?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
Frafra potato (Solenostemon rotundifolius) is an underutilized climate-resilient tuber crop commonly cultivated in the tropics. Different accessions have been identified and bred to broaden its application in food. This study characterized the starches extracted from ten accessions, from Ghana (released) and Burkina Faso (unreleased), in terms of yield, physicochemical, and microstructural characteristics. The starches did not differ significantly (p < .05) in color (L*) and amount (35 to 39% dry matter basis). Furthermore, there were no significant differences (p < .05) among them in amylose/amylopectin ratio, syneresis %, granule types and shapes, except for one of the unreleased accessions (E82), which had a significantly (p < .05) smaller granule size. There were, however, significant differences (p < .05) in paste clarity of the starch gels, ranging from 51 to 63% of the starch gels, as well as in the thermal properties of the starches. The XRD and FTIR spectra showed the starches to be A-type, typical of tuber starches, with relative crystallinities ranging between 30.5 to 33.5%. The ten Frafra potato cultivars were clustered into two groups using PCA procedures; one group (Maa-Lana and Nutsugah) clustering on thermal properties of starch, while the other group (E82, E111, E132, E134, E145, Manga, Naachem-Tiir, and WAAPP) on paste clarity and change in temperature. The variations in granule size and thermal characteristics of the starches could impact the performance, cooking and textural properties, of these Frafra potato accessions in food applications.
Introduction
Frafra potato (Solenostemon rotundifolius) is a neglected climate-smart crop cultivated in parts of Africa and Asia. It is a valuable tuber crop mainly cultivated in Northern Ghana[Citation1–4]. The crop has excellent potential for ameliorating malnutrition and enhancing food and nutrition security in Ghana and other developing countries.[Citation1–3] Although it has not been extensively explored in industrial applications, it has the potential to act as a source of flour and starch.[Citation2,Citation4,Citation5] Frafra potato’s major biomolecule is starch, which accounts for about 80% of the edible root’s dry mass. The crystalline structure, granular characteristics, among other starch qualities, play an important role in the quality and end-user experience of starchy food products.[Citation5–7]
Several new accessions with higher protein content have been developed, through traditional and contemporary breeding methods, to increase the usage and nutritional value of Frafra potatoes. While the root tubers are said to contain more iron and other minerals, they often have a low dry matter content. Since there is a genetic relationship between starch content and dry matter content in tuber crops like Frafra potato and sweetpotato, this drop in dry matter ultimately affects the starch content.[Citation1,Citation3,Citation8] Studies involving tuber crops like potato and sweetpotato have revealed that the development of new accessions of tuber crops have an impact on the functional behavior of biomolecules like starch. Texture, appearance, and cooking qualities are only a few of these variations that are impacted by starch. New lines of Frafra potato are being researched to boost the fight against malnutrition by employing improved crop varieties. But their starch qualities will have a significant impact on how these accessions are used in food. Therefore, it is critical to thoroughly evaluate the starch from Frafra potato genotypes to understand their behavior, as this will inform targeted food and industrial applications, as well as for subsequent crop improvement.[Citation9–13] The study’s aim was to determine the physicochemical, functional, and microstructural characteristics of ten Frafra potato accessions.
Materials and methods
Source of raw materials
Approximately twenty-five[Citation14] kg each of ten[Citation10] cultivars of Frafra potato (FP) were obtained from the Council for Scientific and Industrial Research (CSIR) -Savanna Agricultural Research Institute (CSIR-SARI), Manga in the Upper East Region of Ghana. These cultivars were WAAPP Piesa, Maa-Lana Piesa, Naachem-Tiir Piesa, Nutsugah Piesa, Manga Moya Piesa (released cultivars from Ghana), E82, E111, E132, E134, E145 (unreleased cultivars from Burkina Faso). All the cultivars have whitish flesh. The tubers were transported (about 8 hours) to the food processing laboratory at CSIR-Food Research Institute, Accra, packed in perforated paper cartons (aeration) for processing.
Isolation of Frafra potato starch (FPS)
The procedure of Gayin et al.[Citation15] was used for starch isolation with modifications. Frafra potato tubers were washed in potable water, weighed, hand-peeled, washed, weighed, and sliced into thin slices (5 mm thick) using a mechanical slicer (SLP45HC, Foshan Sorumpor Electrical, Guangdong, China). The FP slices were wet milled at low speed using a laboratory-scale mill (Knife Mill Blender Pulverisette 11, Laval, Canada) with 1:2 w/v of water for 2 min and sifted through a cheesecloth fitted in a standard 100 µ sieve. The residue was wet-milled and filtered thrice, and the suspension was kept overnight, at 7°C, for the starch to settle. The supernatant was decanted, and the residue was purified with repeated suspension in water (1:2 v/v) followed by the settling for 3 h. The purified starch was dried at 35°C in a mechanical dryer (TM1006, NEUE HERBOLD, Sinsheim-Reihen, Germany), packed in high-density polyethylene (HDPE) bags, sealed airtight with an impulse sealer (“Oalink-QNS-3200HI,” Accra, Ghana), and put in storage at room temperature (25 ± 0.53°C) for further tests.
Starch yield of Frafra potato
The yield of FPS and the proportion of peels, moisture and fiber removed during processing of the ten[Citation10] varieties was estimated as a percentage of the unpeeled FP weight.
Colour of FPS
According to Stevenson et al.,[Citation16] the color of FPS was determined using a Chromameter (CR-400 Chroma Meter, Konica Minolta, Tokyo, Japan). The instrument was calibrated against the standard white tile (L × 0 = 98.93, a × 0 = 0.31, and b × 0 = 4.63) before use. FPS samples were contained in a transparent petri dish and covered with the same. FPS color was also described using C* (Chroma) and h* (Hue angle) notations. Paste Clarity (PC) of FPS was determined as follows; briefly, 5 mL of 1% starch suspension in 15 mL screw-capped centrifuge tubes were incubated in a boiling water bath for 30 min, with continual shaking. The starch solutions were cooled to room temperature (25 ± 0.53°C), and their transmittance (%) was measured against a water blank at 650 nm on a UV-VIS spectrophotometer (Shimadzu 1800, Tokyo, Japan).
Amylose/Amylopectin ratio of FPS
The Amylose/Amylopectin ratio was determined using the method of Kowsik and Mazumder,[Citation17] with modifications. One hundred milligram (100 mg) of FPS was dissolved in 9 mL of 90% dimethyl sulfoxide (DMSO) in screw-capped tubes and vortexed. The suspension was heated in a water bath at 85°C for 15 min, with continual shaking. The solution was allowed to cool to room temperature (25 ± 0.53°C) and diluted with water to 25 mL in a volumetric flask. An aliquot (5 mL) of the dilute solution was pipetted into a separate 100 mL standard flask and 1 mL of 1 M acetic acid, followed by 5 mL of iodine solution was added before making up to the mark with distilled water. The resulting solution was, allowed to stand for 20 min for color development before absorbance measurement at 620 nm with a UV-VIS spectrophotometer (Shimadzu 1800, Tokyo, Japan). Amylopectin was calculated as the difference between the total amylose content and 100%.
Resistant starch determination of FPS
Total resistant starch was determined with a Megazyme-resistant starch assay kit following the procedure established by the manufacturer. Fifty gram (50 g) of starch was dissolved and homogenized. Pancreatic α-amylase and amyloglucosidase were added and then incubated in a water bath, shaking for 17 h at 37°C. In that time, nonresistant starch was solubilized and hydrolyzed to D-glucose by the combined action of the two enzymes. The reaction was completed by adding an equal volume of ethanol, and the RS was recovered as a pellet on centrifugation. The pellets were dispersed in 2 M KOH by stirring in an ice-water bath over a magnetic stirrer. This solution was neutralized with acetate buffer (pH of 4.5), and the starch was quantitatively hydrolyzed to glucose with amyloglucosidase. D-Glucose was measured with glucose oxidase/peroxidase reagent, which was a measure of the resistant starch content of the samples.
Retrogradation (syneresis %) of FPS gels
Retrogradation of FPS gels was determined using the method of Ezekiel and Singh.[Citation18] A 12% suspension of FPS was kept at 95°C for 15 min in a water-bath, cooled to 50°C, and kept at this temperature for 15 min. Fifty milliliters was sampled into test tubes and kept at 4°C. The retrogradation of pastes prepared from FPS was measured by determining the amount of water expelled during storage at 4°C, known as syneresis. Retrogradation was expressed as Syneresis (%) and was computed as follows:
Thermal characteristics of FPS
Thermal characteristics (TC) of FPS were determined using a Thermogravimetric Analyzer (TGA-DSC SDT Q600 V20.9 Build 20, TA Instruments, New Castle, DE USA) following the procedure established by the manufacturer. FPS (2.5 mg) was weighed into an aluminum pan, and double deionized water (7.5 µL) was added. The pan was hermetically sealed and equilibrated at 25 ± 0.53°C for about 1 h for moisture balance and then heated at the rate of 5°C/min from 25–100°C with a blank as reference. Thermal parameters such as onset (To), peak (Tp), conclusion (Tc) temperatures, range of gelatinization temperature ΔT (ΔT = Tc - To) and enthalpy of gelatinization (ΔH) were recorded.
FTIR spectroscopy of FPS
FTIR Spectroscopy of FPS was determined using a Fourier Transform Infrared (FTIR) spectrometer (UATR Two, PerkinElmer, United Kingdom 105,024) with a deuterated triglycine sulfate detector, following the procedure established by the manufacturer. Original spectra were corrected by subtraction of the baseline in the region from 4000 to 500 cm−1 before deconvolution was applied using Resolutions Pro FTIR software (version 5). The assumed line shape was Lorentzian with a half-width of 19 cm−1 and a resolution enhancement factor of 1.9. Intensity measurements were performed by recording the height of the absorbance spectra.
XRD spectroscopy of FPS
XRD Spectroscopy of FPS was determined using an X-ray powder diffraction spectroscope (Empyrean Series 2 XRD, Malvern Panalytical, United Kingdom). The starches were kept in a desiccator where a saturated solution of NaCl maintained a constant relative humidity of 75% for one week at 26 ± 0.41°C. XRD recorded the X-ray diffraction spectra. The diffraction angle and intensity measurements were recorded.
Scanning electron microscopy (SEM)
Granular morphology of FPS was characterized by SEM (Phenom ProX World Desktop SEM + EDS, Thermo Fisher Scientific, USA) following the procedure established by the manufacturer. Samples were first coated with gold dust (15 nm) using Emitech K550X Sputter Coater (Quorum Technologies Limited, Kent, UK). An accelerating voltage of 15 kv was used for imaging at x2300 magnification. The average diameter of granules at 30 µm was reported as the granule size (GS) of FPS.
Statistical analysis
The data were subjected to Analysis of Variance (ANOVA) using “R” statistical software for Windows pc version 4.1.1 (R Project, Bell Laboratories, USA), and Duncan Multiple Range Test (DMRT) was performed to separate varieties with significantly different (p < .05) means. Principal components analysis (PCA), in XLSTAT 2018 for windows pc, was used to cluster samples with close associations based on their physicochemical properties.
Results
Starch content of Frafra potato (FPS)
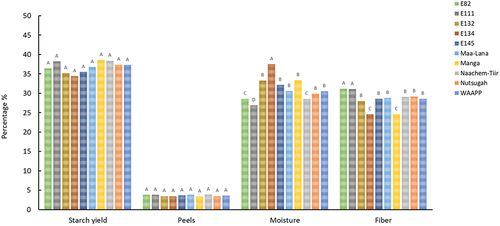
Starch yield and proportion of peels, moisture content and fiber from Frafra potato tubers are summarized in .
The starch yield of the tubers ranged from 35 to 39% (). There was no significant difference (p ≤ .05) between the starch yield and peels among the Frafra potato varieties. However, there were significant differences between moisture, ranging from 27% (E111) to 38% (E134) and fiber ranging from 25% (Manga) to 31% (E111) removed during processing among the Frafra potato varieties. The differences observed in the moisture content can be attributed to varietal differences reported in similar root and tuber starch isolation studies.[Citation7,Citation10] The starch content of the Frafra potatoes was lower than that of some varieties of other root and tuber crops, such as sweet potato, which recorded starch yield ranging from 49% to 77%.[Citation9] As a source of starch for food applications, the implications of a relatively lower starch yielding tuber are unfavorable as it will mean a relatively higher cost of production.[Citation7,Citation10] Therefore, there is the need to enhance the starch content of Frafra potato accessions in Ghana by developing cultivars with improved starch yielding qualities. Higher starch yielding cultivars will help position Frafra potato as an affordable source of starch for food applications in Ghana and Sub-Sahara Africa.
Colour of FPS
The color parameters of FPS is summarized in . The L* values ranged between 97 to 98, indicating that the different cultivars’ starches were white (). Except for the paste clarity, the starches had similar (p ≤ .05) L*, a*, b*, h*, and C × .They had a similar intensity of white color when observed with naked eyes. The differences observed in the paste clarity of the FPS cultivars may be attributed to variations in their carotenoid concentrations.[Citation7] Paste clarity is an essential property of starch gels, especially for food applications, and varies among different botanical sources or cultivars.[Citation9] The results showed that the starch formed clear pastes characteristic of starches from most root and tuber crops.[Citation7,Citation9] Starch from “Maa-Lana” was the most transparent, with a transmittance of nearly 63%, whereas E82 was the least transparent, with about 51% transmittance. Paste clarity is particularly beneficial in food systems that require high transparency[Citation7,Citation9]; hence FPS could be suitable in food applications without impacting the color of the food, such as in jellies and fruit pastes.
Table 1. Color of FPS.
Physicochemical characteristics of FPS
The physicochemical properties of Frafra potato starches are summarized in . There were no significant differences (p ≤ .05) between the amylose/amylopectin content and the relative crystallinity among the starches of the FP cultivars (). However, there were significant differences (p ≤ .05) in the resistant starch content among the cultivars, with the released cultivars of Frafra potato having higher resistant starch (19% to 21%) relative to the E-group cultivars (17% to 18%) except for E132, which had the highest (22%) resistant starch. The granule sizes of FPS were significantly different, ranging from 11.5 µm to 13.8 µm; however, most cultivars had similar average granule sizes. The similar granule sizes could be one of the reasons why FPS had similar paste clarity () since granules of the same size under the same conditions have similar transmittance, hence, similar paste clarity.[Citation10,Citation19]
Table 2. Physicochemical characteristics of FPS.
Syneresis % (retrogradation) of FPS gels
Retrogradation occurs when the amylose and amylopectin chains in cooked, gelatinized starch realign themselves to a crystalline structure as the cooked starch cools.[Citation18] The syneresis % of FPS is summarized in . The syneresis % value of the cooked pastes from the different FPS were comparable until day 10 when significant differences (p ≤ .05) began to show (). The syneresis of cooked pastes from FPS increased progressively during storage, with cultivars recording syneresis ranging from about 4% on day 2 to 27% by Day 10. FPS cultivars had similar syneresis % till storage day 8; however, at storage day 10, cultivars E82, E111, Maa-Lana, and Naachem-Tiir recorded significantly lower syneresis % (p ≤ .05). It was observed that FPS cultivars containing large-sized starch granules, such as WAAPP, E132, and Nutsugah (), showed higher syneresis values at day 10. In contrast, those containing small-sized starch granules, such as E82 and E111 (), showed lower syneresis % at storage day 10. The observed syneresis % of FPS shows that the retrogradation properties are not different from starches from other tropical tubers such as cassava, sweet potato, and yam.[Citation9]
Table 3. Syneresis % of FPS Gels.
Thermal characteristics of FPS
The thermal characteristics of Frafra potato starch are summarized in . FPS showed similar thermal properties (). However, two cultivars (Maa-Lana and Nutsugah) had significantly higher (p ≤ .05) onset, peak, and conclusion temperatures. These are the temperatures at which starch granules absorb water and swell, resulting in increased viscosity, to the point where the number of swollen intact granules is maximum, and the holding period at which usually leads to further disruption of granules and amylose leaching, respectively. The cultivars with low peak viscosity can be used in the production of weaning meals.[Citation20] Also, WAAPP had a significantly higher (p ≤ .05) gelatinization range, and Nutsugah had the least. The released Frafra potato cultivars had significantly higher (p ≤ .05) enthalpies of gelatinization, except for Nutsugah, which was lower but similar to that of the unreleased E-group cultivars. The thermal attributes of starch are associated with several factors, such as the amylose/amylopectin content, granule size, ultrastructure of the starch granules and proportion and kind of crystalline organization.[Citation7,Citation15,Citation18] In this study, FPS had similar amylose content (), crystal type (), relative crystallinity () and granule morphological structure (), but their granule size distribution was different (), which might result in the different thermal characteristics in FPS. These parameters are useful in food processing; this is because gelatinization reduces the crystallinity of starch granules while increasing their non-crystalline amorphous content. This causes visible changes in the optical and rheological properties of food systems.[Citation15,Citation18]
Table 4. Thermal characteristics of FPS.
XRD spectroscopy of FPS
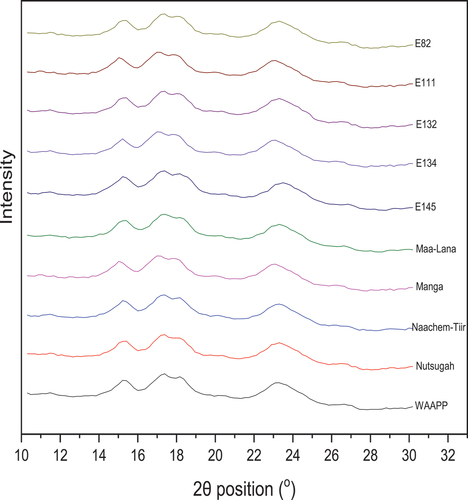
The importance of X-ray diffraction (XRD) in food analyses cannot be overstated. Polymorphism, crystallinity, and amorphism can all be determined using XRD. These parameters aid in controlling the texture and stability of foods under varying processing and storage conditions. XRD has aided in the study of common food ingredients such as starches and fats. XRD enhances FTIR and differential scanning calorimetry (DSC) studies on starch gelatinization in foods. XRD was used to characterize the X-ray diffraction pattern and crystal type of the Frafra potato starches in .
According to the XRD patterns, three types of starch crystallinity are reported, known as A-, B- and C-type.[Citation15,Citation17,Citation21] C-type starch is a mixture of A- and B-type crystallinities and can be further classified to CA-type (closer to A-type), C-type and CB-type (closer to B-type) according to the proportion of A- and B-type allomorphs. A-type allomorphs show strong diffraction peaks at about 15° and 23° and a doublet at around 17° and 18° 2θ. However, B-type allomorphs show strong diffraction peaks at about 5° 2θ.[Citation17,Citation21,Citation22]
The XRD patterns for FPS () showed strong diffraction peaks at about 15° and 23° and a doublet at around 17° and 18° 2θ. The display of strong peaks at these sites is characteristic of A-type allomorphs, implying that the FPS cultivars are A-type starches. The relative crystallinities of starches calculated from the ratio of diffraction peak area and total diffraction area were given in . FPS cultivars had similar (p < .05) relative crystallinity, typical of A-type starches.[Citation21]
FTIR spectroscopy of FPS
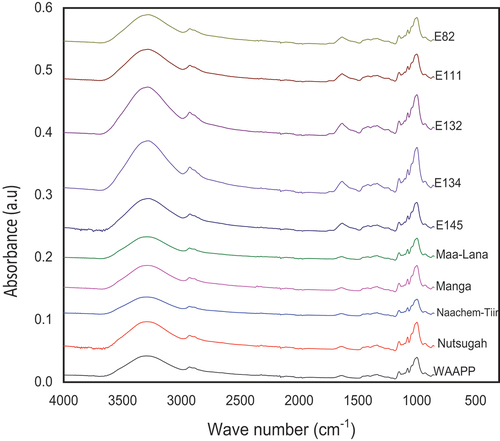
FTIR spectroscopy is helpful in starch modification for detecting changes in molecular and structural conformation.[Citation15,Citation17,Citation22] It may also be used for ascertaining the quality of starch for use in culinary and pharmaceutical purposes.[Citation15,Citation17,Citation22] The structural order of the external regions of FPS was characterized by FTIR spectra in .
All FPS cultivars showed similar ordered structures in the outer granule region (). Even though FTIR is not able to differentiate starch crystal type, native starches with the same crystal type always show a similar FTIR spectrum, the band at 1000/1022 cm-1 is more pronounced in A-type starch than in B-type or C-type starches.[Citation7,Citation15,Citation17,Citation22] This result confirms that FPS has an A-type crystallinity.
Scanning electron microscopy (SEM)
Micrographs of FPS were captured using a scanning electron microscope. This was done to determine the microstructure of FPS. shows the micrographs of FPS. Micrographs of FPS granules () show that all the cultivars had loosely and densely packed small and large granules. FPS cultivars primarily consisted of spherical granules with smooth surfaces. Some oval shape types measuring nearly 14 µm on their central axis and damaged granules (WAAPP) and a few with faceted sides were observed in FPS. Other partly truncated and semi-oval granules were also observed. Generally, the starch granules were within the size range of small and medium.[Citation23,Citation24] Granule diameters ranging between about 12 to 14 µm (mean 12.9 µm) and 11 to 13 µm (mean 12.4 µm) were, respectively, recorded for FPS from the released cultivars and unreleased cultivars. The sizes, shapes, and surface features of FPS were typical of starches from tropical tubers such as sweet potato, cassava, and cocoyam, which are characterized by smooth spherical surfaces, some partly truncated and semi-oval granules, and granule sizes ranging from about 11 µm to 13 µm.[Citation14,Citation17,Citation25]
Principal Components Analysis of FPS characteristics
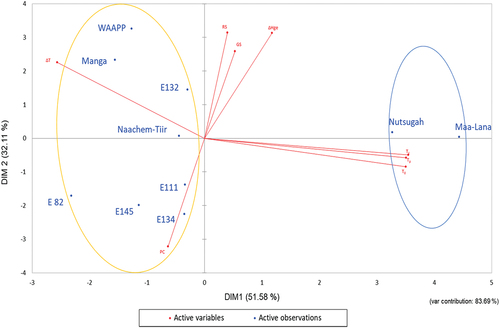
The Principal Component Analysis (PCA) was used to objectively determine which features significantly impacted the differences and similarities amongst the starches from the ten cultivars of Frafra potato. PCA map positioned the 10 FPS samples based on their physicochemical characteristics. Majority of the variance (83.69%) was explained in the first 2 dimensions (DIM1 = 51.58%), DIM2 = 32.11%) as shown in .
PCA clustered starches from the ten cultivars into two main groups, with Nutsugah and Maa-Lana in one group and the others (E82, E111, E132, E134, E145, Manga, Naachem-Tiir, and WAAPP) in the other group (). Nutsugah and Maa-Lana starches were mainly characterized by their onset, peak and concluding temperatures. In contrast, the second group was characterized by their paste clarity and change in temperature. According to Gayin et al.,[Citation15] Ezekiel and Singh,[Citation17] and Ikegwu et al.,[Citation26] these characteristics significantly influence the differences in starches. They are also the critical factors considered in starch modification programs for culinary and pharmaceutical applications.
Conclusion
Frafra potato starch was of the A-type, with similar amylose content and degree of crystallinity. Starch granules from all ten cultivars were mainly spherical or oval-shaped, with smooth surfaces. PCA grouped starches from the ten cultivars into two clusters defined by their thermal properties and paste clarity. These characteristics provide essential information for practical applications of the starches. They may also influence the advanced selection of accessions of interest for developing suitable cultivars with higher starch yield and cooking quality and specific end-use. Starches with excellent clarity, for example, can be utilized as a thickening or coating in culinary and medicinal applications, whilst those with low peak viscosity can be used in the production of weaning meals. Those with high amylose and/or low digestibility are also ideal for food applications targeted at people suffering conditions such as celiac sprue and/or diabetes.
Acknowledgement
The support provided by the Carnegie “Next Generation of Academics in Africa” project (BANGA-Africa Project) at the University of Ghana is duly acknowledged.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Sugri, I.; Kusi, F.; Kanton, R. A. L.; Nutsugah, S. K.; Zakaria, M. Sustaining Frafra Potato (Solenostemon Rotundifolius Poir.) in the Food Chain: Current Opportunities in Ghana. J. Plant Sci. 2013, 1, 68–75.
- Akanlu, S.; Ellis, W. O.; Oduro, I. Frafra Potato – the Neglected Tuber. College of Science Research Conference, Kumasi, Ghana, 2005, 116–126.
- Quainoo, K.; Bayorbor, T. B. The Frafra Potato: A Neglected Crop in Ghana. Agri. Res. Ext. Net. News. 2002, 46, 13.
- Tetteh, J. P.; Guo, J. I. Problems of Frafra Potato Production in Ghana. Ghana J. Agri. Sci. 1997, 30(2), 107–113. DOI: 10.4314/gjas.v30i2.1962.
- Osei Tutu, C.; Amissah, J. G. N.; Amissah, J. N.; Saalia, F. K. Physicochemical and Sensory Characteristics of Bread Made from Wheat-Frafra Potato (Solenostemon rotundifolius) Composite Flour. Sci. Dev. 2019, 3, 20–29.
- Tortoe, C.; Akonor, P. T.; Kusi, F.; Anabire, P. A.; Owusu, R. K.; Boateng, C. Unearthing the Potential of the Frafra Potato (Solenostemon rotundifolius) Flour in Culinary Application: Sensory and Nutritional Analysis of Its Pastry Products. J. Culinary Sci. Technol. 2020, 18(1), 1–12. DOI: 10.1080/15428052.2018.1495588.
- Peroni, F. H. G.; Rocha, T. S.; Franco, C. M. L. Some Structural and Physicochemical Characteristics of Tuber and Root Starches. Food Sci. Technol. Int. 2006, 12(6), 505–513. DOI: 10.1177/1082013206073045.
- Nkansah, G. O. Solenostemon rotundifolius (Poir.) J. K. Morton. In PROTA 2: Vegetables/Légumes. [CD-Rom]; G. J. H. G. Denton O A, Eds.; PROTA: Wageningen, Netherlands, 2004.
- Tortoe, C.; Akonor, P. T.; Koch, K.; Menzel, C.; Adofo, K. Amylose and Amylopectin Molecular Fractions and Chain Length Distribution of Amylopectin in 12 Varieties of Ghanaian Sweet Potato (Ipomoea Batatas) Flours. Int. J. Food Prop. 2017, 20(12), 3225–3233. DOI: 10.1080/10942912.2017.1283326.
- Aprianita, A.; Vasiljevic, T.; Bannikova, A.; Kasapis, S. Physicochemical Properties of Flours and Starches Derived from Traditional Indonesian Tubers and Roots. J. Food Sci. Technol. 2013, 51(12), 3669–3679. DOI: 10.1007/s13197-012-0915-5.
- Chinma, C. E.; Abu, J. O.; James, S.; Iheanacho, M. Chemical, Functional and Pasting Properties of Defatted Starches from Cowpea and Soybean and Application in Stiff Porridge Preparation. Niger. Food J. 2012, 30(2), 80–88. DOI: 10.1016/S0189-7241(15)30039-4.
- Adebowale, K. O.; Olu-Owolabi, B. I.; Olawumi, E. K.; Lawal, O. S. Functional Properties of Native, Physically and Chemically Modified Breadfruit (Artocarpus artilis) Starch. Ind. Crops Prod. 2005, 21(3), 343–351. DOI: 10.1016/j.indcrop.2004.05.002.
- Ratnayake, W. S.; Jackson, D. S. STARCH: Sources and Processing. Encycl. Food Sci. Nutr. 2003, 2, 5567–5572.
- Noda, T.; Kimura, T.; Otani, M.; Ideta, O.; Shimada, T.; Saito, A.; Suda, I. Physicochemical Properties of Amylose-Free Starch from Transgenic Sweet Potato. Carbohydr. Polym. 2002, 49(3), 253–260. DOI: 10.1016/S0144-8617(01)00343-5.
- Gayin, J.; Bertoft, E.; Manful, J.; Yada, R. Y.; Abdel-Aal, E. S. M. Molecular and Thermal Characterisation of Starches Isolated from African Rice (Oryza Glaberrima). Starch. staerke. 2016, 68(1–2), 9–19. DOI: 10.1002/star.201500145.
- Stevenson, D. G.; Jane, J.-L.; Inglett, G. E. Structures and Physicochemical Properties of Starch from Immature Seeds of Soybean Varieties (Glycine Max (L.) Merr.) Exhibiting Normal, Low-Linolenic or Low-Saturated Fatty Acid Oil Profiles at Maturity. Carbohydr. Polym. 2007, 70(2), 149–159. DOI: 10.1016/j.carbpol.2007.03.016.
- Kowsik, P. V.; Mazumder, N. Structural and Chemical Characterisation of Rice and Potato Starch Granules Using Microscopy and Spectroscopy. Microsc. Res. Tech. 2018, 81(12), 1533–1540. DOI: 10.1002/jemt.23160.
- Ezekiel, R.; Singh, N. Use of Potato Flour in Bread and Flat Bread. Flour Breads. Their. Fortifi. Health Dis. Prev. 2011, 5, 247–259.
- Tattiyakul, J.; Pradipasena, P.; Asavasaksakul, S. Taro Colocasia Esculenta (L.) Schott Amylopectin Structure and Its Effect on Starch Functional Properties. Starch. Stärke. 2007, 59(7), 342–347. DOI: 10.1002/star.200700620.
- Ugwu, F. M. The Potentials of Roots and Tubers as Weaning Foods. Pak. J. Nutr. 2009, 8(10), 1701–1705. DOI: 10.3923/pjn.2009.1701.1705.
- Huang, J.; Zhao, L.; Huai, H.; Li, E.; Zhang, F.; Wei, C. Structural and Functional Properties of Starches from Wild Trapa Quadrispinosa, Japonica, Mammillifera and Incisa. Food. Hydrocoll. 2015, 48, 117–126. DOI: 10.1016/j.foodhyd.2015.02.003.
- Zhu, F.; Yang, X.; Cai, Y. Z.; Bertoft, E.; Corke, H. Physicochemical Properties of Sweet Potato Starch. Starch. Stärke. 2011, 63(5), 249–259. DOI: 10.1002/star.201000134.
- Seetharaman, S.; Waduge, K.; Xu, R. Iodine Absorption Properties and Its Effect on the Crystallinity of Developing Wheat Starch Granules. Carbohydr. Polym. 2010, 82(3), 786–794. DOI: 10.1016/j.carbpol.2010.05.053.
- Lindeboom, N.; Chang, P. R.; Tyler, R. T. Analytical, Biochemical and Physicochemical Aspects of Starch Granule Size, with Emphasis on Small Granule Starches: A Review. Starch. staerke. 2004, 56(3–4), 89–99. DOI: 10.1002/star.200300218.
- Marquezi, M.; Gervin, V. M.; Watanabe, L. B.; Bassinello, P. Z.; Amante, E. R. Physical and Chemical Properties of Starch and Flour from Different Common Bean (Phaseolus Vulgaris L.) Cultivars. Braz. J. Food Technol. 2016, 19, 67–78. DOI: 10.1590/1981-6723.0516.
- Ikegwu, O. J.; Okechukwu, P. E.; Ekumankana, E. O. Physico-Chemical and Pasting Characteristics of Flour and Starch from Achi Brachystegia Eurycoma Seed. J. Food Technol. 2010, 8(2), 58–66. DOI: 10.3923/jftech.2010.58.66.