unchanged the WHC of surimi gel, while surimi gels had the higher WHC with 2.5 g/100 g UE-PA or UE-SU. Three emulsified oils could maintain or enhance the rheological characteristics of surimi paste, especially UE-SU. The addition of three ultrasonically emulsified oils increased storage modulus (G’) of surimi pastes during heating from 20 to 90°C. The addition of UE-SU (2.5 g/100 g) increased hydrogen bond in surimi gels more than other two ultrasonically emulsified oils, while the addition of UE-PE (2.5 g/100 g) increased disulfide bond in surimi gels. The lower addition of UE-PE (2.5 g/100 g) could significantly decrease α-helix and random coil but increase β-sheet and β-turn of surimi gel. The addition of UE-SU showed the most uniform distribution of oil droplets in the gel network. With ultrasonic emulsification, lower addition of peanut oil and sunflower oil (2.5 g/100 g) could potentially improve the whiteness, textural, rheological, and nutritional quality of surimi-based products.
Authorship contribution statement
Min Chen: Conceptualization, Methodology, Validation, Formal analysis, Writing – Original Draft. Yudong Wang: Investigation, Validation, Software, Data Curation. Yuqian Tong: Software. Qian Zhong: Validation. Yang Zhong: Software. Hong Yang: Funding acquisition, Conceptualization, Investigation, Methodology, Resources, Supervision, Validation, Visualization, Writing – Review & Editing.
Acknowledgments
This work was financially supported by the National Key R&D Program of China (2018YFD0901003).
Disclosure statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Funding
References
- Luo, Y.; Shen, H.; Pan, D.; Bu, G. Gel Properties of Surimi from Silver Carp (Hypophthalmichthys Molitrix) as Affected by Heat Treatment and Soy Protein Isolate. Food. Hydrocoll. 2008, 22(8), 1513–1519. DOI: 10.1016/j.foodhyd.2007.10.003.
- Liu, S.; Xianfeng, W.; Tong, C.; Chenjie, W.; Hong, Y. Effects of Vegetable Oils on Gel Properties of Surimi Gels. LWT Food Sci. Technol. 2014, 57(2), 586–593. DOI: 10.1016/j.lwt.2014.02.003.
- Song, C.; Lin, Y.; Hong, P.; Liu, H.; Zhou, C. Compare with Different Vegetable Oils on the Quality of the Nemipterus Virgatus Surimi Gel. Food. Sci & Nutri. 2022, 2935–2946. DOI: 10.1002/fsn3.2889.
- Jimenez-Colmenero, F.; Herrero, A.; Pintado, T.; Solas, M. T.; Ruiz-Capillas, C. Influence of Emulsified Olive Oil Stabilizing System Used for Pork Backfat Replacement in Frankfurters. Food Res. Int. 2010, 43(8), 2068–2076. DOI: 10.1016/j.foodres.2010.06.010.
- Elwell, M. W.; Roberts, R. F.; Coupland, J. N. Effect of Homogenization and Surfactant Type on the Exchange of Oil Between Emulsion Droplets. Food. Hydrocoll. 2004, 18(3), 413–418. DOI: 10.1016/j.foodhyd.2003.05.001.
- Abismail, B.; Canselier, J. P.; Wilhelm, A. M.; Delmas, H.; Gourdon, C. Emulsification by Ultrasound: Drop Size Distribution and Stability. Ultrason. Sonochem. 1999, 6(1–2), 75–83. DOI: 10.1016/S1350-4177(98)00027-3.
- Liu, C.; Xu, Y.; Xia, W.; Jiang, Q. Enhancement of Storage Stability of Surimi Particles Stabilized Novel Pickering Emulsions: Effect of Different Sequential Ultrasonic Processes. Ultrason. Sonochem. 2021, 79, 105802. DOI: 10.1016/j.ultsonch.2021.105802.
- Hu, S.; Wu, J.; Zhu, B.; Du, M.; Wu, C.; Yu, C. Low Oil Emulsion Gel Stabilized by Defatted Antarctic Krill (Euphausia Superba) Protein Using High-Intensity Ultrasound. Ultrason. Sonochem. 2021, 70, 105294. DOI: 10.1016/j.ultsonch.2020.105294.
- Arzeni, C.; Martínez, K.; Zema, P.; Arias, A.; Pérez, O. E.; Pilosof, A. M. R. Comparative Study of High Intensity Ultrasound Effects on Food Proteins Functionality. J. Food Eng. 2012, 108(3), 463–472. DOI: 10.1016/j.jfoodeng.2011.08.018.
- Higuera-Barraza, O. A.; Del Toro-Sanchez, C. L.; Ruiz-Cruz, S.; Márquez-Ríos, E. Effects of High-Energy Ultrasound on the Functional Properties of Proteins. Ultrason. Sonochem. 2016, 31, 558–562. DOI: 10.1016/j.ultsonch.2016.02.007.
- Awad, T. S.; Moharram, H. A.; Shaltout, O. E.; Asker, D.; Youssef, M. M. Applications of Ultrasound in Analysis, Processing and Quality Control of Food: A Review. Food Res. Int. 2012, 48(2), 410–427. DOI: 10.1016/j.foodres.2012.05.004.
- Li, K.; Li, Y.; Liu, C. L.; Fu, L.; Zhao, Y. Y.; Zhang, Y. Y. Improving Interfacial Properties, Structure and Oxidative Stability by Ultrasound Application to Sodium Caseinate Prepared Pre-Emulsified Soybean Oil. LWT Food Sci. Technol. 2020, 131, 109755. DOI: 10.1016/j.lwt.2020.109755.
- Xu, Y.; Lv, Y.; Zhao, H.; He, X.; Li, X.; Yi, S. Diacylglycerol Pre-Emulsion Prepared Through Ultrasound Improves the Gel Properties of Golden Thread Surimi. Ultrason. Sonochem. 2022, 82, 105915. DOI: 10.1016/j.ultsonch.2022.105915.
- Silva, M.; Zisu, B.; Chandrapala, J. Interfacial and Emulsification Properties of Sono-Emulsified Grape Seed Oil Emulsions Stabilized with Milk Proteins. Food. Chem. 2020, 309, 125758. DOI: 10.1016/j.foodchem.2019.125758.
- Fang, Q.; Shi, L.; Ren, Z.; Hao, G.; Chen, J.; Weng, W. Effects of Emulsified Lard and TGase on Gel Properties of Threadfin Bream (Nemipterus Virgatus) Surimi. LWT Food Sci. Technol. 2021, 146, 111513. DOI: 10.1016/j.lwt.2021.111513.
- Asuming-Bediako, N.; Jaspal, M. H.; Hallett, K.; Bayntun, J.; Baker, A.; Sheard, P. R. Effects of Replacing Pork Backfat with Emulsified Vegetable Oil on Fatty Acid Composition and Quality of UK-Style Sausages. Meat. Sci. 2014, 96(1), 187–194. DOI: 10.1016/j.meatsci.2013.06.031.
- Zhou, X.; Jiang, S.; Zhao, D.; Zhang, J.; Gu, S.; Pan, Z. Changes in Physicochemical Properties and Protein Structure of Surimi Enhanced with Camellia Tea Oil. LWT Food Sci. Technol. 2017, 84, 562–571. DOI: 10.1016/j.lwt.2017.03.026.
- Gani, A.; Benjakul, S.; Nuthong, P. Effect of Virgin Coconut Oil on Properties of Surimi Gel. J. Food Sci. Technol. 2018, 55(2), 496–505. DOI: 10.1007/s13197-017-2958-0.
- Pietrowski, B. N.; Tahergorabi, R.; Jaczynski, J. Dynamic Rheology and Thermal Transitions of Surimi Seafood Enhanced with Omega-3-Rich Oils. Food Hydrocolloids. 2012, 27(2), 384–389. DOI: 10.1016/j.foodhyd.2011.10.016.
- Gani, A.; Benjakul, S. Impact of Virgin Coconut Oil Nanoemulsion on Properties of Croaker Surimi Gel. Food. Hydrocoll. 2018, 82, 34–44. DOI: 10.1016/j.foodhyd.2018.03.037.
- Zheng, J.; Sun, D.; Li, X.; Liu, D.; Li, C.; Zheng, Y. The Effect of Fatty Acid Chain Length and Saturation on the Emulsification Properties of Pork Myofibrillar Proteins. LWT Food Sci. Technol. 2021, 139, 110242. DOI: 10.1016/j.lwt.2020.110242.
- Chang, T.; Wang, C.; Wang, X.; Shi, L.; Yang, H.; Cui, M. Effects of Soybean Oil, Moisture and Setting on the Textural and Color Properties of Surimi Gels. J. Food Qual. 2015, 38(1), 53–59. DOI: 10.1111/jfq.12121.
- Gu, R.; Xiao, X.; Sun, J.; Shi, L.; Yang, H. Effects of Rice Residue on Physicochemical Properties of Silver Carp Surimi Gels. Int. J. Food Prop. 2018, 21(1), 1743–1754. DOI: 10.1080/10942912.2016.1214146.
- Lowry, O. H.; Rosebrough, N. J.; Farr, A. L.; Randall, R. J. Protein Measurement with the Folin Phenol Reagent. J. Biol. Chem. 1951, 193(1), 265–275. DOI: 10.1016/s0021-9258(19)52451-6.
- Jia, D.; Huang, Q.; Xiong, S. Chemical Interactions and Gel Properties of Black Carp Actomyosin Affected by MTGase and Their Relationships. Food. Chem. 2016, 196, 1180–1187. DOI: 10.1016/j.foodchem.2015.10.030.
- Zhao, F.; Liu, X.; Ding, X.; Dong, H.; Wang, W. Effects of High-Intensity Ultrasound Pretreatment on Structure, Properties, and Enzymolysis of Soy Protein Isolate. Molecules. 2019, 24(20), 3637. DOI: 10.3390/molecules24203637.
- Zhuang, X.; Han, M.; Kang, Z. -L.; Wang, K.; Bai, Y.; Xu, X. -L. Effects of the Sugarcane Dietary Fiber and Pre-Emulsified Sesame Oil on Low-Fat Meat Batter Physicochemical Property, Texture, and Microstructure. Meat. Sci. 2016, 113, 107–115. DOI: 10.1016/j.meatsci.2015.11.007.
- Aslan, D.; Dogan, M. The Influence of Ultrasound on the Stability of Dairy-Based, Emulsifier-Free Emulsions: Rheological and Morphological Aspect. Eur. Food Res. Technol. 2018, 244(3), 409–421. DOI: 10.1007/s00217-017-2966-3.
- Kaci, M.; Meziani, S.; Arab-Tehrany, E.; Gillet, G.; Desjardins-Lavisse, I.; Desobry, S. Emulsification by High Frequency Ultrasound Using Piezoelectric Transducer: Formation and Stability of Emulsifier Free Emulsion. Ultrason. Sonochem. 2014, 21(3), 1010–1017. DOI: 10.1016/j.ultsonch.2013.11.006.
- Tadros, T.; Izquierdo, R.; Esquena, J.; Solans, C. Formation and Stability of Nano-Emulsions. Adv. Coll. Interf. Sci. 2004, 108, 303–318. DOI: 10.1016/j.cis.2003.10.023.
- Silva, E. K.; Thereza, M.; Gomes, M. S.; Hubinger, M. D.; Cunha, R. L.; Angela, M. Ultrasound-Assisted Formation of Annatto Seed Oil Emulsions Stabilized by Biopolymers. Food. Hydrocoll. 2015, 47, 1–13. DOI: 10.1016/j.foodhyd.2015.01.001.
- Taha, A.; Ahmed, E.; Ismaiel, A.; Ashokkumar, M.; Xu, X.; Pan, S. Ultrasonic Emulsification: An Overview on the Preparation of Different Emulsifiers-Stabilized Emulsions. Trends in Food. Sci & Technol. 2020, 105, 363–377. DOI: 10.1016/j.tifs.2020.09.024.
- Euston, S. R.; Hirst, R. L. Comparison of the Concentration-Dependent Emulsifying Properties of Protein Products Containing Aggregated and Non-Aggregated Milk Protein. Int. Dairy J. 1999, 9(10), 693–701. DOI: 10.1016/S0958-6946(99)00138-7.
- Watanabe, T.; Kawai, T.; Nonomura, Y. Effects of Fatty Acid Addition to Oil-In-Water Emulsions Stabilized with Sucrose Fatty Acid Ester. J. Oleo Sci. 2017, 67(3), 307–313. DOI: 10.5650/jos.ess17097.
- Liu, X.; Ji, L.; Zhang, T.; Xue, Y.; Xue, C. Effects of Pre-Emulsification by Three Food-Grade Emulsifiers on the Properties of Emulsified Surimi Sausage. J. Food Eng. 2019, 247, 30–37. DOI: 10.1016/j.jfoodeng.2018.11.018.
- Chen, Y.; Liang, Y.; Jia, F.; Chen, D.; Zhang, X.; Wang, Q. Effect of Extrusion Temperature on the Protein Aggregation of Wheat Gluten with the Addition of Peanut Oil During Extrusion. Int. J. Biol. Macromol. 2021, 166, 1377–1386. DOI: 10.1016/j.ijbiomac.2020.11.017.
- Mourtzinos, I.; Kiosseoglou, V. Protein Interactions in Comminuted Meat Gels Containing Emulsified Corn Oil. Food. Chem. 2005, 90(4), 699–704. DOI: 10.1016/j.foodchem.2004.05.021.
- Chemat, F.; Zill e, H.; Khan, M. K. Applications of Ultrasound in Food Technology: Processing, Preservation and Extraction. Ultrason. Sonochem. 2011, 18(4), 813–835. DOI: 10.1016/j.ultsonch.2010.11.023.
- Jimenez-Colmenero, F. Healthier Lipid Formulation Approaches in Meat-Based Functional Foods. Technological Options for Replacement of Meat Fats by Non-Meat Fats. Trends in Food. Sci & Technol. 2007, 18(11), 567–578. DOI: 10.1016/j.tifs.2007.05.006.
- Zhao, Y. -Y.; Wang, P.; Zou, Y. -F.; Li, K.; Kang, Z. -L.; Xu, X. -L. Effect of Pre-Emulsification of Plant Lipid Treated by Pulsed Ultrasound on the Functional Properties of Chicken Breast Myofibrillar Protein Composite Gel. Food Res. Int. 2014, 58, 98–104. DOI: 10.1016/j.foodres.2014.01.024.
- Liu, W.; Lanier, T. C.; Osborne, J. A. Capillarity Proposed as the Predominant Mechanism of Water and Fat Stabilization in Cooked Comminuted Meat Batters. Meat. Sci. 2016, 111, 67–77. DOI: 10.1016/j.meatsci.2015.08.018.
- Zhang, T.; Xue, Y.; Li, Z.; Wang, Y.; Xue, C. Effects of Deacetylation of Konjac Glucomannan on Alaska Pollock Surimi Gels Subjected to High-Temperature (120 °C) Treatment. Food. Hydrocoll. 2015, 43, 125–131. DOI: 10.1016/j.foodhyd.2014.05.008.
- Asir, G.; Soottawat, B. Impact of Virgin Coconut Oil Nanoemulsion on Properties of Croaker Surimi Gel. Food. Hydrocoll. 2018, 82, 34–44. DOI: 10.1016/j.foodhyd.2018.03.037.
- Debusca, A.; Tahergorabi, R.; Beamer, S. K.; Partington, S.; Jaczynski, J. Interactions of Dietary Fibre and Omega-3-Rich Oil with Protein in Surimi Gels Developed with Salt Substitute. Food. Chem. 2013, 141(1), 201–208. DOI: 10.1016/j.foodchem.2013.02.111.
- Liu, R.; Zhao, S. M.; Xiong, S. B.; Xie, B. J.; Liu, H. M. Studies on Fish and Pork Paste Gelation by Dynamic Rheology and Circular Dichroism. J. Food Sci. 2007, 72(7), E399–403. DOI: 10.1111/j.1750-3841.2007.00470.x.
- Mangang, W.; Youling, L. X.; Jie, C.; Xueyan, T.; Guanghong, Z. Rheological and Microstructural Properties of Porcine Myofibrillar Protein-Lipid Emulsion Composite Gels. J. Food Sci. 2009, 74(4), E207–217. DOI: 10.1111/j.1750-3841.2009.01140.x.
- Ko, W. -C.; Yu, C. -C.; Hsu, K. -C. Changes in Conformation and Sulfhydryl Groups of Tilapia Actomyosin by Thermal Treatment. LWT Food Sci. Technol. 2007, 40(8), 1316–1320. DOI: 10.1016/j.lwt.2006.10.002.
- Sun, X. D.; Arntfield, S. D. Molecular Forces Involved in Heat-Induced Pea Protein Gelation: Effects of Various Reagents on the Rheological Properties of Salt-Extracted Pea Protein Gels. Food. Hydrocoll. 2012, 28(2), 325–332. DOI: 10.1016/j.foodhyd.2011.12.014.
- Zhu, S.; Chen, X.; Zheng, J.; Fan, W.; Ding, Y.; Zhou, X. Emulsion Surimi Gel with Tunable Gel Properties and Improved Thermal Stability by Modulating Oil Types and Emulsification Degree. Foods. 2022, 11, 179. DOI: 10.3390/foods11020179.
- Yan, B.; Jiao, X.; Zhu, H.; Wang, Q.; Huang, J.; Zhao, J. Chemical Interactions Involved in Microwave Heat-Induced Surimi Gel Fortified with Fish Oil and Its Formation Mechanism. Food. Hydrocoll. 2020, 105, 105779. DOI: 10.1016/j.foodhyd.2020.105779.
- Meng, G. T.; Chan, J. C. K.; Rousseau, D.; Li-Chan, E. C. Y. Study of Protein - Lipid Interactions at the Bovine Serum Albumin/Oil Interface by Raman Microspectroscopy. J. Agric. Food Chem. 2005, 53(4), 845–852. DOI: 10.1021/jf040259r.
- Liu, Q.; Chen, Q.; Kong, B.; Han, J.; He, X. The Influence of Superchilling and Cryoprotectants on Protein Oxidation and Structural Changes in the Myofibrillar Proteins of Common Carp (Cyprinus Carpio) Surimi. LWT Food Sci. Technol. 2014, 57(2), 603–611. DOI: 10.1016/j.lwt.2014.02.023.
- Lu, Y.; Zhu, Y.; Ye, T.; Nie, Y.; Jiang, S.; Lin, L. Physicochemical Properties and Microstructure of Composite Surimi Gels: The Effects of Ultrasonic Treatment and Olive Oil Concentration. Ultrason. Sonochem. 2022, 88, 106065. DOI: 10.1016/j.ultsonch.2022.106065.
- Visschers, R. W.; de Jongh, H. H. J. Disulphide Bond Formation in Food Protein Aggregation and Gelation. Biochem. Adv. 2005, 23(1), 75–80. DOI: 10.1016/j.biotechadv.2004.09.005.
- Xiong, Y. L.; Blanchard, S. P.; Ooizumi, T.; Ma, Y. Hydroxyl Radical and Ferryl-Generating Systems Promote Gel Network Formation of Myofibrillar Protein. J. Food Sci. 2010, 75(2), C215–221. DOI: 10.1111/j.1750-3841.2009.01511.x.
- Zhang, T.; Guan, E.; Yang, Y.; Zhang, L.; Liu, Y.; Bian, K. Underlying Mechanism Governing the Influence of Peanut Oil Addition on Wheat Dough Viscoelasticity and Chinese Steamed Bread Quality. LWT Food Sci. Technol. 2022, 156, 113007. DOI: 10.1016/j.lwt.2021.113007.
- Shao, J. -H.; Zou, Y. -F.; Xu, X. -L.; Wu, J. -Q.; Zhou, G. -H. Evaluation of Structural Changes in Raw and Heated Meat Batters Prepared with Different Lipids Using Raman Spectroscopy. Food Res. Int. 2011, 44(9), 2955–2961. DOI: 10.1016/j.foodres.2011.07.003.