?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
The objective of this study was to detect the changes in physicochemical and functional properties of raw and processed divergent honey varieties after adulterating with different sugar syrups. Specifically, when 25% to 55% cane syrup was added to raw Acacia honey, the HMF content increased from 46.25 to 101.6 mg/kg. In processed honey that contained 55% cane syrup, HMF content was even higher, reaching up to 402.47 mg/kg. The reported values for HMF (>80 mg/kg) content and DN (>8 DN) were above the described quality evaluation standard of honey. The results indicated that adulterating Acacia honey with up to 55% corn and corn syrup caused a reduction in diastase content to below 3.5 DN, and an increase in HMF content above 93.28 mg/kg. Similarly, adding 10% corn syrup to raw Ziziphus honey resulted in a decrease in both DPPH and ABTS+ values from 83.16% and 88.58%, respectively, to 72.83% and 76.97%. The findings in this study demonstrated that the addition of malt syrup (55%) to raw Trifolium honey resulted in an increase in the phytochemical content, with the TPC value reaching 805.15 mg GAE/100 g. However, processing Trifolium honey caused a significant decrease in TPC content to 505.15 mg GAE/100 g. This work highlights the need for more research into unexplored honey varieties and adulteration methods to improve quality evaluation standards and mitigate authenticity issues.
Introduction
Honey is a natural sweetener and a produce of Apis mellifera from the plant nectar, which the bees collect, transmute by a combination of their definite substances, deposit, store, dry out, and mature and ripen in honeycombs[Citation1,Citation2]. It has been consumed since the primitive period and is considered the oldest natural sweetening agent. Honey has various nutritious and therapeutic compounds, the ratio of which varies according to geographical and botanical origins.[Citation3,Citation4] The main constituents of honey are carbohydrates, including monosaccharides (fructose and glucose), different disaccharides (including sucrose and maltose,) and oligosaccharides (terlose, maltotriose and raffinose).[Citation5] The process of maturation during honey production involves several changes in its composition, with specific characteristics varying depending on the source of the nectar.[Citation6] As the nectar matures in the comb, its moisture content decreases by 3.4 times and the sugar composition changes, resulting in an increase in total sugar content from 41.5% to 81.0%.[Citation7,Citation8] The nectar is primarily composed of sucrose, which undergoes hydrolysis to form fructose, glucose, and other minor sugars. These changes contribute to the unique taste and nutritional properties of honey, making it a valuable food source with various health benefits.[Citation9]
Numerous types of research have reported that raw honey contains around 200 essential phytochemicals and bioactive compounds in minor proportion, including amino acids, vitamins, minerals, organic acids, and enzymes.[Citation10–12] The presence of polyphenols, especially phenolic acids (majorly Gallic acid), flavonoids and enzymes (catalase, peroxidase, and glucose oxidase) are primarily accountable for its antioxidant activity besides bioactive properties.[Citation13,Citation14] In addition, it has also been utilized for different purposes, such as wound healing, bacterial infections, hepatoprotective, hypoglycemic, diabetes, CVD and cancer-like chronic diseases[Citation15–17] because of its natural remedial components in honey. According to the report of the National Bee Board of India,[Citation18] the total honey production in India during 2020–21 was 1.25 lakh metric tonnes compared to 1.20 lakh metric tonnes in 2018–2019. It is reported that India exported 74,413.05 metric tonnes of natural honey to the world for a value Rs. 1,221.17 Crore/163.73 USD Millions during the year 2021–22. The most exported destinations were the USA, Arab Emirates, Morocco, and Quarter data represented by processed food and export development authority. The commercial value and market price of honey are going higher daily compared to other low-priced sweetening agents because of its medicinal properties and unique flavor. Therefore, honey has become one of the superior food products targeted for economically motivated adulteration (EMA) to reduce honey’s price and gain more market profit.[Citation19]
Since the lack of awareness and improper implications of government policies, honey adulteration is practised worldwide.[Citation20] A common adulteration exercise followed with honey is to extend volume with common sugar syrups; corn syrup, high fructose corn syrup (HFCS), inverted sugar syrup (ISS) and cane sugar syrup. Besides, glucose, beet, inulin, rice syrup, date and jaggery syrup are also included in the list.[Citation21–23] In India, honey adulteration with corn, cane, rice and beet syrups are common because of high availability and low market price; this leads to falsification practices. Nevertheless, conventional heating treatments can compromise honey authenticity.[Citation23,Citation24] Hydroxymethylfurfural content and diastase activity are two principal compounds of honey used to determine authenticity in processed and raw honey. Adulteration and overheating of honey in unacceptable conditions can lead to hydroxymethylfurfural (HMF) formation and reduced or eliminated diastase enzyme. For unprocessed honey, HMF content remains within the specified limit set by Codex Alimentarius Commission[Citation25] and FSSAI.[Citation2] Hence, it is essential to control and assure the quality of honey before marketing. Recent advancements have been made in detecting the excessive processing and ensuring the authenticity of honey. For instance, Yan et al.[Citation7] developed an Amadori compound (N-(1-deoxy-1-fructosyl) phenylalanine (Fru-Phe)) to detect artificial heating of Acacia honey. Additionally, Yuanyuan Gao et al.[Citation8] identified specific biomarkers in nectar that can be used to determine the authenticity of honey.
Numerous methods have emerged for honey adulterants detection; among them, Stable carbon isotope ratio analysis and Nuclear magnetic resonance spectroscopy are commonly used.[Citation26–29] Moreover, researchers are exploring the exogenous DNA and miRNA of adulterant sugars in honey.[Citation6,Citation30] But these techniques remain expensive, time-consuming and not affordable. Thus, qualitative testing of honey could be an alternative to analyzing the adulteration effect in honey. In this study, our objective is to investigate the impact of different syrup adulterants, namely cane syrup, corn syrup, rice syrup, and malt syrup, on the chemical and functional properties of both raw and processed honey. The findings of this study can be utilized to determine the authenticity of honey and assess the effects of adulteration and processing on the overall quality of various honey varieties. These results can serve as a foundation for researchers and regulatory agencies to explore further in this direction and establish improved standards for unexplored unifloral honey varieties. Ultimately, this will facilitate fair trading and marketing of honey on a global scale.
Materials and methods
The present study was based on four honey varieties: multi-floral, Acacia nilotica, Ziziphus mauritiana and Trifolium alexandrinum (Barseem), which were collected from different locations in Punjab and Rajasthan. After collection, the honey samples were filled in glass bottles, followed by airtight sealing and then appropriately stored.
Honey adulteration with different concentrations of sugar syrups
Different adulterants (corn syrup, cane syrup, rice syrup and malt syrup) were sealed within glass bottles and acquired from the local market (Punjab, India). These syrups didn’t contain any diastase activity. No polyphenol or flavonoid content was found in cane syrup, corn syrup and rice syrup, but the total phenolic and flavonoid content of malt syrup was 165 mg GAE/100 g and 121 mg QE/100 g, respectively. Honey was adulterated by adding corn syrup (Brand: Karo), cane syrup (Brand: Dhampur), rice syrup (Brand: Urban Plater), and malt syrup (Brand: Urban Plater), which were procured from certified and reliable vendors. For the analyses, one sample from each variety was adulterated with four different sugar syrups.
These sugar syrups were mixed with pure honey samples with different concentrations, i.e., 10%, 25%, 40% and 55%. For thermal processing of Acacia, Ziziphus, multifloral and Trifolium Honey were adulterated with cane, corn, rice and malt syrup with different concentrations (10%, 25%, 40% and 55%). And then, it was processed at 60°C for 20 min in a double-jacketed vat to analyze the heating effect on functional and chemical properties of both processed and unprocessed honey before and after adulteration.
Chemicals, standards, and reagents
All reagents, chemicals and standards were of analytical grade. Sodium chloride, Sodium hydroxide, sodium acetate, ethanol, and petroleum ether were obtained from Loba Chemie (Mumbai, India). All solutions were prepared in Ultrapure water from Alfa Aesar (Haverhill, Massachusetts, USA). Antioxidant activity and polyphenolic compounds, quercetin, Gallic acid and 2,2-diphenyl-1-picrylhydrazyl (DPPH) for determination of antioxidants were acquired from Sigma-Aldrich (St. Louis, Missouri, USA)
Hydroxymethylfurfural (HMF) (mg/kg) content
International Honey Commission[Citation31] guidelines were used to determine HMF content in honey samples. According to the white method in the IHC, the content of HMF was analyzed by spectrophotometer at 284 nm and 336 nm absorbance values, where a honey sample of 5 g was taken in a beaker and mixed with 0.5 ml of both Carraz I and Carraz II solution followed by dilution with distilled water. HMF was determined by subtracting the sample absorbance from the absorbance of reference and calculated as follows:
where, 149.7 = constant; D = Dilution factor if required; A336 = absorbance at 336 nm; A284 = absorbance at 284 nm; M = mass (g) of the honey sample and 5 = nominal sample weight;
Diastase activity
The diastase Activity or Number of Honey was analyzed according to International Honey Commission[Citation31] guideline. Honey solution (10 ml) was pipetted and placed in a water bath at 40°C with a second flask with starch solution (10 ml). Starch solution (5 ml) was mixed into the honey solution after 15 min, and the timer was started. At intervals of 5 min, 0.5 ml of aliquot parts were removed and rapidly transferred to diluted iodine solution (5 ml). The absorbance at 660 nm was taken against blank using a Spectrophotometer (Hach Lange DR6000 UV-VIS, Germany). The results are presented in diastase number (DN):
where tx is the reaction period in minutes.
Total phenolic content (mg GAE/100 g)
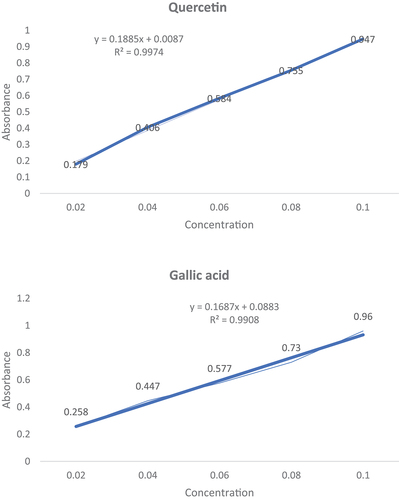
The total phenolic content of honey was analyzed according to the Folin-Ciocalteu method studied by Meda et al..[Citation32] The honey solution was prepared by adding 0.1 g/ml of honey to distilled water. The stock solution (0.5 ml) was mixed with 2 Folin-Cioclateu reagents (.5 ml of 0.2 N) and Na2CO3 (2 ml of 75 g/l). The solution was incubated for 2 hours and at 760 nm. The absorbance of the mixture was analyzed against a methanol blank. They were using a Spectrophotometer of Hach Lange DR6000 UV-VIS (Dusseldorf, Germany). The Gallic acid curve for the standard was prepared for quantification using a concentration range within 0–100 mg/L (, and the results were presented as mg GAE/100 g. ()
Total flavonoid content (mg QE/100 g)
The total flavonoid content of honey was analyzed by the aluminum chloride method, where quercetin was used as standard.[Citation33,Citation34] First, a honey sample (5 ml) was added to water (50 ml) to prepare a honey solution. Next, that honey solution (5 ml) was added to 2% AlCl3 (5 ml), followed by incubation for 20 min. After incubation, an absorbance reading was taken against 415 nm in the spectrophotometer. A standard curve with quercetin was prepared for quantification using range of concentration from 0 to 100 mg/L (), and the obtained results are expressed in mg QE/100 g.), and the obtained results are expressed in mg QE/100 g. ()
DPPH radical scavenging activity
To conduct a DPPH assay sample (0.6 g) of honey was dissolved in methanol (4 ml). After this, 0.75 ml of honey-methanol solution was dissolved in 1.5 ml of DPPH reagent solution (0.02 mg/mL), and after adequately capping, the samples were incubated for 15 min in a dark place at room temperature.[Citation35,Citation36] The solution was measured at an absorbance of 517 nm against a methanol blank using a Spectrophotometer by Hach Lange DR6000 UV-VIS (Dusseldorf, Germany). The DPPH radical scavenging activity was calculated from the equation and presented as % DPPH inhibition. The DPPH radical scavenging activity was calculated from the equation and presented as % DPPH inhibition. And the ascorbic acid calibration curve (0–10 mg/L) was used to analyze the required concentration of honey for scavenging 50% DPPH (EC50).[Citation35]
where the Abs sample is the absorbance of the sample and Abs control is the absorbance of the control at 517 nm.
ABTS+
The antioxidant activity was determined by using ABTS assay as described by Meda et al.[Citation32] and Dhillon et al.[Citation36] with slight modifications. The stock solutions were prepared from 7.4 mM ABTS solution and 2.45 mM potassium persulfate solution. Both solutions are then mixed in 1:1 ratio and incubated in the dark at room temperature and reaction time is 15–16 h from the formation of ABTS+ The solution was then diluted by mixing 1 ml ABTS+ solution with methanol (100%) to obtain an absorbance of 0.700 at 734 nm using the spectrophotometer. Fruit extracts 100 µL were allowed to react with 3.9 ml of the ABTS+ solution for 10 to 15 min in a dark condition at room temperature. Then the absorbance was observed at 734 nm using the spectrophotometer. The ABTS+ radical-scavenging activity of the samples was expressed as percent antioxidant activity (AA%)
where Acontrol is the absorbance of the control, and Atest is Extracted with reagent after 15 min.
Pollen analysis
The botanical authentication of honey samples was determined using Harmonized Melissopalynology Methods,[Citation37] which involved calculating the pollen spectrum (expressed as a percentage) of the honey based on the frequency of single pollen grains. The frequency classes used were important minor pollen (3–15%), minor pollen (<3%), secondary pollen (16–45%), and predominant pollen (>45% of pollen grains counted)
Statistical analysis
All the analytical findings were conducted in triplicate. The obtained data were statistically analyzed by using Statistica.v.12 (Stat Soft, Tulsa, Oklahoma, USA) in which two-way ANOVA was followed by Duncan’s multiple range test (DMRT) (p < .05) to find out the significance level.
Results
Pollen analyses
The classification of honey is based on the botanical source and can be categorized as unifloral or multifloral. According to the International Honey Commission (IHC), honey with more than 45% pollen count from a single botanical origin is classified as unifloral, and if the pollen percentage of one varietal source is less than 45%, it is categorized as multifloral.[Citation2,Citation37] In this study, four varieties of honey were analyzed for their classification, out of which three were unifloral and one was classified as multifloral. The multifloral honey had an absolute pollen count of 8750/g, with pollen from Pennisetum sativum, Cucumis sativus, Acacia spp., Gossypium herbacum, and Ziziphus spp. Three samples were classified as unifloral, namely Acacia (pollen percentage 89%, absolute pollen count 1170/g), Ziziphus (pollen percentage 67% and absolute pollen count 6700/g), and Trifolium (pollen percentage 98% and absolute pollen count 26,000/g).
Hydroxymethylfurfural content of honey (mg/kg)
Analysis of Hydroxymethylfurfural (HMF) content is essential in investigating honey’s freshness and adulteration. According to our study, all the honey varieties were found with HMF levels less than the permissible limit (40 mg/kg), as suggested by both the Codex Alimentarius Commission[Citation25] and FSSAI.[Citation2] After statistical Analysis, a significant difference among all four varieties was reported (P < .05). The HMF content of Ziziphus honey (10.12 mg/kg) was maximum compared with other samples, whereas multifloral honey showed minimum HMF content (5.29 mg/kg), as reported in . Olmez,[Citation29] conducted a similar study, based on the chemical characteristics of 16 honey varieties from Turkey and resulted in the HMF content of the samples has lied between 1.34 mg/kg to 31.28 mg/kg.
Table 1. Physicochemical Analysis of Raw Honey Adulterated with Different Sugar Syrups.
Table 2. Physicochemical Analysis of Raw Honey Adulterated with Different Sugar Syrups.
Effect of Concentration of Sugar Syrups on the Hydroxymethylfurfural (HMF) (mg/kg) Content of Raw and Processed Honey from Different Botanical Origins
In our study, the effect of cane syrup on the hydroxymethylfurfural (HMF) content of different honey was studied, and it was reported that the concentration of cane syrup and HMF content of honey had a direct relation. depict that, with the addition of cane syrup with different concentration from 10% to 55%, assimilated HMF content in Acacia, Ziziphus, multifloral and Trifolium Honey from 14.81 mg/kg to 101.6 mg/kg, 11.09 mg/kg to 94.56 mg/kg, 13.23 mg/kg to 97.51 mg/kg and 17.21 mg/kg to 94.32 mg/kg respectively, and found exceeding of standard limit (<40 mg/kg) suggested by both the Codex Alimentarius Commission[Citation25] and FSSAI[Citation2] (<80 mg/kg). The results also reported that, after adding cane syrup, a maximum increment of HMF content was found in Acacia honey compared to other honey. Moreover, interpret that a significant increment in HFM content of processed honey was seen after increasing the cane syrup concentration from 10% to 55% in all four honey varieties. In Acacia, Ziziphus, multifloral and Trifolium honey from 63.07 mg/kg to 402.47 mg/kg, 85.58 mg/kg to 371.47 mg/kg, 51.46 mg/kg to 382.41 mg/kg and 81.43 mg/kg to 411.49 mg/kg, respectively.
Table 3. Physicochemical Analysis of Processed Honey Adulterated with Different Sugar Syrups.
Table 4. Physicochemical Analysis of Processed Honey Adulterated with Different Sugar Syrups.
After studying the effect of corn syrup on the hydroxymethylfurfural (HMF) content of different honey, it was reported that corn syrup concentration was directly proportional to the HMF content of honey. Maintained in , with the addition of corn syrup concentrating from 10% to 55% increased HMF content in Acacia, Ziziphus, multifloral and Trifolium honey from 8.73 to 26.71 mg/kg, 16.73 to 24.73 mg/kg, 6.73 to 14.73 mg/kg and 11.73 to 18.56 mg/kg, respectively, which were within the limit (<40 mg/kg) suggested by both the Codex Alimentarius Commission[Citation25] and FSSAI.[Citation2] However, after thermal processing of honey adulterated with cone syrup, concentrations of 10 to 55% showed significantly higher results of HMF. As per the standards of FSSAI, the maximum limit for HMF is <80 mg/kg. According to , HMF content in Acacia, Ziziphus, multifloral and Trifolium Honey from 45.95 mg/kg to 95.35 mg/kg, 32.59 mg/kg to 67.25 mg/kg, 14.95 mg/kg to 41.35 mg/kg and 54.15 mg/kg to 141.29 mg/kg, respectively. After the statistical Analysis was found that the effect of concentration of corn syrup on HMF content of raw and processed Acacia, Ziziphus, multifloral and Trifolium Honey was significantly different (p < .05).
According to , a significant rise in HMF content in Acacia, Ziziphus, multifloral and Trifolium raw honey was observed from 3.65 to 52.55 mg/kg, 6.24 to 63.22 mg/kg, 3.22 to 51.60 mg/kg and 7.22 to 38.59 mg/kg, respectively. Whereas, after thermal processing of all four honey samples increased HMF content from 51.42 mg/kg to 121.55 mg/kg, 24.72 mg/kg to 61.73 mg/kg, 11.62 mg/kg to 41.75 mg/kg and 56.23 mg/kg to 108.59 in Acacia, Ziziphus, multifloral and Trifolium, respectively, which was higher than raw samples with the same rice syrup concentration (). The HMF content of all honey samples exceeded the maximum recommended limits (<40 mg/kg) of both the Codex Alimentarius Commission[Citation25] and FSSAI,[Citation2] except Trifolium raw honey.
Adulterating honey with 10% malt syrup had not increased the HMF content. However, when the concentration of malt syrup goes beyond 55%, a gradual increase is observed. The values were 26.78, 27.03, 31.22 and 36.19 mg/kg for Raw Acacia, Ziziphus, multifloral and Trifolium Honey, respectively. Moreover, these values were below the maximum limits prescribed by the Codex Alimentarius Commission[Citation25] and FSSAI.[Citation2] It is concluded that processing and adulteration of honey increased the HMF content. As per the observations, hydroxymethylfurfural (HMF) content of processed Acacia, Ziziphus, multifloral and Trifolium honey samples were increased after the addition of malt syrup with different concentrations (10%, 25%, 40% and 55%). Processed Acacia, Ziziphus, multifloral, and Trifolium honey adulterated with 55% malt syrup had HMF content of 278.23, 274.13, 377.32 and 382.21 mg/kg, respectively (). The statistical analysis established that the concentration of malt syrup on the HMF content of Acacia, Ziziphus, multifloral and Trifolium Honey was significantly different (p < .05).
Diastase number (DN) of honey
Diastase activity and invertase activity are both essential enzymes in honey and are the quality indicators against adulteration, heating intensity during honey processing, and storage. In our study, the diastase activity of all tested raw honey samples was above the minimum limit of both Codex Standard[Citation25] (>8 DN) and FSSAI[Citation2](2018) (>3 DN), indicating honey freshness (). Invertase number (IN) of honey samples was also found above the standard of Codex Alimentarius Commission (2001) (>4 IN), and it ranged from 9.61 IN (Acacia) to 17.53 IN (Ziziphus). Both IN > 4 and DN > 8 indicated that all the honey samples were fresh and unprocessed. Our result was significant to the reporting values of Andrade et al..[Citation33] Andrade et al.,[Citation33] investigated the physicochemical properties and pollen spectrum of twenty Portuguese heather honey and found that diastase activity of all samples ranged between 13 and 15.1 DN. Czipa et al.[Citation34] also found that the diastase activity was placed between 15.02 to 20.5 DN after studying the physicochemical properties of Hungarian Acacia honey.
Effect of concentration of sugar syrups on the diastase activity of raw and processed honey from different botanical origins
The concentration of cane syrup in raw Acacia, Ziziphus, multifloral and Trifolium Honey was concentrated at 55%, diastase number reduced to 4.28, 16.28, 11.28 and 6.18, respectively, from 11.51, 21.03, 21.52 and 12.53 found during 10% concentrated cane syrup adulterated samples, and it was also reported that maximum deterioration of diastase enzyme was observed in Acacia honey samples (). Our findings were significant to Zábrodská et al.,[Citation32] who reported that diastase activity (8.15–11.20 DN) of honey from citruses, rosemary, eucalyptus and honeydew was affected significantly after adulteration with cane syrup as well as after thermal processing. However, after thermal processing of all four adulterated samples with the same cane syrup, there was a significant decline in diastase activity. Acacia, Ziziphus, multifloral and Trifolium honey from 10% to 55% reduced diastase number from 5.19 to 2.95, 12.71 to 6.29, 13.79 to 6.95 and 8.19 to 5.35, respectively. Hebbar et al.[Citation33] and Kowalski et al.[Citation34] reported the same destruction and concluded that diastase enzyme concentration reduced more during thermal treatment of rock bee honey (16.6–7) and Polish Honey (15.2–5.1) due to the inactivation of enzymes. The effect of concentration of cane syrup on diastase activity of Acacia, Ziziphus, multifloral and Trifolium raw and processed honey was found to be significantly different after conducting statistical Analysis (p < .05).
The concentration of cane syrup in Acacia, Ziziphus, multifloral and Trifolium Honey was increased from 10% to 55%, and the diastase number reduced from 9.13 to 3.88, 16.31 to 3.18, 16.73 to 5.24 and 12.73 to 5.35 respectively (), which existed below the standard limit of Codex Alimentarius Commission[Citation25] (>8 DN) but above the limit of FSSAI.[Citation2] In addition, a rapid diastase enzyme reduction in each honey sample was observed after thermal processing. After adulterating processed Acacia, Ziziphus, multifloral and Trifolium 5honey with 10% to 55% concentration of cone syrup, a considerable drop in DN was seen from 5.82 to 2.45, 7.95 to 2.15, 8.82 to 2.41 and 8.51 to 2.23, respectively. Statistical analysis revealed that the effect of concentration of corn syrup on diastase activity of raw and processed Acacia, Ziziphus, multifloral and Trifolium Honey was significantly different (p < .05).
This study () interprets the reduction in DN after increasing the concentration (10%, 25%, 40%, and 55%) of rice syrup in four raw honey samples. Acacia, Ziziphus, multifloral and Trifolium honey. In Acacia, Ziziphus, multifloral and Trifolium honey diastase numbers were reduced gradually from 10.16 to 5.42, 19.16 to 4.27, 22.16 to 6.67 and 13.16 to 5.81, respectively; similarly, at 25% and 40%, a related trend of diastase reduction was determined, which existed below the standard limit of Codex Alimentarius Commission[Citation25] (>8 DN) but above the limit of FSSAI[Citation2] (>3 DN). In thermally processed honey, heat and rice syrup adulteration in honey samples showed a significant reduction in diastase activity compared to raw samples. direct that the diastase numb reduced after elevating the concentration of corn syrup (10% to 55%) in Acacia, Ziziphus, multifloral, and Trifolium Honey from 6.64 to 3.46, 9.22 to 3.16, 10.64 to 5.85 and 8.64 to 3.46 respectively. Similarly, pure processed Acacia (7.35 DN), Ziziphus (14.36 DN), multifloral (15.15 DN) and Trifolium (9.35 DN) honey samples indicated a rapid reduction of diastase activity in raw samples. The effect of the concentration of rice syrup on diastase activity was found to be significantly different after statistical Analysis (p < .05).[Citation38]
After addition of malt syrup (10% to 55%) in raw honey and processing showed a negative result with the reduction in DN. render DN values of adulterated Raw Acacia, Ziziphus, multifloral and Trifolium Honey with different malt syrup concentrations reduced from 8.41 to 3.41 DN, 18.41 to 11.53 DN, 23.67 to 13.22 DN and 13.31 to 9.53 DN, respectively. In contrast, a rapid reduction was observed in processed samples of the same four honey types and adulterated with the same concentration of malt syrup. , processed Acacia, Ziziphus, multifloral and Trifolium Honey had a significant drop in diastase from 6.33 to 2.53 DN, 14.03 to 6.51 DN, 14.08 to 4.45 DN and 8.17 to 5.75 DN, respectively. Statistical analysis revealed significant differences in the concentration of malt syrup on diastase activity of raw and processed honey from different origins (p < .05).
Phytochemical activity (TPC and TFC) of honey
Honey is an excellent source of secondary metabolites, such as flavonoids and polyphenols, which incorporate sufficient antioxidant activity in honey.[Citation39,Citation40] According to our study, it was observed that the multifloral honey sample was rich in total phenolic content (TPC) (155 mg GAE/100 g), whereas minimum phenolic content was found in Acacia honey (36.71 mg GAE/100 g). Kavanagh et al.[Citation35] conducted a related study that characterized honey from diverse floral origins in Ireland and reported that the highest phenolic content (68.16 mg GAE/100 g) was in Heather honey. Other honey resulted in phenolic content ranging from (62.43 mg GAE/100 g to 20.32 mg GAE/100 g), which was lower than our reported values. Flavonoid compounds, a subgroup of polyphenolic compounds, also incorporate the antioxidant activity of honey.[Citation40] In four varieties of Honey, TFC (total flavonoid content) varied between 8.87 and 90.96 mg QE/100 g (). The total flavonoid content was found to be maximum in multifloral honey (90.96 mg/100 g).
Effect of concentration of sugar syrups on the total phenolic content (mg gae/100 g) of raw and processed honey from different botanical origins
Honey adulteration and processing can degrade the total phenolic content. It was observed that cane syrup at a concentration of 55% in Acacia, Ziziphus, multifloral and Trifolium Honey showed decreased total phenolic content, 9.36, 42.13, 64.80 and 14.80 mg/100 g, respectively (). Moreover, after thermal treatment of adulterated Acacia, Ziziphus, multifloral and Trifolium Honey along with adulteration of cane syrup, the TPC was found at 25, 71.89, 125 and 23.12 mg GAE/100 g at 10% concentration, which was further reduced to 13.26, 17.67, 13.27 and 10.27 mg GAE/100 g when the concentration of syrups was extended to 55% (). It is observed that both heat and cane syrup harmed the phenolic content of honey from different origins.In this study, it was revealed that increasing the concentration of corn syrup in honey can affect the total phenol content of honey. At 10% concentration of corn syrup, the polyphenol content of raw Acacia, Ziziphus, multifloral and Trifolium Honey was 32.26, 109.26, 129.26 and 29.26 mg GAE/100 g, respectively, whereas, at 55% concentration, polyphenol content was reduced enormously to 3.12, 11.16, 10.16 and 4.16 mg GAE/100 g respectively (). However, the exact impact was observed after processing of same honey samples. Processed Acacia, Ziziphus, multifloral and Trifolium honey TPC was found at 24.73, 74.53, 114.43 and 24.54 mg GAE/100 g at 10% concentration, which was reduced to 3.57, 6.92, 3.92 and 4.06 mg GAE/100 g when the concentration of syrup was extended to 55% (). Statistical analysis revealed significant differences among all variables (p < .05)During this research, the TPC content decreased with increasing the concentration of rice syrup in honey. add rice syrup in Acacia, Ziziphus, multifloral and Trifolium Honey with different concentrations (10%, 25%, 40% and 55%). It resulted that, at 10% rice syrup concentration, phenolic content of Acacia, Ziziphus, multifloral and Trifolium Honey was 29.16, 106.39, 129.39 and 41.29 mg GAE/100 g, respectively, which was reduced significantly to 13.57, 59.08, 79.80 and 29.80 mg GAE/100 g, respectively, after raising sugar concentration to 55%. TPC content of thermally processed honey adulterated with the same concentration of rice syrup showed a considerable decline in TPC, as reported in . At 10% rice syrup concentration, phenolic content of Acacia, Ziziphus, multifloral and Trifolium Honey was observed 28.63, 68.63, 108.63 and 26.29 mg GAE/100 g, respectively, whereas the values were reduced to 1.85, 17.23, 7.45 and 17.35 mg GAE/100 g respectively, when the concentration of rice syrup was reached to 55%. The variable of raw and processed honey resulted in statistically significantly different (p < .05).
After observing the effect of malt syrup on the phenolic content of honey, it was revealed that altering the concentration of malt syrup in honey from 10% to 55% significantly elevated the phenolic content of honey. At 10% concentration, phenolic content of Acacia, Ziziphus, multifloral and Trifolium Honey was observed 228.60, 333.96, 348.16 and 353.96 mg GAE/100 g, respectively, which were increased to 531.25, 778.85, 786.32 and 805.15 mg GAE/100 g respectively, after concentrating the malt syrup to 55% in Honey (). The malt syrup enhanced the total phenolic content in honey, after thermal processing, the destruction of polyphenolic content was observed after comparing the result observed in with . Therefore, from the investigation, it was found that malt syrup had a direct interference in increasing the phenolic content, whereas thermal heating negatively correlated with honey’s phenolic content.
Effect of concentration of sugar syrups on the total flavonoid content (mg QE/100 g) of raw and processed honey from different botanical origins
The total flavonoid content of honey can be reduced by the adulteration and thermal processing of cane syrup. In our study, flavonoid content in honey was gradually reduced with the addition of cane syrup by 10%, 25%, 40% and 55%. , showed that total flavonoid content was reduced in Acacia, Ziziphus, multifloral, and Trifolium honey from 6.23 to 2.25 mg QE/100 g, 15.31 to 69.17 mg QE/100 g, 86.93 to 69.17 mg QE/100 g and 8.31 to 5.17 mg/100 g, respectively, as the concentration of cane syrup was increased from 10% to 55%. Additionally, a rapid reduction in sample TFC values was observed after thermal processing of all adulterated honey samples with the syrup. From , total flavonoid content was reduced in Acacia, Ziziphus, multifloral, and Trifolium Honey from 6.23 to 2.25 mg QE/100 g, 15.31 to 69.17 mg QE/100 g, 86.93 to 69.17 mg QE/100 g and 8.31 to 5.17 mg QE/100 g respectively, as the concentration of cane syrup was increased from 10% to 55%. Raw and processed honey Variables resulted in statistically significant differences (p < .05).
Our study revealed that enhancing the concentration of corn syrup in honey from 10% to 55% significantly affected the TFC of Honey. At 10% concentration of corn syrup, flavonoid content of Acacia, Ziziphus, multifloral and Trifolium Honey were 7.92 mg/100 g, 15.72, 89.72 and 10.12 mg QE/100 g, respectively. Whereas, after the interference of corn syrup to 55% concentration, flavonoid content was reduced enormously to 4.02, 5.31, 65.42 and 6.55 mg QE/100 g, respectively (). Honey Acacia, Ziziphus, multifloral and Trifolium adulterated with corn syrup at 60°C for 20 min. TFC were found at 6.78, 11.24, 69.78 and 6.74 mg QE/100 g at 10% concentration, which was reduced to 2.96, 3.02, 55.96 and 4.16 mg QE/100 g when the concentration of syrups was extended up to 55%, which was much lower than raw samples and statistical Analysis revealed significant difference among flavonoid content on the concentration of corn syrup (p < .05) as shown in .
Adding rice syrup with different concentrations (10%, 25%, 40% and 55%) in Acacia, Ziziphus, multifloral, and Trifolium honey gradually reduced the samples’ TFC. From , it resulted that, at 10% rice syrup concentration, flavonoid content of Acacia, Ziziphus, multifloral and Trifolium Honey was 7.43, 14.34, 90.36 and 9.43 mg QE/100 g, respectively, which was reduced significantly to 4.52, 8.32, 68.92 and 6.92 mg QE/100 g, respectively, after raising rice sugar concentration to 55%. Thermal processing of adulterated honey samples showed a considerable rapid reduction in TFC value then unprocessed adulterated samples. According to , at 10% rice syrup concentration, the total flavonoid content of Acacia, Ziziphus, multifloral and Trifolium Honey was observed 6.81, 12.31, 82.36 and 6.31 mg QE/100 g, respectively, and reduced progressively to 3.52, 8.46, 58.46 and 3.34 mg QE/100 g, respectively, when the concentration of cane syrup was reached to 55%.
As per our investigation, adding malt syrup to honey from 10% to 55% significantly elevated the flavonoid content of honey. At a 10% concentration of malt syrup, total flavonoid content of Acacia, Ziziphus, multifloral and Trifolium Honey was observed 138.68, 84.14, 142.68 and 131.57 mg QE/100 g, respectively, which were increased to the 298.27 QE/100 g, 311.27 QE/100 g, 296.27 QE/100 g and 473.59 mg QE/100 g respectively, after concentrating the malt syrup to 55% in Honey (). The malt syrup enhanced the total flavonoid content of honey. After thermal processing, the obliteration of flavonoid content is observed in . Therefore, the investigation found that malt syrup directly increased the flavonoid content, whereas thermal heating negatively correlated with the flavonoid content.
Antioxidant activity (DPPH and ABTS+) of honey
The antioxidant activity of honey varies widely depending on factors, such as geographical origins, floral sources, climatic conditions, storage conditions, and processing methods.[Citation39] In this study, we evaluated the antioxidant activity of the honey samples by measuring their DPPH scavenging activity and ABTS+ radical scavenging activity. Among the samples tested, Ziziphus honey (83.16%) and the multifloral honey (79.38%) exhibited the highest DPPH scavenging activity, while Ziziphus honey (88.58%) and the multifloral honey (81.98%) showed the highest ABTS+ radical scavenging activity. These findings are in line with the results of a study conducted by Dzugan et al.,[Citation40] who investigated the antioxidant activity of various types of honey and reported that the DPPH and ABTS+ scavenging activity of the analyzed samples ranged from 17.34% to 82.41%. Furthermore, the ascorbic acid value of EC50 was lower than the honey samples.[Citation35] The lower the value of EC50 the greater will be the scavenging power of honey. shows the EC50 of Cane syrup in raw acacia honey has the value of 22.5, which was increased to 28.5 after adulteration, which is the thermal effect that lower down the reducing power. Moreover, the maximum reducing power was observed in malt syrup in all four varieties of processed and raw honey. The raw acacia, ziziphus, multifloral and Trifolium exhibits EC50 value of 8.21, 14.32, 16.02, 10.02, respectively (). Whereas, corn syrup showed maximum value of EC50 (acacia (42.11), ziziphus (62.45), multifloral (49.75) and Trifolium (52.45)), therefore the reducing power is lower in honey adulterated with corn syrup. The obtained results were in accordance with previous studies conducted on Acacia honey.[Citation35] However, no evidence found in literature related to the effect on EC50 value of thermally processed honey.
Table 5. EC 50 (mg/ml) values of reducing power of honey adulterated with different sugar syrups.
Effect of concentration of sugar syrups on the dpph and abts+ scavenging activity of raw and processed honey from different botanical origins
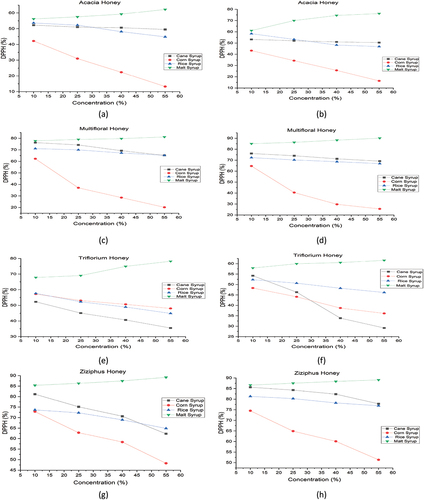
In our result, pure Acacia, Ziziphus, multifloral and Trifolium Honey showed high free radical scavenging activity and significant according to Blasa et al.,[Citation41] who found a similar result for Millefiori and Acacia Honey, which reported high antioxidant activities also. But this activity was reduced gradually from 52.26% to 49.51% (Acacia), 81.16% to 62.32% (Ziziphus), 76.28% to 65.23% (multifloral) and 52.26% to 35.51% (Trifolium) as concentration of cane syrup was increased from 10% to 55%. At 25% and 40% of cane syrup concentration, DPPH scavenging activity was found to be much lower than in raw honey, which might be due to the elimination of some antioxidant-generating compounds in honey. The effect of concentration of sugar syrups on the DPPH activity of different honey varieties is shown in .
Figure 2. The effect of sugar syrups on the DPPH% of various raw and processed honey varieties; (a) Raw Acacia, (b) Processed Acacia, (c) Raw Multifloral, (d) Processed Multifloral, (e) Raw Troflorium, (f) Processed Troflorium, (g) Raw Ziziphus, and (h) Processed Ziziphus.
Discussion
This study aimed to investigate the impact of various sugar syrups on the physicochemical properties of different raw and processed honey varieties. Results showed that the sugar syrups had a significant effect on the overall quality of honey, with considerable effects on HMF content, Diastase Number, TPC, TFC and DPPH activity. Cane syrup was found to significantly affect the quality of raw honey, particularly in Acacia honey. When the concentration of cane syrup in honey exceeded 25%, the HMF content increased above the international standards set by the Codex Alimentarius Commission[Citation2] (40 mg/kg). In contrast, adulteration of Acacia honey with other syrups had little effect on the HMF content of the honey. Rice syrup adulteration also increased the HMF content of honey beyond food safety standards.[Citation2,Citation25] Similar effects of rice syrup adulteration were observed in raw Ziziphus, multifloral, and Triflorium Honey. Thermal processing of honey also increased the HMF content of processed honey, and when combined with sugar syrup adulteration, the HMF content exceeded acceptable limits set by FSSAI[Citation25] and the Codex Alimentarius Commission.[Citation2] Cane syrup had the most significant effect on the HMF content in all four varieties of processed honey, with concentrations over 25% resulting in HMF content exceeding 100 mg/kg. Adulteration of Acacia honey with different syrups like corn, rice, and malt syrup resulted in high HMF content compared to processed Ziziphus, multifloral, and Triflorium Honey. Therefore, the adulteration of honey with different sugar syrups has a significant effect on the HMF content and can be used to evaluate the quality of raw and processed honey.[Citation45,Citation46] It is worth noting that processing honey at 60°C for 20 min increased the production of HMF content in the honey, which was much greater in cane syrup adulteration than other syrup types.[Citation32–34]
Furthermore, the addition of sugar syrups significantly reduced the diastase number (DN) in both raw and processed honey. As the concentration of the sugar syrup increased, the DN decreased accordingly. The study found that adulteration of Acacia honey with corn syrup had the greatest impact on the DN of the honey, with concentrations above 25% resulting in DN values below the permissible standards set by FSSAI[Citation25] and the Codex Alimentarius Commission.[Citation2] However, the adulteration of Acacia honey with cane, rice, and malt syrups showed a lesser effect on the quality of honey, as concentrations above 40% resulted in a significant reduction in DN below the required standards for determining honey quality.[Citation37,Citation39,Citation40] In addition, the study observed a direct effect of thermal processing on the DN value of honey, as thermal processing destroys the diastase enzyme. Moreover, the thermal processing of adulterated honey further reduced the DN value. Adulteration of Acacia honey with corn syrup had a drastic effect on the DN of the honey, with syrup concentrations above 10% reducing the DN value below the Indian and international standards. Similarly, Triflorium Honey adulterated with rice syrup at concentrations of 40% or above showed DN values below the standards set by FSSAI[Citation2] and the Codex Alimentarius Commission.[Citation25] Overall, the study found that the addition of all four varieties of sugar syrup (cane, corn, rice, and malt) to honey significantly reduced the DN, which is one of the primary criteria for determining honey quality.
Honey is known for its good phytochemical activity due to various polyphenols and flavonoids, but its phytochemicals are heat-labile in nature. Therefore, gentle heat treatment can slightly lower its overall antioxidant activity.[Citation47] Honey processing at the industrial level involves passing raw honey through different levels of filtration, thermal processing, and moisture reduction. In this study, the thermal processing was carried out at 60°C for 20 min. The effect of heating on raw and adulterated honey was clearly observed in the phytochemicals and antioxidant activity. Raw Acacia, Ziziphus, multifloral, and Triflorium honey adulterated with corn, cane, and rice syrup significantly reduced phytochemical contents, as determined by TPC and TFC.[Citation48,Citation49] Corn syrup adulteration in all kinds of raw honey showed the maximum reduction in TPC content. Both cane and rice syrup reduced the TFC value in all four honey varieties. However, in the case of honey adulteration with malt syrup, TPC and TFC content showed a drastic increase.[Citation50] As the concentration of malt syrup increases in all honey varieties, the TFC and TPC content increases significantly. The identical effect of adulterant syrups was observed in this study on the total antioxidant properties of all four honey varieties. Rice, cane, and corn syrup reduced the ABTS+ and DPPH scavenging activity, whereas as the malt syrup concentration in honey increased, the antioxidant value of the honey increased sharply.
The thermal processing of honey directly affects the quality of raw honey.[Citation48] The thermally processed raw and adulterated honey with different sugar syrups in this study showed a remarkable reduction in respective honey varieties and phytochemical and antioxidant activity. The maximum effect on TPC and TFC content of processed multifloral and Acacia honey was adulterated with a cane and corn syrup, whereas rice syrup adulteration in Ziziphus honey had a maximum reduction in the TPC and TFC values.[Citation50] Whereas the processing of honey adulterated with malt syrup had the opposite effect; as the concentration of malt syrup increased in the honey, a spike increases in the TPC and TFC content was observed.[Citation51,Citation52] Similarly, the processing of honey decreased the antioxidant activity as compared to raw honey, which is further reduced after adulteration of respective honey varieties with cane, corn, and rice syrup. Whereas the processing of honey adulterated with malt syrup showed increased values of antioxidant activity by ABTS+ and DPPH with increased syrup concentration. This can be due to the heat treatment, which increased the total phenol content present in malt syrup.[Citation42–47] The therapeutic and antibiotic properties of honey are due to the present phytochemicals and antioxidant properties.[Citation45,Citation53] Due to the adulteration of honey along with thermal processing, these beneficial components were destroyed, by which the functional value of honey was reduced and a reduction in the quality of respective honey.[Citation50] Furthermore, the EC50 was evaluated to determine the radical scavenging capacity of honey and the effect of thermal processing and adulteration on the scavenging of honey. The identical trend compared to antioxidant activity was observed in degradation of scavenging capacity based on EC50 of honey samples. Moreover, the sugar syrups showed different results on the scavenging power of each honey variety. The corn syrup had maximum effect on the reducing power of honey, the higher value of EC50 of each honey adulterated with corn syrup was observed. In contrast, the malt syrup had minimum value of EC50 which depict the increase in reducing power of adulterated honey. Considering the effect of thermal processing of on adulterant syrups in honey increase the EC50 which is directly related to the reduction in reducing power due the action of heat on bioactive compounds.[Citation45,Citation48,Citation49]
This study highlights the need for exploring the effect of different sugar syrups on raw and processed honey’s physicochemical and functional properties. Adding specific sugar syrup to a certain concentration in a specific variety of honey showed a remarkable effect, such as increased HMF and decreased DN, which are primarily analytical methods. These parameters can be applied to determine honey authenticity.[Citation53] Moreover, this outcome also provides guidance to researchers and governing bodies for outlining specifications for standardization of various Indian unifloral honey varieties, which are undermined and need more exploitation in the research field to cover the gap between honey authentication and to establish specific standards in the market. This work helps researchers focus on these factors and assists the honey market in starting production and processing unique unifloral honey varieties for economic support and diversification of honey varieties.
Conclusion
Based on the research conducted, significant changes in the functional and chemical activity of honey were observed when adulterated with different concentrations of all four sugar syrups. Parameters such as TPF, TPC, DPPH, ABTS+, and diastase activity of honey decreased with an increase in syrup concentration, except for malt syrup, which showed an increase in TPC, DPPH, and TFC content due to its high values in particular parameters. However, after processing, values of these parameters decreased due to thermal effects. Additionally, HMF content increased in both processed and adulterated test samples. As authenticity is a primary concern for consumers, the current methods for detecting honey adulteration are expensive, less available, and more sophisticated. Therefore, this study emphasizes the need for a simple and accessible process validation method for honey authentication. Moreover, the research highlights the divergent effect on the quality of different honey varieties, which could lead to the development and evaluation of precise standards for the authentication of multi- and uni-floral honey and their proper adulteration detection methods by researchers and regulatory bodies. This study paves the way for future research and the development of standard protocols for honey authentication and quality control.
Acknowledgments
The investigation was supported by the Bhabha Atomic Research Centre, Trombay, Mumbai, under the BRNS (Board of Research in Nuclear Sciences) project (sanction letter: 55/14/16/2020) awarded to Dr. Vikas Nanda. We would like to express our gratitude to the Bhabha Atomic Research Centre and the BRNS for their support in this research.
Disclosure statement
No potential conflict of interest was reported by the authors.
Data availability statement
Although adequate data has been given in the form of tables and figures, however, all authors declare that if more data are required then the data will be provided on a request basis.
References
- Council Directive of the European Union. Council Directive 2001/110/EC of 20 December 2001 Relating to Honey. Off. J. Euro. Commun. 2001, 10, 47–52.
- Standards for honey revised to build public interest in quality of honey in market. Food Safety and Standards (Food Products Standards and Food Additives) Ninth Amendment Regulations; FSSAI: New Delhi, India, 2018. https://www.pfndai.org/Document/Gazette/FSSAI/Gazette_Notification_Pulses_Grains_Maize_14_08_2018.pdf (Accessed 10 December 2021).
- De la Fuente, E.; Ruiz-Matute, A. I.; Valencia-Barrera, R. M.; Sanz, J.; Martínez Castro, I. Carbohydrate Composition of Spanish Unifloral Honey. Food. Chem. 2011, 129(4), 1483–1489. DOI: 10.1016/j.foodchem.2011.05.121.
- Ouchemoukh, S.; Schweitzer, P.; Bachir Bey, M.; Djoudad-Kadji, H.; Louaileche, H. HPLC Sugar Profiles of Algerian Honey. Food. Chem. 2010, 121, 561–568. DOI: 10.1016/j.foodchem.2009.12.047.
- Kang, K. M.; Yoo, B. Dynamic Rheological Properties of Honey at Low Temperatures as Affected by Moisture Content and Temperature. Food Sci. Biotechnol. 2008, 17(1), 90–94.
- Gismondi, A.; De Rossi, S.; Canuti, L.; Novelli, S.; Di Marco, G.; Fattorini, L.; Canini, A. From Robinia pseudoAcacia L. Nectar to Acacia Monofloral Honey: Biochemical Changes and Variation of Biological Properties. J. Sci. Food Agric. 2018, 98(11), 4312–4322. DOI: 10.1002/jsfa.8957.
- Gao, Y.; Xue, A.; Li, X.; Huang, X.; Ning, F.; Zhang, X.; Luo, L., Chen, H., Luo, L. Analysis of Chemical Composition of Nectars and Honeys from Citrus by Extractive Electrospray Ionization High Resolution Mass Spectrometry. LWT. 2020, 131, 109748. DOI: 10.1016/j.lwt.2020.109748.
- Yan, S.; Wang, X.; Wu, Y.; Wang, K.; Shan, J.; Xue, X. A Metabolomics Approach Revealed an Amadori Compound Distinguishes Artificially Heated and Naturally Matured Acacia Honey. Food. Chem. 2022, 385, 132631. DOI: 10.1016/j.foodchem.2022.132631.
- Adgaba, N.; Al-Ghamdi, A.; Tadesse, Y.; Getachew, A.; Awad, A. M.; Ansari, M. J.; Alqarni, A. S.; Mohammed, S. E. A.; Alqarni, A. S. Nectar Secretion Dynamics and Honey Production Potentials of Some Major Honey Plants in Saudi Arabia. Saudi J. Biol. Sci. 2017, 24(1), 180–191. DOI: 10.1016/j.sjbs.2016.05.002.
- Da Silva, P. M.; Gauche, C.; Gonzaga, L. V.; Costa, A. C. O.; Fett, R. Honey: Chemical Composition, Stability and Authenticity. Food. Chem. 2016, 196, 309–323. DOI: 10.1016/j.foodchem.2015.09.051.
- Czipa, N.; Andrási, D.; Kovács, B. Determination of Essential and Toxic Elements in Hungarian Honey. Food . Chem. 2015, 175, 536–542. DOI: 10.1016/j.foodchem.2014.12.018.
- Escuredo, O.; Míguez, M.; Fernández-González, M.; Carmen Seijo, M. Nutritional Value and Antioxidant Activity of Honey Produced in a European Atlantic Area. Food. Chem. 2013, 138(2), 851–856. DOI: 10.1016/j.foodchem.2012.11.015.
- Ajibola, A.; Chamunorwa, J. P.; Erlwanger, K. H. Nutraceutical Values of Natural Honey and Its Contribution to Human Health and Wealth. Nutri. Metabolism. 2012, 9(1), 61. DOI: 10.1186/1743-7075-9-61.
- Gül, A.; Pehlivan, T. Antioxidant Activities of Some Monofloral Honey Types Produced Across Turkey. Saudi J. Biol. Sci. 2018, 25(6), 1056–1065. DOI: 10.1016/j.sjbs.2018.02.011.
- Samarghandian, S.; Farkhondeh, T.; Samini, F. Honey and Health: A Review of Recent Clinical Research. Pharmacogn. Res. 2017, 9(2), 121–127.
- Nayik, G. A.; Shah, T. R.; Muzaffar, K.; Wani, S. A.; Gull, A.; Majid, I.; Bhat, F. M. Honey: Its History and Religious Significance: A Review. Univ. J. Pharma. 2014, 3, 5–8.
- Ahmed, S.; Othman, N. H. Review of the Medicinal Effects of Tualang Honey and a Comparison with Manuka Honey. Malay. J. Med. Sci. 2013, 20(3), 6–13.
- Agricultural and Processed Food Products Export Development Authority. http://apeda.gov.in/apedawebsite/SubHead_Products/Natural_Honey.htm (Accessed 20 November 2022).
- Kennedy, S. Economically Motivated Adulteration of Honey: Quality Control Vulnerabilities in the International Honey Market. Food. Protect. Trends. 2014, 34(1), 8–14.
- Gebremariam, T.; Brhane, G. Determination of Quality and Adulteration Effects of Honey from Adigrat and Its Surrounding Areas. Int. J. Tech. Enhance. Emerg. Engineer. Res. 2014, 2(10), 2347–4289.
- Amiry, S.; Esmaiili, M.; Alizadeh, M. Classification of Adulterated Honey by Multivariate Analysis. Food. Chem. 2017, 224, 390–397. DOI: 10.1016/j.foodchem.2016.12.025.
- Mishra, S.; Kamboj, U.; Kaur, H.; Kapur, P. Detection of Jaggery Syrup in Honey Using Near-Infrared Spectroscopy. Int. J. Food Sci. Nutr. 2010, 61(3), 306–315. DOI: 10.3109/09637480903476415.
- Fauzi, N. A.; Farid, M. M.; Silva, F. V. M. High-Pressure Processing of Manuka Honey: Improvement of Antioxidant Activity, Preservation of Color and ?ow Behavior. Food Bioprocess. Technol. 2014, 7(8), 2299–2307. DOI: 10.1007/s11947-013-1204-7.
- Chaikham, P.; Prangthip, P. Alteration of Antioxidative Properties of Longan Flower-Honey After High Pressure, Ultra-Sonic and Thermal Processing. Food. Biosci. 2015, 10, 1–7. DOI: 10.1016/j.fbio.2015.01.002.
- Codex Alimentarius Commission. Revised Standards for Honey. Codex Standard 12–1981. In Rev; FAO: Rome, 2001.
- Gan, Z.; Yang, Y.; Li, J.; Wen, X.; Zhu, M.; Jiang, Y.; Ni, Y. Using Sensor and Spectral Analysis to Classify Botanical Origin and Determine Adulteration of Raw Honey. J. Food Eng. 2016, 178, 151–158. DOI: 10.1016/j.jfoodeng.2016.01.016.
- Du, B.; Wu, L.; Xue, X.; Chen, L.; Li, Y.; Zhao, J.; Cao, W. Rapid Screening of Multiclass Syrup Adulterants in Honey by Ultrahigh-Performance Liquid Chromatography/Quadrupole Time of Flight Mass Spectrometry. J. Agric. Food Chem. 2015, 63(29), 6614–6623. DOI: 10.1021/acs.jafc.5b01410.
- Puscas, A.; Hosu, A.; Cimpoiu, C. Application of a Newly Developed and Validated High-Performance Thin-Layer Chromatographic Method to Control Honey Adulteration. J. Chromatography. A. 2013, 132–135. DOI: 10.1016/j.chroma.2012.11.064.
- Bertelli, D.; Lolli, M.; Papotti, G.; Bortolotti, L.; Serra, G.; Plessi, M. Detection of Honey Adulteration by Sugar Syrups Using One-Dimensional and Two-Dimensional High-Resolution Nuclear Magnetic Resonance. J. Agric. Food Chem. 2010, 58(15), 8495–8501. DOI: 10.1021/jf101460t.
- Smith, C.; Cokcetin, N.; Truong, T.; Harry, E.; Hutvagner, G.; Bajan, S. Cataloguing the Small RNA Content of Honey Using Next Generation Sequencing. Food. Chemis. Mol. Sci. 2021, 2, 100014. DOI: 10.1016/j.fochms.2021.100014.
- International Honey Commission (IHC). 2009. Harmonized Methods of the International Honey Commission. http://www.bee-hexagon.net/en/network.htm (Accessed 15 December 2021).
- Meda, A.; Lamien, C. E.; Romito, M.; Millogo, J.; Nacoulma, O. G. Determination of the Total Phenolic, Flavonoid and Proline Contents in Burkina Fasan Honey, as Well as Their Radical Scavenging Activity. Food. Chem. 2005, 91(3), 571–577. DOI: 10.1016/j.foodchem.2004.10.006.
- Andrade, P. B.; Amaral, M. T.; Isabel, P.; Carvalho, J. C. M. F.; Seabra, R. M.; Proença da Cunha, A. Physicochemical Attributes and Pollen Spectrum of Portuguese Heather Honey. Food. Chem. 1999, 66(4), 503–510. DOI: 10.1016/S0308-8146(99)00100-4.
- Czipa, N.; Phillips, C. J. C.; Kovács, B. Composition of Acacia Honey Following Processing, Storage and Adulteration. J. Food Sci. Technol. 2019, 56, 1245–1255. DOI: 10.1007/s13197-019-03587-y.
- Chua, L. S.; Rahaman, N. L. A.; Adnan, N. A.; Eddie Tan, T. T. Antioxidant Activity of Three Honey Samples in Relation with Their Biochemical Components. J. Anal. Methods Chem. 2013, 2013, 1–8. DOI: 10.1155/2013/313798.
- Dhillon, B.; Sodhi, N. S.; Singh, D.; Kaur, A. Analyses of Functional Diets Formulated for Dysphagia Patients Under International Dysphagia Diet Standardization Initiative (IDDSI) Level 3 to Level 7. J. Food Meas. Charact. 2022, 16(5), 3537–3546. DOI: 10.1007/s11694-022-01454-7.
- Von Der Ohe, W.; Oddo, L. P.; Piana, M. L.; Morlot, M.; Martin, P. Harmonized Methods of Melissopalynology. Apidologie. 2004, 35(Suppl. 1), S18–S25. DOI: 10.1051/apido:2004050.
- Cozmuta, A. M.; Cozmuta, L. M.; Varga, C.; Marian, M.; Peter, A. Effect of Thermal Processing on Quality of Polyfloral Honey. Romanian J. Food. Sci. 2011, 1(1), 45–52.
- Escriche, I.; Kadar, M.; Juan-Borrás, M.; Domenech, E. Suitability of Antioxidant Capacity, Flavonoids and Phenolic Acids for Floral Authentication of Honey. Impact of Industrial Thermal Treatment. Food. Chem. 2014, 142, 135–143. DOI: 10.1016/j.foodchem.2013.07.033.
- Dżugan, M.; Tomczyk, M.; Sowa, P.; Grabek-Lejko, D. Antioxidant Activity as Biomarker of Honey Variety. Molecules. 2018, 23(8), 2069. DOI: 10.3390/molecules23082069.
- Blasa, M.; Candiracci, M.; Accorsi, A.; Piacentini, M. P.; Albertini, M. C.; Piatti, E. Raw Millefiori Honey is Packed Full of Antioxidants. Food. Chem. 2006, 97(2), 217–222. DOI: 10.1016/j.foodchem.2005.03.039.
- Turkmen, N.; Sari, F.; Poyrazoglu, E. S.; Velioglu, Y. S. Effects of Prolonged Heating on Antioxidant Activity and Colour of Honey. Food. Chem. 2006, 95(4), 653–657. DOI: 10.1016/j.foodchem.2005.02.004.
- Arif, M.; Bangash, J. A.; Khan, F.; Abid, H. Effect of Soaking and Malting on the Selected Nutrient Profile of Barley. Paki. J. Biochemis. Mol. Biol. 2011, 44(1), 18–21.
- Goupy, P.; Hugues, M.; Boivin, P.; Amiot, M. J. Antioxidant Composition and Activity of Barley (Hordeum vulgare) and Malt Extracts and of Isolated Phenolic Compounds. J. Sci. Food Agric. 1999, 79(12), 1625–1634. DOI: 10.1002/(SICI)1097-0010(199909)79:12<1625::AID-JSFA411>3.0.CO;2-8.
- Hebbar, H. U.; Nandini, K. E.; Lakshmi, M. C.; Subramanian, R. Microwave and Infrared Heat Processing of Honey and Its Quality. Food Sci. Technol. Res. 2003, 9(1), 49–53. DOI: 10.3136/fstr.9.49.
- Kowalski, S.; Lukasiewicz, M.; Bednarz, S.; Panuś, M. Diastase Number Changes During Thermaland Microwave Processing of Honey. Czech J. Food Sci. 2012, 30(1), 21–26. DOI: 10.17221/123/2010-CJFS.
- Noor, N.; Sarfraz, R. A.; Ali, S.; Shahid, M. Antitumour and Antioxidant Potential of Some Selected Pakistani Honey. Food. Chem. 2014, 143, 362–366. DOI: 10.1016/j.foodchem.2013.07.084.
- Zarei, M.; Fazlara, A.; Alijani, N. Evaluation of the Changes in Physicochemical and Antioxidant Properties of Honey During Storage. FFHD. 2019, 9(9), 593–605. DOI: 10.31989/ffhd.v9i9.616.
- Šarić, G.; Marković, K.; Vukičević, D.; Lež, E.; Hruškar, M.; Vahčić, N. Changes of Antioxidant Activity in Honey After Heat Treatment. Czech J. Food Sci. 2013, 31(6), 601–606. DOI: 10.17221/509/2012-CJFS.
- Payet, B.; Shum Cheong Sing, A.; Smadja, J. Comparison of the Concentrations of Phenolic Constituents in Cane Sugar Manufacturing Products with Their Antioxidant Activities. J. Agric. Food Chem. 2006, 54(19), 7270–7276. DOI: 10.1021/jf060808o.
- McMurrough, I.; Loughrey, M. J.; Hennigan, G. P. Content of (+)-Catechin and Proanthocyanidins in Barley and Malt Grain. J. Sci. Food Agric. 1983, 34(1), 62–72. DOI: 10.1002/jsfa.2740340110.
- Bonoli, M.; Verardo, V.; Marconi, E.; Caboni, M. F. Antioxidant Phenols in Barley (Hordeum Vulgare L.) Flour: Comparative Spectrophotometric Study Among Extraction Methods of Free and Bound Phenolic Compounds. J. Agric. Food Chem. 2004, 52(16), 5195–5200. DOI: 10.1021/jf040075c.
- Brar, D. S.; Pant, K.; Krishna, R.; Kaur, S.; Rasane, P.; Nanda, V.; Gautam, S.; Gautam, S. A Comprehensive Review on Unethical Honey: Validation by Emerging Techniques. Food. Control. 2022, 109482. DOI: 10.1016/j.foodcont.2022.109482.