?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
The bioactive constituents of Nettle (Urtica dioica L.) plants were extracted by green and environmentally friendly methods using several deep eutectic solvents (DESs) followed by liquid chromatography-mass spectrometry separation and identification. To optimize the extraction process, bio-solvents composed of organic acids such as hydrogen bond donors (HBD) and mannitol/menthol/sugars as HBA were selected and coupled with ultrasound-assisted extraction. The maximum bioactive compound contents were TPC 2423 mg GAE/100 g, TFC 134.71 mg CE/100 g, and DPPH 1071.05 mol TE/mL. The optimum extraction parameters to achieve the highest values of phenolic compounds from Nettle (Urtica dioica L.) via UAE-DES were obtained at an extraction temperature 70°C, extraction time of 30 min, and water addition on DES 20%. The ideal experimental conditions were confirmed using the Central Composite Design (CCD) of the Response Surface Methodology (RSM) model to analyze the significance of extraction parameters demonstrating high prediction levels of the bioactive compounds. Liquid chromatography coupled with diode array detection and electrospray ionization tandem mass spectrometry (LC-DAD-ESI-MS/MS) was used to identify the extracts. The results showed that quercetin-3-O-rutinoside, 5-ocaffeoylquinic acid, and caffeoyl malic acid were the three compounds with the highest abundance. For the first time, the extraction of bioactive compounds from nettle using a food-grade DES that contains GRAS components is reported. According to these findings, the best solvent for extracting bioactive substances for food-grade applications is DESCAMAL (citric acid/maltose).
Introduction
The constituents extracted from roots and above-ground plant parts of medicinal plants contain secondary metabolites, also known as bioactive compounds or phytochemicals encompass a large variety of natural compounds, including phenols, phenolic acids, flavonoids, flavonolignans, saponins, alkaloids, tannins, glycosides, lignins, steroids, phenylpropanoid glycerols, and isoprene-derived terpenoids (also known as isoprenoids) such as monoterpenes, sesquiterpenes, diterpenes and triterpenes.[Citation1]
Phenolic compounds (including flavonoids and tannins) play numerous molecular and physiological roles in plants, such as free radical scavenging, signaling, mediating auxin transport, and plant defense. These compounds likewise contribute significantly to health promotion and disease prevention as well as therapeutic applications in human especially due to antioxidant, antimicrobial, anticarcinogenic, and anti-inflammatory properties.[Citation1,Citation2]
The most common spontaneous perennial herb in the world is nettle (Urtica dioica L.), which is from the Urticaceae family and the genus Urtica.[Citation3] This plant has been shown to have a variety of chemical components, including polyphenols, flavonoids, organic acids, tannins, lignans, alkaloids, chlorophylls, volatile compounds, fatty acids, essential amino acids, polysaccharides, proteins, vitamins, terpenes, sterols, and minerals.[Citation4] The leaves of the Urtica dioica L. plant are rich in carotenoids such as lutein, lutein isomers, and β-carotene. Also, some minerals such as iron, calcium, magnesium, manganese, zinc, potassium, phosphorus, and selenium are present.[Citation5] As phytopharmaceuticals and nutraceuticals, nettle (Urtica dioica L.) extracts are widely used in the different sectors like food industries, pharmaceutical, cosmetic ect. It is used as a food additive as a green color (chlorophyll E140), in cosmetics to make shampoos and lotions to prevent hair loss and dandruff, in the kitchen to make a variety of dishes, and as a natural flavor for food.[Citation6–9] Nettle extracts’ antioxidant and anti-aging capabilities are one of the most intriguing effects they have on cosmetic applications.[Citation10] Nettles accumulate a number of scavengers of reactive oxygen species, which can reduce skin damage from free radicals and therefore have anti-aging effects.[Citation5] The antiaging efficacy may also occur from the suppression of deteriorating enzymes like elastase or collagenase, whose actions cause a loss of skin elasticity and wrinkles.[Citation10,Citation11] Health benefits of Nettle (Urtica dioica L.) including protective properties against hepatic ischemia-reperfusion, arthritic pain, hypoglycemic properties, anti-proliferative, immunological stimulant, hypotensive, anti-ulcer, anti-bacterial, anti-infectious, and prevention of cardiovascular diseases like hypertension.[Citation12–14] On the other hand, the chemical compounds in nettle (Urtica dioica L.) have a number of health benefits for women such as the relief of unpleasant symptoms before and during menstruation, reducing the intensity of hormonal changes in the body in menopausal women, as a coagulant can help prevent excessive bleeding.[Citation4,Citation15] The selection of methods and solvents is crucial for maximizing the extraction of phytochemicals from plants.[Citation16]
Green solvents have attracted a lot of scientific attention as a potential replacement for traditional organic solvents for extracting bioactive chemicals from natural resources due to crucial qualities like chemical soundness, thermal stability, non-flammability, non-toxicity, biodegradability, low toxicity, low volatility, and ease of preparation, as well as due to high environmental concerns.[Citation17–21] There are many conventional and modern techniques used to extract phytochemicals from plants. These compounds are held tightly within plant cells and are often difficult to extract in large quantities.[Citation22, Citation23] It is crucial to investigate new extraction techniques since conventional extraction methods have a number of drawbacks, including a high solvent need, a time and energy requirement, and environmental risks.[Citation24,Citation25] Efficiency, safety, and environmental friendliness are desired characteristics in an extraction technique.[Citation16] Ultrasound-assisted extraction (UAE), which releases bioactive components from plant material and natural products by using ultrasound waves, is one of the contemporary techniques with these qualities.[Citation26] Ultrasonic waves give the required force to liberate natural compounds from plant biomass according to green chemistry principles, making UAE a productive and environmentally friendly process.[Citation13,Citation16] Also, extraction sonification is suitable for improved nutrient preservation.[Citation27] For this purpose, UAE-DES has been used in recent years due to its lower energy consumption and the use of a small amount of solvent, resulting in higher extraction yields.[Citation28] While the response surface methodology (RSM) was used as a statistical tool for the optimization of the extraction conditions. To the best of our knowledge, there is no research report on the optimization of UAE-DES for the extraction of phenolic compounds and the identification of major phenolics from this plant.
Materials and methods
Nettle (Urtica dioica L.) extract preparation
Nettle (Urtica dioica L.) was obtained from spontaneous flora in May 2022. Samples are selected without any mechanical damage. The phenolic components in the nettle were extracted using the ultrasound-assisted extraction (UAE) technique. An ultrasonic bath at a temperature of 30–90°C for 10–60 min was used to extract about 1.0 g of plant material using the suitable solvent (10–30 mL).
Preparation of DES as bio solvent
Prior to usage, the HBA, which was citric acid, was kept in an electric drying oven at 40°C for 1 hour. Citric acid and lactic acid were mixed with one or more HBD (mannitol, menthol, glucose, and maltose) at a particular molar proportion. The mixture was kept in a water bath at 80°C and stirred continuously for 30–40 minutes to form a homogeneous, transparent, colorless solution that showed no signs of crystallization after 12 hours. In , the names of DES and their molar ratios are presented.
Table 1. Name, compositions of DES and mole ratios.
Bioactive compound using UAE-DES
Total phenolic content (TPC)
The method by Singleton et al.[Citation29] with some changes was utilized to determine total phenolic content. About 200 µL of the extract of Nettle was placed in a test tube, then 600 µL of ten times diluted Folin-Ciocalteu solution was added after 6 minutes 450 µL of 7.5% sodium carbonate solution was added and after 60 minutes the absorbance was measured at 756 nm using UV-VIS spectrophotometry GENESYSTM10S. TPC concentration was measured in mg GAE/g.
Total flavonoid content (TFC)
Koraqi et al.[Citation30] previously employed a modified method to determine the concentration of total flavonoid content. Briefly, 25 µL of 5% sodium nitrite was added to the 500 µL of extract of nettle (Urtica dioica L.) prepared with DESs, which was then mixed. Five minutes later, 25 µL of 10% aluminum chloride was added, and five more minutes later, 100 µL of sodium hydroxide was added. Wavelength 510 was used to measure the absorbances of the samples using GENESYSTM10S UV-VIS spectrophotometer. mg CE/g was the unit of measurement for TFC.
Antioxidant activity (AA)
The DPPH free radical scavenging assay, a method previously described by Xiao et al.[Citation31] was used to assess antioxidant activity. In a test tube, 2,000 µl of DPPH (2,2-diphenyl-1-picryl-hydrazyl-hydrate) solution and 700 µLof Tris-HCl buffer (pH 7.4) were added. 200 µL of the testing sample solution was added after that, and it was quickly mixed. The solution was kept at room temperature for thirty minutes. Using a spectrophotometer (GENESYSTM 10S), the solution’s absorbance was measured at 517 nm. The blank was a mixture of 900 µl of Tris-HCl buffer (pH 7.4) and 100 µL of ethanol.
Extraction method optimization
The solvent type and concentration, extraction time, and temperature are just a few of the variables that might determine how effective an extraction is.[Citation32] To explore the interaction between different factors, it is crucial to utilize an appropriate experimental design technique.[Citation33] Three independent variables were chosen for the central composite design (CCD) based on response surface methodology (RSM): extraction temperature (A), extraction time (B), and water addition on DES (C). The extraction conditions of the UAE-DES method were optimized using the three-factor and three-level response surface analysis approach; the independent variables and coded factors are provided in . To improve the extraction technique, 20 runs of experiments with 6 centers and 14 non-centers per block were carried out, and the results were fitted to a second-order polynomial. To generate a quadratic polynomial mathematical model to explain the effects of the interaction between the process parameters on the extraction of the phenolic compounds, the ANOVA was performed using the Design-Expert (Version 13.0) program. The model’s capability was predicted using the determination’s value (R2) and the model P value.
Table 2. The independent variables and coded factors in the CCD of the response surface.
Liquid chromatography coupled to diode array detection and electrospray ionization tandem mass spectrometry (LC-DAD-ESI-MS/MS) analysis
For the analysis, an Agilent 1100 HPLC system was used. For elution of the components, Agilent Zorbax Eclipse Plus C18 column (150 mm × 4.6 mm, 5 µm) and two mobile phases A (1% (V/V) HCOOH in water) and B (1% HCOOH (V/V) in methanol) were used. The elution started in the first 10 min isocratic with 10% B, and linear increase to 80% B to 20 min, and then to 100% B from 20 to 25 min. The last 5 min isocratic elution with 100% B was used. The injection volume, the flow rate and temperature of the column were adjusted at 10 µL, 0.5 mL/min and 40°C, respectively. The chromatograms were recorded at 280, 330 and 350 nm and the spectra were collected in the UV/Vis range from 190 to 600 nm. For the MS detection, an ion trap mass detector with electrospray ionization in negative mode was used, with nitrogen as nebulizing gas at a pressure of 50 psi and a flow rate of 12 L/min. Spectra were collected in the m/z range from 15 to 800. The LC-DAD-ESI-MS/MS conditions were the same as that described by Petreska Stanoeva et al.[Citation34]
Results and discussion
DESs system selection for extraction bioactive compounds from nettles (Urtica dioica L.)
Effect of different types of DESs
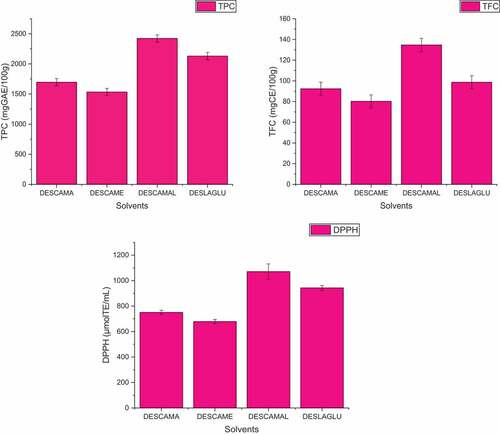
Due to the use of different types of HBD and HBA, DESs vary in terms of polarity, solubility, viscosity, surface tension and physicochemical interactions.[Citation35] Considering that the extraction efficiency is determined by the composition of the DES, it is important to identify the most efficient DES for the extraction of plant constituents. In this study, the design of DES was organic acids such as citric acid and lactic acid were used as HBA, and mannitol, menthol and sugars (maltose, glucose) were used as HBD and their extraction efficiencies were compared. As shown in , among the four DES, the content of bioactive compounds extracted using DESCAMAL was the highest, followed by DESCAMA, DESCAME and DESLAGLU. DESs as a class of “green solvents” with special advantages in physicochemical properties, such as density, surface tension, viscosity, vapor pressure, low toxicity, non-flammability, etc. have been suggested for the extraction of phenolic compounds as an alternative to conventional organic solvents, offering increased extraction efficiency and extract quality.[Citation7,Citation19] In our study, the combination of innovative extraction techniques (UAE) with DES is proposed for the extraction of phenolic compounds from Nettle (Urtica dioica L.). By applying UAE and a DES composed of citric acid and maltose (DESCAMAL molar ratio of 1:2), the bioactive compounds (TPC and TFC) and antioxidant activity of extracts from nettle (Urtica dioica L.) were higher than those of obtained from conventional solvents (ethanol, methanol and water) and conventional extraction methods in previous publications.[Citation5,Citation9,Citation10,Citation36–39]
Optimization of bioactive compounds extraction using CCD
Statistical analysis and modeling of extraction of total phenolic content (TPC)
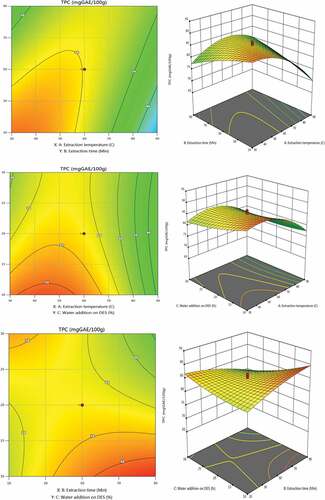
Nettle (Urtica dioica L.). extracts obtained using UAE are shown in by their total phenolic content. The experimental results were subjected to regression analysis, and the model coefficients’ relevance in the extraction of phenolic compounds was assessed. Indicating a good correlation between the model and the experimental data for the selected parameters, this method’s coefficient of determination (R2) was 0.9510 (). The relationship between extraction temperature, extraction time, and water addition on DESs for the extraction of plant constituents is shown in the following equation:
Table 3. Effect of DES type on TPC, TFC and DPPH of extracts from Nettle (Urtica dioica L.).
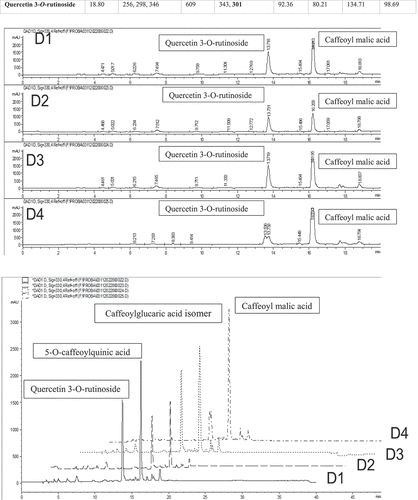
Table 4. Structural characterization (tR, UV max, MS data) and content (mg/100 g) of phenolic compounds in different deep eutectic solvents in Urtica dioica L. (Nettle).
The parameters in the quadratic model significantly impacted the extraction of phenolic compounds (p ˂ 0.0001), as shown by the coefficients of the aforementioned equation and the p-values. There were both positive and negative effects of the extraction conditions in the quadratic and interaction terms. Water addition on DESs is the factor that has the second-highest positive impact on TPC after the extraction temperature.
shows that the amount of TPC starts to decrease as the extraction time, which was a negative quadratic term, increases, while extraction temperature and water addition on DESs showed positive quadratic effects on the extraction of TPC. Multiple regression analysis is consistent with the 2D contour plots and 3D response surface plots analyses in .TPC content in relation to extraction time is shown in ; a higher content of phenolic compounds was obtained at the extraction time of 30 min. Our results demonstrate that extra time is unnecessary to extract more phenolic compounds. The longer the extraction time, the more likely phenolic compounds will oxidize.[Citation40]
Extraction temperature has a favorable effect and is a more important quadratic parameter than others. The maximum TPC levels, according to this research, were attained at an optimum at extraction temperature 70°C. Using the following experimental conditions: 70°C extraction temperature,30 min of extraction time, and 20% water addition on DESs the maximum TPC value (TPC = 2423 mg GAE/100 g) was attained.
Water added to DESs has a positive effect on the extraction process, causing a decrease in viscosity and an increase in solvent polarity.[Citation35,Citation41] The greatest polyphenol extraction yield and solvent extraction have a correlation, but the environmental and health dangers caused by the food, drug, and cosmetic industries also need to be taken into account. It is helpful to select an alternative and stable extraction solvent to reduce such dangers. In addition to being more environmentally friendly than other organic solvents, DESs also have the benefit of just needing a small amount for the UAE.[Citation26,Citation42,Citation43] In our experiment, we used both DESs and UAE, which was consistent with green extraction. According to our knowledge, until today there are no data on the green extraction of bioactive compounds such as TPC from Nettle (Urtica dioica L.). in the literature. Furthermore, according to other studies, phenolic profiles differ according to the plant’s variety, cultivation, extraction solvent, and extraction circumstances.[Citation44,Citation45]
Our study’s maximum TPC levels were higher than previously reported by Shonte et al.[Citation46] whose results of TPC ranged from TPC 118.4 mg GAE/g as solvent mixture 80% methanol-water and lyophilization method. Moreira et al.[Citation47] reported in ethanolic extract TPC 4.07 mgGAE/g by using high hydrostatic pressure extraction; Elez Garofulic et al.[Citation13] reported TPC values of 1044.18 mg GAE/100 g in water extract, 1724.91 mgGAE/100 g in 30% ethanolic extract, and 2012.55 mgGAE/100 g in 30% acetone using Microwave Assisted Extraction. Moreover, Jeszka-Skowran et al.[Citation21] reported a TPC value of water extract of 3.1 mg GAE 100 mL−1 by conventional extraction.
Statistical analysis and modeling of extraction of total flavonoid content (TFC)
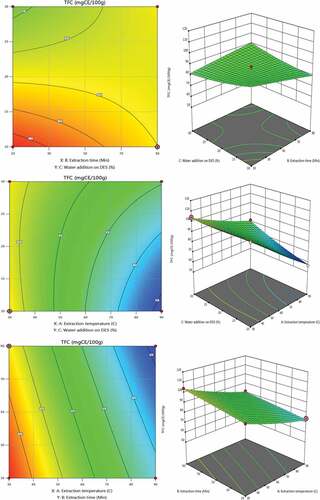
shows the total flavonoid content (TFC) of nettle (Urtica dioica L.) extracts acquired via the UAE. On the experimental data, regression analysis was conducted, and the model coefficients were assessed for significance in the extraction of total flavonoid content. The coefficient of determination (R2) for this approach was 0.9904, demonstrating a sufficient correlation between the model predictions and the experimental findings for the selected parameters (). The relationship between extraction temperature, extraction time, and water addition on DESs for flavonoid content extraction can be represented by the equation below:
The extracting of total flavonoid content was significantly affected by quadratic model parameters (p˂0.0001), as shown by the coefficients of the aforementioned equation and the p-values. Extraction temperature has the most positive effect on TFC, followed by extraction time. Quadratic and interaction terms’ extraction conditions exhibited both positive and negative effects. The negative quadratic term was the water addition on DESs. By increasing the addition of water to DESs, the amount of flavonoid extraction decreased. Multiple regression analysis is consistent with the 2D contour plots and 3D response surface plots analyses in . TFC content increases with water content, as shown in .
The positive linear effect, extraction temperature is a more significant quadratic variable than others. This research found that TFC levels peaked in temperature 80°C after 60 minutes of extraction and that 20% (v/v) of DESs’ water addition. The following experimental parameters were used: extraction time 80°C, 60 min of extraction time, and 20% water addition on DESs to get the greatest TFC value (TFC = 134.71 ± 0.23 mg CE/100 g).
Nettle (Urtica dioica L.) green extract’s total flavonoid content has not yet been documented in any literature. This fact makes the comparison difficult due to differences in parts of the studied material, extraction methods and conditions, and the solvent used for extraction. Furthermore, some other studies show that the flavonoid content of Nettle (Urtica dioica L.) extracts varies depending on, variety, cultivation, extraction solvent, and conditions of extraction.
Šic Žlabur et al.[Citation48] reported in ethanolic extract TFC value ranges from 148.07 to 186.66 mg CTH/100 g by using solid-liquid extraction, and 26.19 to 365.32 mg CTH/100 g by ultrasonic-assisted extraction; as well as Güder et al.[Citation49] who reported the TFC values of 365.8 mgQE/g dw in aqueous ethanolic extract using Soxhlett extraction.
Statistical analysis and modeling of antioxidant activity (AA) by DPPH free radical scavenging assay
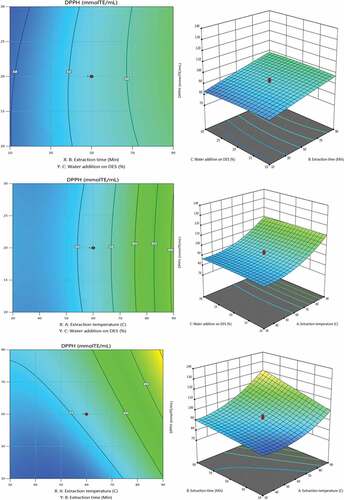
The experimental data for (AA) of Nettle extracts are presented in . A satisfactory relationship between predicted and experimental data was demonstrated by R2 values of 0.9874 DPPH responses. According to equation models, AA is significantly affected by the quadratic effects of all parameters (p˂ 0.0001 and p˂0.05), including the quadratic effects of extraction temperature, extraction time, and water addition at DESs.
Jeszka-Skowran et al.[Citation21] reported an AA by DPPH method value of water extract 2.5 mg TE/100 mL by conventional extraction. The highest results for the antioxidant activity of nettle extracts with DPPH scavenging (1071.05 μmolTE/mL) were obtained under the conditions when the extraction time was 60 min, the extraction temperature was 90°C and the addition of water to DESs 30% ().
The results further demonstrate that stronger AA was not associated with higher TPC of Nettle (Urtica dioica L.) extracts. Of the many biological activities that Nettle (Urtica dioica L.) extracts have, it cannot be denied that the antioxidant activity is the most studied. Our findings closely resemble those of previous studies,[Citation49–51] suggesting that these potentials may be related to their polyphenolic profile.
LC-DAD-ESI-MS/MS analysis
Chromatogram and structural characterization (tR, UV max, MS data) and content (mg/100 g) of phenolic compounds in different deep eutectic solvents in Nettle (Urtica dioica L.) identified by LC-DAD-ESI-MS/MS are presented in and . The main identified phenolic compounds were caffeoyl malic acid (1557.55 mg100/g), and 5-O-caffeoylquinic acid (426.55 mg/100 g, whereas Quercetin 3-O-rutinoside (134.71 mg/100 g) was the dominated flavonoid. Repajic et al.[Citation52] studied the phenolic profile of Nettle (Urtica dioica L.) in ethanolic extract. They identified cinnamic acids, flavonols, flavones, flavan-3-ols, and benzoic acids. Koczkodaj et al.[Citation24] also showed that four phenolic acids were detected caffeoyl malic, chlorogenic, neo-chlorogenic and cichoric acids were the main phenolic acids in Nettle (Urtica dioica L.) methanolic extract whereas the major flavonoids were rutoside, and hyperoside. Jeszka-Skowron et al.[Citation21] studied the phenolic profile of Nettle (Urtica dioica L.) in infusions (water extract) by LC-MS/MS and reported identified quinic, protocatechuic, and 3-caffeoylquinic acids. The most abundant of flavonols analyzed was rutin. This variation in the phenolic content of the extracts may be due to plant species, the section of the plant being extracted, and the solvent type. There have been no studies on the application of DES to extract the phenolic content and flavonoids from Nettle (Urtica dioica L.).
Conclusion
In this present study, bioactive compounds were extracted from Nettle (Urtica dioica L.) using UAE-DES. The central composite design (CCD) by response surface methodology (RSM) was used as a statistical tool for the optimization of the extraction conditions. According to the results, the optimum extraction conditions for UAE-DES were extraction temperature of 70°C, extraction time of 60 min, and water addition on DES was 20%. This is the first investigation of bioactive compounds extraction from Nettle (Urtica dioica L.) using a deep eutectic solvent (DES). Regarding the effectiveness of the solvent, DESCAMAL was more effective in extracting bioactive compounds from Nettle. LC-DAD-ESI-MS/MS analysis indicated that the extract obtained at optimal conditions was rich in caffeoyl malic acid, quercetin 3-O-rutinoside and 5-O-caffeoylquinic acid. According to these results, nettle extracts have the ability to scavenge DPPH radicals, making them a potential natural antioxidant source. These findings suggest that the UAE-DES system works well as a green substitute for organic solvents in the extraction of sustainable bioactive substances. This technology adheres to green analytical chemistry principles and is quick and easy to use. The potential for UAE-DES extraction in the food industry is encouraging since it could improve its sustainability by employing processing by-products and substituting renewable GRAS components for organic solvents. Furthermore, innovative functional food additives derived from DES extracts using GRAS components may be developed.
Author contributions
H.K performed the methods and investigation. B.Q and W.K conceptualization, funding acquisition and writing of original draft. J.P.S and F.S helped in writing of this manuscript. While C.C, A.S and K.A.K helped in software, M.A.R, I.H and E.Z supported in analysis and supervision of research work.
Acknowledgments
The authors are highly obliged to the UBT-Higher Education Institution, Ss. Cyril and Methodius University in Skopje, Library Department, University of Poonch Rawalakot, Azad Kashmir, University of Lahore, Government College University Faisalabad, Pakistan and Higher Education Commission (HEC, Islamabad) for access to journals, books and valuable database. The authors extend their appreciation to the Deanship of Scientific Research at King Khalid University Saudi Arabia for funding this work through Large Groups Project under grant number RGP2/495/44.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The data it is availability within this study.
References
- Soleimani, M.; Arzani, A.; Arzani, V.; Roberts, T. H. Phenolic Compounds and Antimicrobial Properties of Mint and Thyme. J. Herb. Med. 2022, 36, 100604. DOI: 10.1016/j.hermed.2022.100604.
- Potter, C. M.; Jones, D. L. Polyphenolic Profiling of Green Waste Determined by UPLC-HDMSE. Processes. 2021, 9(5), 824. DOI: 10.3390/pr9050824.
- Adhikari, B. M.; Bajracharya, A.; Shrestha, A. K. Comparison of Nutritional Properties of Stinging Nettle (Urtica dioica) Flour with Wheat and Barley Flours. Food Sci. Nutr. 2015, 4(1), 119–124. DOI: 10.1002/fsn3.259.
- Bhusal, K. K.; Magar, S. K.; Thapa, R.; Lamsal, A.; Bhandari, S.; Maharjan, R.; Shrestha, S.; Shrestha, J. Nutritional and Pharmacological Importance of Stinging Nettle (Urtica Dioica L.): A Review. Heliyon. 2022 Jun 22; 8(6), e09717. DOI: 10.1016/j.heliyon.2022.e09717.
- Bourgeois, C.; Leclerc, É. A.; Corbin, C.; Doussot, J.; Serrano, V.; Vanier, J.; Seigneuret, J.; Auguin, D.; Pichon, C.; Lainé, É., et al. Nettle (Urtica Dioica L.) as a Source of Antioxidant and Anti-Aging Phytochemicals for Cosmetic Applications. C. R. Chim. 2016, 19(9), 1090–1100. DOI: 10.1016/j.crci.2016.03.019.
- Carreira-Casais, A.; Otero, P.; Garcia-Perez, P.; Garcia-Oliveira, P.; Pereira, A. G.; Carpena, M.; Soria-Lopez, A.; Simal-Gandara, J.; Prieto, M. A. Benefits and Drawbacks of Ultrasound-Assisted Extraction for the Recovery of Bioactive Compounds from Marine Algae. Int. J. Environ. Res. Public Health. 2021, 18(17), 9153. DOI: 10.3390/ijerph18179153.
- Chanioti, S.; Katsouli, M.; Tzia, C. Novel Processes for the Extraction of Phenolic Compounds from Olive Pomace and Their Protection by Encapsulation. Molecules. 2021, 26(6), 1781. DOI: 10.3390/molecules26061781.
- Chaves, J. O.; de Souza, M. C.; da Silva, L. C.; Lachos-Perez, D.; Torres-Mayanga, P. C.; Machado, A. P. F.; Forster-Carneiro, T.; Vázquez-Espinosa, M.; González-de-Peredo, A. V.; Barbero, G. F., et al. Extraction of Flavonoids from Natural Sources Using Modern Techniques. Front. Chem. 2020, 8, 507887. DOI: 10.3389/fchem.2020.507887.
- Chemat, F.; Abert Vian, M.; Fabiano-Tixier, A. S.; Nutrizio, M.; Režek Jambrak, A.; Munekata, P. E. S.; Lorenzo, J. M.; Barba, F. J.; Binello, A.; Cravotto, G. A Review of Sustainable and Intensified Techniques for Extraction of Food and Natural Products. Green Chem. 2020, 22(8), 2325–2353. DOI: 10.1039/c9gc03878g.
- De Vico, G.; Guida, V.; Carella, F. Urtica Dioica (Stinging Nettle): A Neglected Plant with Emerging Growth Promoter/Immunostimulant Properties for Farmed Fish. Front. Physiol. 2018, 9, 285. DOI: 10.3389/fphys.2018.00285.
- Devkota, H. P.; Paudel, K. R.; Khanal, S.; Baral, A.; Panth, N.; Adhikari-Devkota, A.; Jha, N. K.; Das, N.; Singh, S. K.; Chellappan, D. K., et al. Stinging Nettle (Urtica Dioica L.): Nutritional Composition, Bioactive Compounds, and Food Functional Properties. Molecules. 2022, 27, 5219. DOI: 10.3390/molecules27165219.
- Dhouibi, R.; Moalla, D.; Ksouda, K.; Ben Salem, M.; Hammami, S.; Sahnoun, Z.; Zeghal, M. K.; Affes, H. Screening of Analgesic Activity of Tunisian Urtica Dioica and Analysis of Its Major Bioactive Compounds by GCMS. Arch. Physiol. Biochem. 2017, 124(4), 335–343. DOI: 10.1080/13813455.2017.1402352.
- Elez Garofulić, I.; Malin, V.; Repajić, M.; Zorić, Z.; Pedisić, S.; Sterniša, M.; Smole Možina, S.; Dragović-Uzelac, V. Phenolic Profile, Antioxidant Capacity and Antimicrobial Activity of Nettle Leaves Extracts Obtained by Advanced Extraction Techniques. Molecules. 2021, 26(20), 6153. DOI: 10.3390/molecules26206153.
- Esposito, S.; Bianco, A.; Russo, R.; Di Maro, A.; Isernia, C.; Pedone, P. V. Therapeutic Perspectives of Molecules from Urtica Dioica Extracts for Cancer Treatment. Molecules. 2019, 24(15), 2753. DOI: 10.3390/molecules24152753.
- Flórez, M.; Cazón, P.; Vázquez, M. Antioxidant Extracts of Nettle (Urtica dioica) Leaves: Evaluation of Extraction Techniques and Solvents. Molecules. 2022, 27(18), 6015. DOI: 10.3390/molecules27186015.
- Garcìa, L. M.; Ceccanti, C.; Negro, C.; De Bellis, L.; Incrocci, L.; Pardossi, A.; Guidi, L. Effect of Drying Methods on Phenolic Compounds and Antioxidant Activity of Urtica Dioica L. Leaves. Horticulturae. 2021, 7(1), 10. DOI: 10.3390/horticulturae7010010.
- Dheyab, A. S.; Abu Bakar, M. F.; Al-Omar, M.; Sabran, S. F.; Muhamad Hanafi, A. F.; Mohamad, A. Deep Eutectic Solvents (DESs) as Green Extraction Media of Beneficial Bioactive Phytochemicals. Separations. 2021, 8(10), 176. DOI: 10.3390/separations8100176.
- Grauso, L.; de Falco, B.; Lanzotti, V.; Motti, R. Stinging Nettle, Urtica Dioica L.: Botanical, Phytochemical and Pharmacological Overview. Phytochem. Rev. 2020, 19, 1341–1377. DOI: 10.1007/s11101-020-09680-x.
- Halder, A. K.; Haghbakhsh, R.; Voroshylova, I. V.; Duarte, A. R. C.; Cordeiro, M. N. D. S. Predicting the Surface Tension of Deep Eutectic Solvents: A Step Forward in the Use of Greener Solvents. Molecules. 2022, 27(15), 4896. DOI: 10.3390/molecules27154896.
- Jan, K. N.; Zarafshan, K.; Singh, S. Stinging Nettle (Urtica Dioica L.): A Reservoir of Nutrition and Bioactive Components with Great Functional Potential. Food Meas. 2017, 11(2), 423–433. DOI: 10.1007/s11694-016-9410-4.
- Jeszka-Skowron, M.; Zgoła-Grześkowiak, A.; Frankowski, R.; Grześkowiak, T.; Jeszka, A. M. Variation in the Content of Bioactive Compounds in Infusions Prepared from Different Parts of Wild Polish Stinging Nettle (Urtica Dioica L.). Molecules. 2022, 27(13), 4242. DOI: 10.3390/molecules27134242.
- Jiskani, A. H.; Aydar, A. Y.; Ahmed, D. Optimization of Ultrasound-Assisted Extraction of Antioxidant Compounds from Rumex Hastatus with Response Surface Methodology. J. Food Process. Preserv. 2021, 45(11), e15983. DOI: 10.1111/jfpp.15983.
- Kainat, S.; Arshad, S. M.; Khalid, W.; Khalid, Z. M.; Koraqi, H.; Afzal, F. M.; Noreen, S.; Aziz, Z.; Al-Farga, A. Sustainable Novel Extraction of Bioactive Compounds from Fruits and Vegetables Waste for Functional Foods: A Review. Int. J. Food Prop. 2022, 25(1), 2457–2476. DOI: 10.1080/10942912.2022.2144884.
- Koczkodaj, S.; Przybył, J. L.; Kosakowska, O.; Węglarz, Z.; Bączek, K. B. Intraspecific Variability of Stinging Nettle (Urtica Dioica L.). Molecules. 2023, 28(3), 1505. DOI: 10.3390/molecules28031505.
- Koina, I. M.; Sarigiannis, Y.; Hapeshi, E. Green Extraction Techniques for the Determination of Active Ingredients in Tea: Current State, Challenges, and Future Perspectives. Separations. 2023, 10(2), 121. DOI: 10.3390/separations10020121.
- Koraqi, H.; Qazimi, B.; Çesko, C.; Trajkovska Petkoska, A. Environmentally Friendly Extraction of Bioactive Compounds from Rosa Canina L. Fruits Using Deep Eutectic Solvent (DES) as Green Extraction Media. Acta Chim. Slov 2022, 69(3), 665–673. DOI: 10.17344/acsi.2022.7559.
- Aziz, A.; Noreen, S.; Khalid, W.; Mubarik, F.; Niazi, M. K.; Koraqi, H.; Ali, A.; Lima, C. M. G.; Alansari, W. S.; Eskandrani, A. A., et al. Extraction of Bioactive Compounds from Different Vegetable Sprouts and Their Potential Role in the Formulation of Functional Foods Against Various Disorders: A Literature-Based Review. Molecules. 2022, 27(21), 7320. DOI: 10.3390/molecules27217320.
- Ayad, R.; Ayad, R.; Bourekoua, H.; Lefahal, M.; Makhloufi, E. H.; Akkal, S.; Medjroubi, K.; Nieto, G. Process Optimization of Phytoantioxidant and Photoprotective Compounds from Carob Pods (Ceratonia Siliqua L.) Using Ultrasonic Assisted Extraction Method. Molecules. 2022, 27(24), 8802. DOI: 10.3390/molecules27248802.
- Singleton, V. L.; Orthofer, R.; Lamuela-Raventos, R. M. Analysis of Total Phenols and Other Oxidation Substrates and Antioxidants by Means of Folin-Ciocalteu Reagent. Methods Enzymol. 1999, 299, 152–178. DOI: 10.1016/S0076-6879(99)99017-1.
- Koraqi, H.; Petkoska, A. T.; Khalid, W.; Sehrish, A.; Ambreen, S.; Lorenzo, J. M. Optimization of the Extraction Conditions of Antioxidant Phenolic Compounds from Strawberry Fruits (Fragaria X Ananassa Duch.) Using Response Surface Methodology. Food Anal. Methods. 2023, 16(6), 1030–1042. DOI: 10.1007/s12161-023-02469-6.
- Xiao, F.; Xu, T.; Lu, B.; Liu, R. Guidelines for Antioxidant Assays for Food Components. Food Front. 2020, 1(1), 60–69. DOI: 10.1002/fft2.10.
- Belwal, T.; Dhyani, P.; Bhatt, I. D.; Rawal, R. S.; Pande, V. Optimization Extraction Conditions for Improving Phenolic Content and Antioxidant Activity in Berberis Asiatica Fruits Using Response Surface Methodology (RSM). Food Chem. 2016, 207, 115–124. DOI: 10.1016/j.foodchem.2016.03.081.
- Lee, J. E.; Yoon, Y. K. Optimization of Extraction Conditions for Functional Compounds from Thinned Unripe Apple Using β-Cyclodextrin-Based Ultrasound-Assisted Extraction. CyTa – J. Food. 2023, 21(1), 10–19. DOI: 10.1080/19476337.2022.2156619.
- Petreska Stanoeva, J.; Stefova, M.; Matevski, V. Extraction, Distribution and Diversity of Phenolic Compounds in Most Widespread Boraginaceae Species from Macedonia. Chem. Biodivers. 2023, 20(5). DOI: 10.1002/cbdv.202201149.
- Şahin, S.; Kurtulbaş, E.; Bilgin, M. Special Designed Deep Eutectic Solvents for the Recovery of High Added-Value Products from Olive Leaf: A Sustainable Environment for Bioactive Materials. Prep. Biochem. Biotechnol. 2020. DOI: 10.1080/10826068.2020.1824162.
- Nallan Chakravartula, S. S.; Moscetti, R.; Farinon, B.; Vinciguerra, V.; Merendino, N.; Bedini, G.; Neri, L.; Pittia, P.; Massantini, R. Stinging Nettles as Potential Food Additive: Effect of Drying Processes on Quality Characteristics of Leaf Powders. Foods. 2021, 10(6), 1152. DOI: 10.3390/foods10061152.
- Nortjie, E.; Basitere, M.; Moyo, D.; Nyamukamba, P. Extraction Methods, Quantitative and Qualitative Phytochemical Screening of Medicinal Plants for Antimicrobial Textiles: A Review. Plants. 2022, 11, 2011. DOI: 10.3390/plants11152011.
- Papaemmanouil, C. D.; Peña-García, J.; Banegas-Luna, A. J.; Kostagianni, A. D.; Gerothanassis, I. P.; Pérez-Sánchez, H.; Tzakos, A. G. ANTIAGE-DB: A Database and Server for the Prediction of Anti-Aging Compounds Targeting Elastase, Hyaluronidase, and Tyrosinase. Antioxidants. 2022, 11(11), 2268. DOI: 10.3390/antiox11112268.
- Paulauskienė, A.; Tarasevičienė, Ž.; Laukagalis, V. Influence of Harvesting Time on the Chemical Composition of Wild Stinging Nettle (Urtica Dioica L.). Plants. 2021, 10(4), 686. DOI: 10.3390/plants10040686.
- Mokrani, A.; Madani, K. Effect of Solvent, Time and Temperature on the Extraction of Phenolic Compounds and Antioxidant Capacity of Peach (Prunus persica L.) Fruit. Sep. Purif. Techn. 2016, 162, 68–76. DOI: 10.1016/j.seppur.2016.01.043.
- Gao, M.; Cui, Q.; Wang, L.; Meng, Y.; Yu, L.; Li, Y.; Fu, Y. A Green and Integrated Strategy for Enhanced Phenolic Compounds Extraction from Mulberry (Morus Alba L.) Leaves by Deep Eutectic Solvent. Microchem. J. 2020, 154, 104598. DOI: 10.1016/j.microc.2020.104598.
- Cannavacciuolo, C.; Pagliari, S.; Frigerio, J.; Giustra, C. M.; Labra, M.; Campone, L. Natural Deep Eutectic Solvents (NADESs) Combined with Sustainable Extraction Techniques: A Review of the Green Chemistry Approach in Food Analysis. Foods. 2023, 12(1), 56. DOI: 10.3390/foods12010056.
- Dong, J.; Wu, G.; Dong, Z.; Yang, D.; Bo, Y.; An, M.; Zhao, L. Natural Deep Eutectic Solvents as Tailored and Sustainable Media for the Extraction of Five Compounds from Compound Liquorice Tablets and Their Comparison with Conventional Organic Solvents. R.S.C. Adv. 2021, 11(59), 37649. DOI: 10.1039/d1ra06338c.
- Shi, L.; Zhao, W.; Yang, Z.; Subbiah, V.; Suleria, H. A. R. Extraction and Characterization of Phenolic Compounds and Their Potential Antioxidant Activities. Environ. Sci. Pollut. Res. 2022, 29(54), 81112–81129. DOI: https://doi.org/10.1007/s11356-022-23337-6.
- Mohammed, E. A.; Abdalla, I. G.; Alfawaz, M. A.; Mohammed, M. A.; Al Maiman, S. A.; Osman, M. A.; Yagoub, A. E. A.; Hassan, A. B. Effects of Extraction Solvents on the Total Phenolic Content, Total Flavonoid Content, and Antioxidant Activity in the Aerial Part of Root Vegetables. Agriculture. 2022, 12(11), 1820. DOI: 10.3390/agriculture12111820.
- Shonte, T. T.; Duodu, G. K.; de Kock, L. H. Effect of Drying Methods on Chemical Composition and Antioxidant Activity of Underutilized Stinging Nettle Leaves. Heliyon. 2020, 6(5), e03938. DOI: 10.1016/j.heliyon.2020.e03938.
- Moreira, S. A.; Pintado, M. E.; Saraiva, J. A. Optimization of High Hydrostatic Pressure Assisted Extraction of Stinging Nettle Leaves Using Response Surface Methodology Experimental Design. Food Meas. 2020, 14(5), 2773–2780. DOI: 10.1007/s11694-020-00522-0.
- Šic Žlabur, J.; Radman, S.; Opačić, N.; Rašic, A.; Dujmovic, M.; Brnčić, M.; Barba, F. J.; Castagnini, J. M.; Voća, S. Application of Ultrasound as Clean Technology for Extraction of Specialized Metabolites from Stinging Nettle (Urtica Dioica L.). Front. Nutr. 2022, 9, 870923. DOI: 10.3389/fnut.2022.870923.
- Güder, A.; Korkmaz, H. Evaluation of in-Vitro Antioxidant Properties of Hydroalcoholic Solution Extracts Urtica Dioica L., Malva Neglecta Wallr. and Their Mixture. Iran. J. Pharm. Res. 2012 Summer, 11(3), 913–923. PMID: 24250519; PMCID: PMC3813119.
- Costea, L.; Chițescu, C. L.; Boscencu, R.; Ghica, M.; Lupuliasa, D.; Mihai, D. P.; Deculescu-Ioniță, T.; Duțu, L. E.; Popescu, M. L.; Luță, E. A., et al. The Polyphenolic Profile and Antioxidant Activity of Five Vegetal Extracts with Hepatoprotective Potential. Plants. 2022, 11, 1680. DOI: 10.3390/plants11131680.
- Rudrapal, M.; Khairnar, S. J.; Khan, J.; Dukhyil, A. B.; Ansari, M. A.; Alomary, M. N.; Alshabrmi, F. M.; Palai, S.; Deb, P. K.; Devi, R. Dietary Polyphenols and Their Role in Oxidative Stress-Induced Human Diseases: Insights into Protective Effects, Antioxidant Potentials and Mechanism(s) of Action. Front. Pharmacol. 2022, 13, 806470. DOI: 10.3389/fphar.2022.806470.
- Repajić, M.; Cegledi, E.; Zorić, Z.; Pedisić, S.; Elez Garofulić, I.; Radman, S.; Palčić, I.; Dragović-Uzelac, V. Bioactive Compounds in Wild Nettle (Urtica Dioica L.) Leaves and Stalks: Polyphenols and Pigments Upon Seasonal and Habitat Variations. Foods. 2021, 10(1), 190. DOI: 10.3390/foods10010190.