?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
Pomegranates, which are renowned for their diverse bioactive phytochemicals with antioxidant, anti-inflammatory, and antimicrobial properties, have garnered significant interest in the field of natural medicine. This study aimed to systematically investigate the phytochemical content, bioavailability, and antimicrobial effects of extracts derived from different parts of three Jordanian pomegranate cultivars, Kodari, Qerati, and Black. Comprehensive quantitative analyses revealed significantly higher phenolic content and antioxidant capacities in peel extracts, particularly in the Black and Qerati cultivars. The total phenolic content was determined using the Folin-Ciocalteu colorimetric assay, and the antioxidant activity was evaluated using the 2,2-diphenyl-1-picrylhydrazyl (DPPH) radical scavenging assay. Bioavailability was determined using stimulated gastrointestinal digestion assays, revealing controlled release in gastric fluid compared to more substantial release in intestinal fluid, thereby suggesting potential implications for bioavailability. Furthermore, antimicrobial assays showed promising antibacterial effects against Staphylococcus aureus in a time-dependent manner, although the efficacy varied among the cultivars and components. These findings underscore the multifaceted bioactive potential of pomegranate extracts, particularly those derived from optimized peel extracts, positioning them as promising natural preservatives or functional ingredients. However, further research is warranted to delve into specific bioactive constituents, absorption mechanisms, and their potential applications in diverse fields, such as foods, nutraceuticals, and pharmaceuticals. By expanding the current understanding of the factors influencing pomegranate bioactivities, this study lays the groundwork for tailored utilization of pomegranate fractions based on ideal phytochemical profiles and targeted effects.
Introduction
Pomegranate (Punica granatum) has become an increasingly popular functional food and source of nutraceuticals, owing to its high antioxidant and anti-inflammatory properties. Various parts of the pomegranate fruit, including the peel, membrane, and arils (juice sacs), are rich sources of diverse phenolic compounds such as flavonoids, anthocyanins, and hydrolyzable tannins[Citation1]. These phytochemicals impart pomegranates both medicinal bioactivities and their distinct red color. Although previous studies have provided valuable insights into the phytochemical composition of various pomegranate components, there remains a need to further explore specific aspects, particularly in the context of comparing the total phenolic content, antioxidant activity, and antimicrobial activity among different types of pomegranate cultivars. Edible pomegranate juice contains high levels of ellagic acid derivatives, such as punicalagins, and flavonoids, such as quercetin and kaempferol.[Citation2] The peel and membrane byproducts are rich sources of unique ellagitannins, gallic acid, and anthocyanin pigments linked to antioxidant capacity.[Citation3] However, research is still limited on the bioavailability of these compounds after pomegranate consumption.[Citation4] Simulated digestion models show that certain ellagitannins, such as punicalins, can be metabolized into free ellagic acid during gastric and small intestinal digestion, which may improve bioabsorption. Overall, the pharmacokinetic data suggest that the absorption of intact pomegranate polyphenols in the stomach and intestines is relatively low.[Citation2] Further human trials are warranted to determine the factors that enhance the bioavailability of pomegranate phytochemicals.
Both in vitro and in vivo studies have provided compelling evidence of the antioxidant and anti-inflammatory activities of pomegranates for potential therapeutic benefits. Using cell culture models, pomegranate extracts have been shown to boost antioxidant status while suppressing inflammatory cytokines through multiple signaling pathways.[Citation5] Animal studies have reported that pomegranate peel and seed oil formulations reduce markers of inflammation and oxidative stress associated with conditions such as fatty liver disease and obesity when administered as a dietary supplement.[Citation6] Again, the limited bioavailability may restrict the direct antioxidant effects of intact pomegranate polyphenols in body tissues. Nevertheless, metabolites, such as ellagic acid, can elicit significant protective actions.[Citation7] Human clinical data have also revealed encouraging anti-inflammatory effects of pomegranate juice supplementation.[Citation8] Further clinical research is required to support the use of pomegranate extracts and isolated compounds as antioxidant and anti-inflammatory nutraceuticals. In addition to their metabolic bioactivities, emerging studies have highlighted the antimicrobial effects of pomegranates against various pathogens. High-molecular-weight hydrolyzable tannins in pomegranate rinds and peels have broad antibacterial activity against both gram-positive and gram-negative species.[Citation9] Other analyses have specifically confirmed the strong antibacterial actions of crude and purified pomegranate extracts against common foodborne pathogens, including Listeria monocytogenes, Yersinia enterocolitica, and Staphylococcus aureus.[Citation10] The antifungal properties of pomegranate against Candida albicans and other sources of candidiasis have also been documented.[Citation11] These antimicrobial effects appear to be closely associated with the direct actions of ellagitannins and other polar polyphenols. Microbial infections, particularly those caused by bacterial pathogens, present significant health challenges globally with varying prevalence rates across different regions.[Citation12] In Jordan, like many other parts of the world, microbial infections, especially those involving bacteria such as Staphylococcus aureus, are a substantial public health concern.[Citation13] In particular, S. aureus is known for its ability to cause a wide range of infections, from mild skin conditions to severe systemic illnesses, and its prevalence in healthcare settings further complicates treatment approaches.[Citation14,Citation15] Therefore, there is a pressing need to explore alternative antimicrobial agents.[Citation12] Natural products have long been recognized for their potential therapeutic properties, and many traditional remedies show promise as sources of novel antimicrobial compounds.[Citation16] Pomegranates (Punica granatum) have a rich history in traditional medicine and are known to contain diverse bioactive phytochemicals with antioxidant, anti-inflammatory, and antimicrobial properties.[Citation17] Given the prevalence of microbial infections and escalating challenge of antibiotic resistance, there is growing interest in exploring the antimicrobial potential of pomegranate extracts. The rationale for choosing pomegranate for evaluation lies in its rich repertoire of bioactive compounds, which have shown promise in combating various pathogens in preliminary studies.[Citation18,Citation19]
Several areas have sparked debate and controversy in the field of pomegranate research, particularly concerning the specific mechanisms of action of bioactive compounds and their potential health effects.[Citation20] One ongoing debate revolves around the optimal extraction methods to preserve the bioavailability and efficacy of phytochemicals from different parts of the pomegranate fruit. Additionally, there is ongoing discussion regarding the translation of in vitro findings to in vivo or clinical outcomes, which underscores the need for more robust human trials to accurately elucidate the health benefits of pomegranate consumption. In recent years, significant advances have been made in our understanding of the bioactive properties of pomegranate. For instance, a groundbreaking study published in 2015 by Rahmanian[Citation21] identified novel bioactive compounds in pomegranate peel extracts and elucidated their potential therapeutic applications for combating oxidative stress and inflammation. Furthermore, 2023 studies conducted by Rizzo[Citation22] shed light on the role of pomegranate-derived compounds in modulating gut microbiota composition, highlighting their potential as prebiotics and their implications for gastrointestinal health. These recent achievements underscore the dynamic nature of pomegranate research and its continued relevance in various fields, including nutrition, medicine, and functional foods
However, considerable gaps in knowledge remain regarding the pharmacokinetics, bioavailability, and specific molecular mechanisms conferring therapeutic action to compounds found uniquely in pomegranate fruit.[Citation23] There is also a need for expanded clinical research to support health claims associated with pomegranate extracts or juice. The proposed work to compare the phytochemical content, bioavailability, and antimicrobial activity of different pomegranate parts against select pathogen will help to address some of these research limitations. These findings could have future applications in the pharmaceutical, nutraceutical, and agricultural industries, aiming to effectively utilize pomegranates as a functional food source or source of antibiotic alternatives to manage diseases.[Citation24–26]
In this study, the phytochemical content of three Jordanian pomegranate cultivars was examined (). Total phenolics and antioxidants were quantified in peel, membrane, and fruit extracts using common colorimetric assays for antioxidant compounds.[Citation27,Citation28] Investigating phytochemical levels across different types and parts of pomegranate provides useful data on the factors influencing pomegranate bioactive composition. Following phytochemical screening, a simulated digestion model was used to assess the bioaccessibility of compounds extracted from the black peel pomegranate fraction after gastric and small-intestinal digestion stages.[Citation29] The model can be screened for bioaccessibility before animal or human trials. Finally, the antimicrobial activity of the pomegranate extracts from each pomegranate part was tested against gram-positive bacteria. Standard disc diffusion assays were used to determine the growth inhibition zones for common causes of food poisoning, such as Staphylococcus aureus.[Citation30] A comparison of antimicrobial actions across fruit components and cultivars has revealed the sources with optimal activity.[Citation31] By systematically investigating the phytochemical content, bioavailability, and antimicrobial effects of different parts of Jordanian pomegranate cultivars, our study aims to contribute to the growing body of evidence supporting the use of pomegranates as natural alternatives to conventional antimicrobial agents.
Materials and method
Chemicals and reagents
High-performance liquid chromatography (HPLC)-grade solvents were used in all experiments. The chemicals gallic acid, Folin-Ciocalteu reagent, and 2,2-diphenyl-1-picrylhydrazyl (DPPH) were obtained from Sigma-Aldrich (POCH, Gliwice, Poland). Peels, membranes, and fruits from three different pomegranate (Punica granatum) cultivars (Kodari, Qerati, and Black) in Jordan were collected as experimental materials, with 1.5 g harvested per cultivar sample.
Extraction procedure
The peel, membrane, and inner fruit of the pomegranate were subjected to exhaustive Soxhlet extraction over two days using 250 ml of 80% methanol solvent at 25°C 13. This involves continuous contact and percolation with an organic polar solvent, allowing for the efficient recovery of phenolics, flavonoids, and other plant secondary metabolites with pharmaceutical relevance.[Citation32] The sample names are given in .
Table 1. The names and abbreviations of the pomegranate samples.
Total phenolic contents
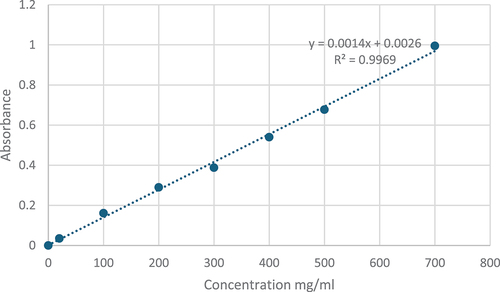
Development of Gallic Acid Standard Curve: A calibration curve using gallic acid as the reference phenolic standard was constructed to quantify the total phenolic content. Gallic acid solutions ranging from 100 to 700 μg/mL were freshly prepared in methanol by serial dilution of a 1 mg/mL gallic acid stock. For each concentration, 150 μL of gallic acid solution was combined with 450 μL of water, followed by the addition of 2.5 mL of Folin-Ciocalteu reagent. After incubation for 5 min, 2 mL of 75 g/L sodium carbonate was added. The solutions were shaken at 30°C for 90 min and the absorbance was measured at 765 nm using UV spectrophotometer.[Citation33] A standard curve was generated by plotting the absorbance against known gallic acid concentrations, and the regression equation was subsequently utilized to interpolate the total phenolic content of the plant extract samples. All gallic acid standard solutions were analyzed in triplicates. The constructed calibration curve and equation are shown in as references for colorimetric quantification of total phenolics.
Total Phenolic Content: The total phenolic content in the pomegranate peel, membrane, and fruit extracts was measured using the Folin-Ciocalteu colorimetric assay. Briefly, 150 μL of each plant extract was combined with 2.5 mL of 0.2 N Folin-Ciocalteu reagent and allowed to stand at room temperature for 5 min. Next, 2 mL of 75 g/L sodium bicarbonate solution was added to the mixture, which was subsequently incubated at 30°C for 90 min. The absorbance was read at 765 nm using a UV/Vis spectrophotometer (model SPUV-26/TECH). Quantification was performed by comparing the absorbance values against a standard curve constructed with gallic acid. The gallic acid curve was prepared using seven concentrations ranging from to 100–700 μg/mL. The total phenolic content in pomegranate extracts was expressed as milligram gallic acid equivalents (mg GAE) per gram of dried plant material, using the gallic acid standard plot for interpolation. Measurements were conducted in triplicates for each sample.[Citation34]
Evaluation of antioxidant activity by DPPH assay
The free radical scavenging capacity of pomegranate extracts was evaluated using the stable 2,2-diphenyl-1-picrylhydrazyl (DPPH) radical. The assay relies on the purple chromophore of DPPH, which strongly absorbs at 517 nm. Upon reduction by an antioxidant compound or plant extract, the DPPH radical gains an electron and converts it to a yellow-colored diphenylpicrylhydrazine molecule.
Briefly, a 0.5 mM DPPH methanolic stock solution was freshly prepared and its initial absorbance was measured at 517 nm using a spectrophotometer. Next, 150 μL of each pomegranate extract was combined with 2 mL of DPPH solution. After rapid mixing, the decrease in absorbance was monitored at zero time and then at 30 min by measuring absorbance at 517 nm. All samples were analyzed in triplicates.[Citation35] The percentage DPPH radical scavenging capacity was determined using the following equation:
where A is the absorbance of DPPH after the addition of extract at time zero, and B is the absorbance of a sample taken after 30 min of reaction with DPPH. Subsequently, the %inhibition/100 mg was calculated using the following equation:
Biological assay
The antibacterial potential of the pomegranate extracts was assessed against the common pathogen Staphylococcus aureus (ATCC 29,213) using standard disc diffusion assays. Extracts were obtained from the peel, inner membrane, and inner fruit fractions of the three Jordanian pomegranate cultivars after exhaustive Soxhlet extraction for 8, 16, 24, and 32 h. For each extraction time point, sterile filter paper discs (6 mm) were impregnated with 10 μL of the extract solutions dissolved in DMSO at a concentration of 100 mg/mL. Discs were allowed to dry and then placed onto Mueller-Hinton agar plates freshly inoculated with a 0.5 McFarland turbidity standardized S. aureus suspension (~1–2 × 108 CFU/mL). After incubation at 37°C for 18–24 hours, the zones of inhibition were measured in millimeters. Cephalosporin discs (30 μg) were used as positive controls. Three experimental replicates were performed for each pomegranate extract against S. aureus. Greater zone diameters indicate enhanced antimicrobial action from pomegranate phytochemical diffusion into the agar media. Differences in the growth inhibition zones were evaluated across cultivars, fruit components, and Soxhlet extraction times.[Citation36]
Dissolution study
The in vitro dissolution profiles of the pomegranate extract were conducted.[Citation37] The cumulative release percentage was evaluated over time. Dissolution studies were conducted utilizing a USP apparatus type 2, also known as the paddle method, maintained at 37°C [Citation37]. Stirring was achieved using a rotating paddle operating at a speed of 100 rpm. For each formulation, 100 mg of the extract was added directly to 900 mL of simulated gastric fluid (SGF) without pepsin. At predetermined time points of 10, 15, 20, 25, 30, 35, 40, 45, 50, 55, and 60 min, aliquots were withdrawn and replaced with fresh SGF to maintain sink conditions throughout the 1 h gastric digestion simulation. Samples were filtered through a 0.45 μm membrane before analysis by UV. Similarly, dissolution tests were performed using simulated intestinal fluid (SIF) to model small intestinal conditions over 1 h. All dissolution experiments were conducted in triplicates for each extract formulation. Differences in the dissolution profiles could be used to assess the potential impact of the sample on the bioaccessibility and delivery of the pomegranate extract under simulated digestive conditions.[Citation38]
Results
Total phenolic content
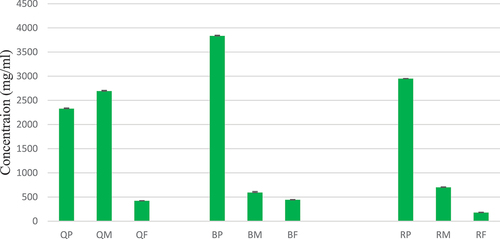
The assessment of total phenolic content across various pomegranate extracts, including the peel, inner membrane, and fruit sections, from Black, Qerati, and Kodari cultivar, revealed variations in phenolic concentrations (). Specifically, the peel extracts from the Black and Kodari cultivars demonstrated higher phenolic levels 3833.81 ± 15 & 2950.47 ± 3.22 respectively, indicating that these outer layers are rich sources of bioactive antioxidants and phytochemicals with prospective health benefits. Of all the samples tested, black pomegranate peel extract possessed the highest total phenolic content, signifying an abundant presence of antioxidant and functional bioactive agents. In contrast, the inner membrane and fruit fractions consistently exhibited lower phenolic contents across the pomegranate varieties analyzed. Furthermore, extracts with elevated phenolic levels appeared to correlate with enhanced antibacterial effects against Staphylococcus aureus in select samples. This relationship highlights the potential contribution of phenolic compounds within the extracts to the observed antimicrobial activity. These findings emphasize that pomegranate peel extracts, especially from Black and Qerati cultivars, are prospective sources of bioactive constituents worthy of further research into their precise phenolic profiles and applicability as functional ingredients in foods or natural preservative agents.
Antioxidant activity
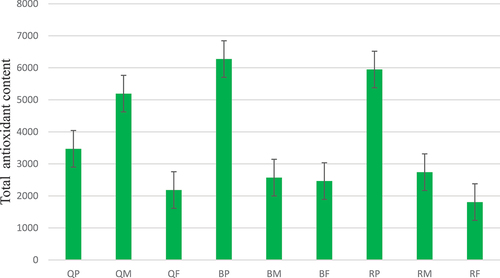
The assessment of antioxidant capabilities across various pomegranate extracts, including peel, inner membrane, and fruit sections, from Black, Qerati, and one unidentified cultivar showed noticeable variations in inhibition percentages. These results highlight the differing extents of antioxidant potential within these extracts . Among the Qerati samples, the inner membrane extract (QM) displayed the highest inhibition rate, indicating significant antioxidant capacity, followed by the peel extract (QP), with both exhibiting substantial inhibitory effects. Conversely, the Qerati fruit extract (QF) reveals a comparatively lower inhibition percentage. Black pomegranate extracts, particularly the peel (BP), demonstrated the highest overall inhibition percentage, signifying a robust antioxidant activity. The Black inner membrane (BM) and fruit extracts (BF) also showed notable but relatively lower antioxidant potentials than the peel. The unidentified pomegranate cultivar extracts also exhibited variable antioxidant effects, with the peel (RP) presenting inhibition percentages comparable to the Qerati and Black peel extracts, while the inner membrane (RM) and fruit (RF) extracts showed relatively weaker antioxidant capacities. These results highlight that pomegranate peel extracts, especially those from the Black and Qerati varieties, possess significant antioxidant capabilities, designating them as potentially valuable sources of antioxidant phytochemicals with prospective health benefits worthy of further research into their diverse applications as functional food ingredients or natural antioxidant additives.
Antibacterial activity
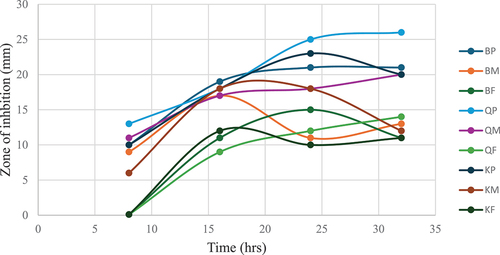
The results demonstrated varying degrees of antibacterial efficacy against Staphylococcus aureus among the different pomegranate extracts tested, including peel, inner membrane, and fruit components from Black, Qerati, and Kodari cultivars (). Specifically, Qerati peel extract consistently displayed the highest inhibition, exhibiting a gradual increase from a 13 mm zone at 8 h to 26 mm at 32 h. This indicates potent antibacterial effects over time for this sample. In contrast, Black and Kodari extracts presented less consistent or weaker inhibition levels, with fluctuations in their antimicrobial effects across the withdrawal periods. Particular extracts displayed time-dependent tendencies in activity, implying that certain antibacterial phytochemicals may require defined durations to elicit optimal effects. However, these kinetic patterns were irregular across all extracts. Ultimately, these findings highlight the differential antibacterial capabilities of the assayed pomegranate extracts, highlighting the need for additional studies. Future studies should focus on identifying the specific bioactive constituents conferring the observed effects and elucidating the mechanisms governing their time-dependent behavior. Such research would deepen the understanding and prospective applications of these natural antibacterial preparations derived from pomegranate fruit fractions.
Dissolution study
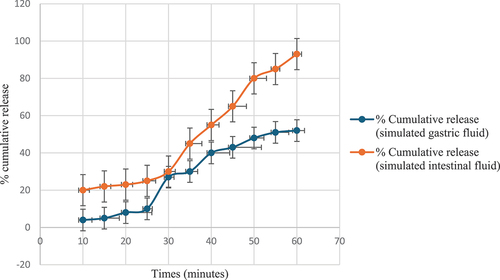
The dissolution study of total phenolic content from black peel pomegranate extract highlighted markedly different release patterns between simulated gastric and intestinal fluids (). The black peel sample was chosen because it had the highest antibacterial activity. In the gastric medium, the release remained low and gradual, failing to surpass 8% even after 60 minutes. This indicated controlled dissolution kinetics in the stomach environment. In contrast, within the simulated intestinal fluid, the release profile differed notably, beginning slowly but then rapidly accelerating to steadily rise until complete 100% dissolution was achieved after one hour. This stark divergence in dissolution behavior underscores the substantial difference in the response of the extract between the two environments. While gastric fluid elicits only a minor release over one hour, intestinal fluid triggers an expedited, continuous release, culminating in full dissolution within the same timeframe. This disparity indicates the potential of the extract for extended, regulated intestinal release compared to restricted, slower gastric release, with implications for bioavailability and physiological impacts in different digestive regions. Comprehending these variances in release dynamics is integral to harnessing the extract’s optimal efficacy and tailored delivery in relevant contexts. Thus, further probing of the absorption mechanisms and prospective biological activity effects of the differential gastrointestinal release profiles warrant investigation.
Figure 6. Dissolution study for the total phenolic content of black peel pomegranate extract in simulated gastric fluid (without pepsin) and in simulated intestinal fluid.
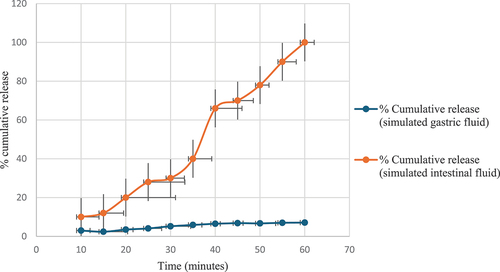
The dissolution profile of black pomegranate peel extract in the simulated gastric fluid indicated a gradual release of antioxidant constituents over time (). The cumulative antioxidant release slowly increased from 4% at 10 minutes to 52% at 60 minutes. The release pattern exhibited some fluctuations, as highlighted by the varying standard deviation values across the time points. However, the overall release remained fairly consistent, signifying the sustained gastric release of antioxidant compounds. In the simulated intestinal fluid, the dissolution behavior differs markedly from that in the gastric fluid. The release kinetics displayed a divergent pattern, initiating with slower release, reaching 27% at 30 min, then accelerating thereafter to culminate at 52% release by 60 min. Intestinal fluid release demonstrated lower variability and maintained a more controlled profile than gastric fluid.[Citation17] Dissolution testing in both simulated digestive fluids revealed contrasting release dynamics of antioxidant agents from the black peel pomegranate extract. In gastric fluid, there is a steady, progressive release, while in intestinal fluid, the release starts slower but then hastens, achieving a comparable cumulative level to gastric fluid by the end of the study. This suggests a potentially delayed but ultimately substantial intestinal release of antioxidants. The distinct dissolution behaviors observed in the gastric and intestinal environments imply that antioxidant compounds from the extract exhibit differential release kinetics depending on the gastrointestinal region. These observations suggest prospective implications for the bioavailability and efficacy of the extract, proposing variable release rates in different digestive compartments. Additional research into absorption mechanisms and subsequent biological effects is vital to comprehend and leverage these dissolution variations for optimized utilization and effectiveness of the black peel pomegranate extract.
Discussion
A comprehensive analysis of the total phenolic content, antioxidant activity, antimicrobial effects, and dissolution behavior of the pomegranate extract revealed a complex interplay among these facets of bioactivity. The strong correlation observed between higher phenolic content and potent antioxidant activity substantiates the pivotal role of phenolic compounds in driving antioxidant potential.[Citation42] This robust association aligns with the higher antioxidant effects of the extract in samples rich in phenolic compounds. However, although a partial alignment exists between phenolic content and antimicrobial effects, variations in antibacterial efficacy across extracts suggest multifactorial influences beyond phenolic compounds in determining antimicrobial potential.[Citation45] Moreover, this dissolution study sheds light on the distinct release patterns of the extract in simulated gastric and intestinal fluids. The slow, limited release in gastric fluid contrasts starkly with the delayed, yet substantial, release observed in the intestinal environment, suggesting differential behavior in gastrointestinal conditions. These diverse behaviors underscore the potential implications of the bioavailability and efficacy of the extract in different regions of the digestive system.[Citation37] We also conducted an ANOVA test to examine the total phenolic content and antioxidant activity of various pomegranate samples, including the peel, inner membrane, and fruit fractions from different cultivars.[Citation38–41 The results revealed significant differences in both the total phenolic content and antioxidant activity among the pomegranate samples (p < .05).[Citation43,Citation44These findings are consistent with previous research indicating variations in bioactive compound levels among pomegranate cultivars.[Citation46] For example, black pomegranate peel extract exhibited the highest total phenolic content and antioxidant activity, followed by the Qerati peel extract. These cultivars may hold promise for applications that require potent antioxidant properties, such as functional foods or nutraceuticals. However, further investigation is needed to elucidate the underlying factors driving these differences and optimize the selection of pomegranate cultivars for specific industrial applications. Future research should explore the genetic and environmental factors influencing the bioactive profiles of pomegranate extracts and investigate their potential health benefits in clinical trials. This comprehensive understanding emphasizes the multifaceted nature of the bioactivity of the extract, with phenolic compounds playing a pivotal yet nuanced role in driving antioxidant and potentially antimicrobial effects.[Citation47] The dissolution dynamics further highlight variations in the release behavior, necessitating in-depth investigations into specific constituents, absorption mechanisms, and their collective impact on biological activity to optimize the utilization of the extract and its targeted efficacy in various applications.
The results showed the potential of optimized pomegranate peel extracts, such as the black peel extract studied here, as promising natural preservatives and functional ingredients. The demonstrated antioxidant, antimicrobial, and modulated release capabilities substantiate its prospective applications in foods, nutraceuticals, and pharmaceuticals. Integrating black peel extract into such products could limit oxidative deterioration while suppressing microbial growth via the controlled release of bioactive phenolics.[Citation48] This can mitigate health risks associated with foodborne infections and toxicity.[Citation49] Before practical utilization, further investigations of the phenolic composition, bioaccessibility under storage conditions, and impact on product quality are warranted.[Citation50] Recent studies on pomegranate have contributed to our understanding of its bioactive properties and its potential applications. Sweidan et al.[Citation51] investigated the antioxidant and antimicrobial activities of pomegranate peel extracts from different cultivars in Australia. Their findings corroborated with ours, showing higher phenolic content and antioxidant capacity in peel extracts, particularly from specific cultivars. However, they reported variations in antimicrobial efficacy against different bacterial strains compared with our study, highlighting the influence of environmental factors and genetic variability in pomegranate cultivars. Similarly, Shukla et al.[Citation29] focused on the bioavailability of pomegranate polyphenols and their metabolites in humans. Although our simulated digestion model provided insights into release kinetics, human trials offer valuable data on actual absorption and metabolism. Patel et al. reported varying degrees of bioavailability of different polyphenols, suggesting that factors such as compound structure and gut microbiota composition may influence absorption rates. Comparing these findings with those of our study could elucidate how in vitro bioaccessibility translates to in vivo bioavailability. Additionally, Abu‐Niaaj et al. Click or tap to enter text. While our study focused on Staphylococcus aureus, Garcia et al. demonstrated antimicrobial activity against multiple foodborne pathogens and Candida species. Comparison of the efficacy of pomegranate extracts against diverse microbial targets could provide insights into their broad-spectrum antimicrobial potential and guide future applications in food preservation and pharmaceuticals. Overall, recent studies on pomegranate have reinforced its multifaceted bioactive potential, with variations observed in the phenolic content, antioxidant activity, antimicrobial efficacy, and bioavailability across different cultivars and experimental conditions. Comparing these findings with those of our study highlights the robustness of certain observations and indicates areas for further investigation and potential applications in various industries.
Conclusion
This study investigated the pharmaceutical prospects of pomegranate antioxidants in combating microbial infections, exploring the phytochemical content, bioavailability, and antimicrobial effects of extracts from different parts of three Jordanian pomegranate cultivars. Our findings underscore the multifaceted bioactive potential of pomegranate extracts, particularly highlighting optimized peel extracts as natural preservatives or functional ingredients with promising antimicrobial properties. The significant variations observed in total phenolic content and antioxidant activity among peel, membrane, and fruit extracts emphasize the importance of specific pomegranate fractions in conferring bioactive benefits. Peel extracts, especially from the Black and Qerati cultivars, exhibited the highest phenolic content and antioxidant capacity, indicating their potential as rich sources of bioactive antioxidants. Antimicrobial assays revealed promising antibacterial effects against Staphylococcus aureus, albeit with variability across the cultivars and components. This underscores the need for further research on the specific bioactive constituents that drive antimicrobial activity and their targeted effects. This dissolution study provided insights into the differential release patterns of phenolic compounds in simulated gastric and intestinal fluids, suggesting potential implications for bioavailability and efficacy in different gastrointestinal regions. Understanding these release dynamics is crucial for optimizing the utilization and effectiveness of pomegranate extracts for various applications. Recent achievements in pomegranate research have shed light on its bioactive properties and potential applications, thereby emphasizing the dynamic nature of this field. However, considerable gaps remain regarding pharmacokinetics, bioavailability, and specific molecular mechanisms, warranting further exploration in future studies. Although this study highlights the potential of pomegranate extracts, particularly peel extracts, as natural antimicrobial agents, several limitations should be noted. This study only examined a limited set of pomegranate cultivars from one region and focused solely on Staphylococcus aureus for antimicrobial testing. Additionally, the absence of HPLC analysis limits the identification and quantification of the specific phytochemicals responsible for bioactivity. Although UV spectrophotometry provides insights, it may not capture the full phytochemical spectrum. Future research should consider broader cultivar sampling, testing against a wider range of pathogens, and employing more advanced analytical techniques for a comprehensive understanding of pomegranate’s pharmaceutical potential. In summary, our study contributes to the growing body of evidence supporting the pharmaceutical prospects of pomegranate antioxidants for combating microbial infections. By elucidating the relationships between phytochemical content, bioavailability, and antimicrobial effects, our findings pave the way for tailored utilization of pomegranate fractions for enhanced health and wellness benefits.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Viuda-Martos, M.; Fernández-Lóaez, J.; Pérez-Álvarez, J. A. Pomegranate and Its Many Functional Components As Related to Human Health: A Review. Compr. Rev. Food Sci. Food Saf. 2010, 9(6), 635–654. DOI: 10.1111/j.1541-4337.2010.00131.x.
- Basu, A.; Penugonda, K. Pomegranate Juice: A Heart-Healthy Fruit Juice. Nutr. rev. 2009, 67(1), 49–56. DOI: 10.1111/j.1753-4887.2008.00133.x.
- Li, Z. J.; Liu, M.; Dawuti, G.; Dou, Q.; Ma, Y.; Liu, H.-G.; Aibai, S. Antifungal Activity of Gallic Acid in vitro and in vivo. Phytotherap. Res. 2017, 31(7), 1039–1045. DOI: 10.1002/ptr.5823.
- Singh, R. P.; Chidambara Murthy, K. N.; Jayaprakasha, G. K. Studies on the Antioxidant Activity of Pomegranate (Punica Granatum) Peel and Seed Extracts Using in vitro Models. J. Agric. Food. Chem. 2002, 50(1), 81–86. DOI: 10.1021/jf010865b.
- Banihani, S. A.; Makahleh, S. M.; El-Akawi, Z.; Al-Fashtaki, R. A.; Khabour, O. F.; Gharibeh, M. Y.; Saadah, N. A.; Al-Hashimi, F. H.; Al-Khasieb, N. J. Fresh Pomegranate Juice Ameliorates Insulin Resistance, Enhances β-Cell Function, and Decreases Fasting Serum Glucose in Type 2 Diabetic Patients. Nutrit. Res. 2014, 34(10), 862–867. DOI: 10.1016/j.nutres.2014.08.003.
- Barghchi, H.; Milkarizi, N.; Belyani, S.; Norouzian Ostad, A.; Askari, V. R.; Rajabzadeh, F.; Goshayeshi, L.; Ghelichi Kheyrabadi, S. Y.; Razavidarmian, M.; Dehnavi, Z., et al. Pomegranate (Punica Granatum L.) Peel Extract Ameliorates Metabolic Syndrome Risk Factors in Patients with Non-Alcoholic Fatty Liver Disease: A Randomized Double-Blind Clinical Trial. Nutr. J. 2023, 22(1), 1–17.
- Islam, F.; Dehbia, Z.; Zehravi, M.; Das, R.; Sivakumar, M.; Krishnan, K.; Billah, A. A. M.; Bose, B.; Ghosh, A.; Paul, S., et al. Indole Alkaloids from Marine Resources: Understandings from Therapeutic Point of View to Treat Cancers. Chem. Biol. Interact. 2023, 383, 110682. DOI: 10.1016/j.cbi.2023.110682.
- Ismail, T.; Sestili, P.; Akhtar, S. Pomegranate Peel and Fruit Extracts: A Review of Potential Anti-Inflammatory and Anti-Infective Effects. J. Ethnopharmacol. 2012, 143(2), 397–405. DOI: 10.1016/j.jep.2012.07.004.
- Salim, A.; Deiana, P.; Fancello, F.; Molinu, M. G.; Santona, M.; Zara, S. Antimicrobial and Antibiofilm Activities of Pomegranate Peel Phenolic Compounds: Varietal Screening Through a Multivariate Approach. J. Bioresourc. Bioprod. 2023, 8(2), 146–161. DOI: 10.1016/j.jobab.2023.01.006.
- Al-Zoreky, N. S. Antimicrobial Activity of Pomegranate (Punica Granatum L.) Fruit Peels. Int. J. Food Microbiol. 2009, 134(3), 244–248. DOI: 10.1016/j.ijfoodmicro.2009.07.002.
- Endo, E. H.; Garcia Cortez, D. A.; Ueda-Nakamura, T.; Nakamura, C. V.; Dias Filho, B. P. Potent Antifungal Activity of Extracts and Pure Compound Isolated from Pomegranate Peels and Synergism with Fluconazole Against Candida albicans. Res. Microbiol. 2010, 161(7), 534–540. DOI: 10.1016/j.resmic.2010.05.002.
- Malkawi, R.; Iyer, A.; Parmar, A.; Lloyd, D.; Leng Goh, E.; Taylor, E.; Sarmad, S.; Madder, A.; Lakshminarayanan, R.; Singh, I., et al. Cysteines and Disulfide-Bridged Macrocyclic Mimics of Teixobactin Analogues and Their Antibacterial Activity Evaluation Against Methicillin-Resistant Staphylococcus Aureus (MRSA). Pharmaceut. 2018, 10(4), 183.
- Malkawi, R.; Jarwan, B.; Tawalbeh, J. Assessing the Awareness, Attitude, and Knowledge of Senior Pharmacy Students in Jordanian Universities Regarding Antibiotic Use and Resistance: A Study of the Medical Curriculum. J. Pharm. Res. Int. 2023, 35(5), 41–52. DOI: 10.9734/jpri/2023/v35i57328.
- Girt, G. C.; Mahindra, A.; Al Jabri Zaaima, J. H.; De Ste Croix, M.; Oggioni, M. R.; Jamieson, A. G. Lipopeptidomimetics Derived from Teixobactin Have Potent Antibacterial Activity Against Staphylococcus Aureus. Chem. Commun. (Camb) 2018, 54(22), 2767–2770. DOI: 10.1039/C7CC06093A.
- Parmar, A.; Lakshminarayanan, R.; Iyer, A.; Mayandi, V.; Leng Goh, E. T.; Lloyd, D. G.; Chalasani, M. L. S.; Verma, N. K.; Prior, S. H.; Beuerman, R. W., et al. Design and Syntheses of Highly Potent Teixobactin Analogues Against Staphylococcus Aureus, Methicillin-Resistant Staphylococcus Aureus (MRSA), and Vancomycin-Resistant Enterococci (VRE) in vitro and in vivo. J. Med. Chem. 2018, 61(5), 2009–2017.
- Radek, K.; Gallo, R. Antimicrobial Peptides: Natural Effectors of the Innate Immune System. Semin. Immunopathol. 2007, 29(1), 27–43. DOI: 10.1007/s00281-007-0064-5.
- Cheng, J.; Li, J.; Xiong, R.-G.; Wu, S.-X.; Huang, S.-Y.; Zhou, D.-D.; Saimaiti, A.; Shang, A.; Feng, Y.; Gan, R.-Y., et al. Bioactive Compounds and Health Benefits of Pomegranate: An Updated Narrative Review. Food Biosci. 2023, 53, 102629. DOI: 10.1016/j.fbio.2023.102629.
- Abu-Niaaj, L. F.; Al-Daghistani, H. I.; Katampe, I.; Abu-Irmaileh, B.; Bustanji, Y. K. Pomegranate Peel: Bioactivities As Antimicrobial and Cytotoxic Agents. Food Sci. Nutr. 2024, 12(4), 2818. DOI: 10.1002/fsn3.3963.
- Malviya, S.; Arvind Jha, A.; Jha, A.; Hettiarachchy, N. Antioxidant and Antibacterial Potential of Pomegranate Peel Extracts. J. Food Sci. Technol. 2014, 51(12), 4132. DOI: 10.1007/s13197-013-0956-4.
- Gandla, K.; Islam, F.; Zehravi, M.; Karunakaran, A.; Sharma, I.; Haque, M. A.; Kumar, S.; Pratyush, K.; Dhawale, S. A.; Nainu, F., et al. Natural Polymers As Potential P-Glycoprotein Inhibitors: Pre-ADMET Profile and Computational Analysis As a Proof of Concept to Fight Multidrug Resistance in Cancer. Heliyon. 2023, 9(9), e19454.
- Rahmanian, N.; Jafari, S. M.; Wani, T. A. Bioactive Profile, Dehydration, Extraction and Application of the Bioactive Components of Olive Leaves. Trends Food Sci. Technol. 2015, 42(2), 150–172. DOI: 10.1016/j.tifs.2014.12.009.
- Rizzo, G.; Pineda Chavez, S. E.; Vandenkoornhuyse, E.; Cárdenas Rincón, C. L.; Cento, V.; Garlatti, V.; Wozny, M.; Sammarco, G.; Di Claudio, A.; Meanti, L., et al. Pomegranate Extract Affects Gut Biofilm Forming Bacteria and Promotes Intestinal Mucosal Healing Regulating the Crosstalk Between Epithelial Cells and Intestinal Fibroblasts. Nutri. 2023, 15(7), 1771.
- Islam, F.; Mitra, S.; Emran, T. B.; Khan, Z.; Nath, N.; Das, R.; Sharma, R.; Awadh Ahmed Abdullah, A.; Park, M. N.; Kim, B. Natural Small Molecules in Gastrointestinal Tract and Associated Cancers: Molecular Insights and Targeted Therapies. Molecul 2022, 27(17), 5686. DOI: 10.3390/molecules27175686.
- Anjum, J.; Mitra, S.; Das, R.; Alam, R.; Mojumder, A.; Emran, T. B.; Islam, F.; Rauf, A.; Hossain, M. J.; Aljohani, A. S. M., et al. A Renewed Concept on the MAPK Signaling Pathway in Cancers: Polyphenols as a Choice of Therapeutics. Pharmacol. Res. 2022, 184, 106398. DOI: 10.1016/j.phrs.2022.106398.
- Emran, T. B.; Islam, F.; Mitra, S.; Paul, S.; Nath, N.; Khan, Z.; Das, R.; Chandran, D.; Sharma, R.; Lima, C. M. G., et al. Pectin: A Bioactive Food Polysaccharide with Cancer Preventive Potential. Molecul. 2022, 27(21), 7405.
- Mitra, S.; Das, R.; Emran, T. B.; Labib, R. K.; Islam, F.; Sharma, R.; Ahmad, I.; Nainu, F.; Chidambaram, K., et al. Diallyl Disulfide: A Bioactive Garlic Compound with Anticancer Potential. Front. Pharmacol. 2022, 13, 943967. DOI: 10.3389/fphar.2022.943967.
- Zhishen, J.; Mengcheng, T.; Jianming, W. The Determination of Flavonoid Contents in Mulberry and Their Scavenging Effects on Superoxide Radicals. Food Chem. 1999, 64(4), 555–559. DOI: 10.1016/S0308-8146(98)00102-2.
- Singleton, V. L.; Orthofer, R.; Lamuela-Raventós, R. M. Analysis of Total Phenols and Other Oxidation Substrates and Antioxidants by Means of Folin-Ciocalteu Reagent. Methods Enzymol. 1999, 299, 152–178.
- Laurindo, L. F.; Barbalho, S. M.; Marquess, A. R.; Grecco, A. I. D. S.; Goulart, R. D. A.; Tofano, R. J.; Bishayee, A. Pomegranate (Punica Granatum L.) and Metabolic Syndrome Risk Factors and Outcomes: A Systematic Review of Clinical Studies. Nutri 2022, 14(8), 1665. DOI: 10.3390/nu14081665.
- Duman, A. D.; Ozgen, M.; Dayisoylu, K. S.; Erbil, N.; Durgac, C. Antimicrobial Activity of Six Pomegranate (Punica Granatum L.) Varieties and Their Relation to Some of Their Pomological and Phytonutrient Characteristics. Molecul 2009, 14, 1808–1817. DOI: 10.3390/molecules14051808.
- Laurindo, L. F.; Barbalho, S. M.; Marquess, A. R.; Grecco, A. I. D. S.; Goulart, R. D. A.; Tofano, R. J.; Bishayee, A. Pomegranate (Punica Granatum L.) and Metabolic Syndrome Risk Factors and Outcomes: A Systematic Review of Clinical Studies. Nutri 2022, 14(8), 1665. DOI: 10.3390/nu14081665.
- Chen, J.; Liao, C.; Ouyang, X.; Kahramanoğlu, I.; Gan, Y.; Li, M. Antimicrobial Activity of Pomegranate Peel and Its Applications on Food Preservation. J. Food Qual. 2020, 2020, 1–8. DOI: 10.1155/2020/8850339.
- Ullah Shirazi, O. Determination of Total Phenolic, Flavonoid Content and Free Radical Scavenging Activities of Common Herbs and Spices. J. Pharmacogn. Phytochem. 2014, 3, 104–108.
- Ghanbari, R.; Anwar, F.; Alkharfy, K. M.; Gilani, A. H.; Saari, N. Valuable Nutrients and Functional Bioactives in Different Parts of Olive (Olea Europaea L.)—A Review. IJMS. 2012, 13(3), 3291–3340. DOI: 10.3390/ijms13033291.
- Katalinic, V.; Milos, M.; Kulisic, T.; Jukic, M. Screening of 70 Medicinal Plant Extracts for Antioxidant Capacity and Total Phenols. Food Chem. 2006, 94(4), 550–557. DOI: 10.1016/j.foodchem.2004.12.004.
- Mudzengi, C. P.; Murwira, A.; Tivapasi, M.; Murungweni, C.; Burumu, J. V.; Halimani, T. Antibacterial Activity of Aqueous and Methanol Extracts of Selected Species Used in Livestock Health Management. Pharmaceut. Biol. 2017, 55(1), 1054. DOI: 10.1080/13880209.2017.1287744.
- Vora, A. K.; Londhe, V. Y.; Pandita, N. S. Preparation and Characterization of Standardized Pomegranate Extract-Phospholipid Complex as an Effective Drug Delivery Tool. J. Adv. Pharm. Technol. Res. 2015, 6(2), 75. DOI: 10.4103/2231-4040.154542.
- Malkawi, R.; Malkawi, W. I.; Al-Mahmoud, Y.; Tawalbeh, J.; Mutalik, S.; Mutalik, S. Current Trends on Solid Dispersions: Past, Present, and Future. Advanc. Pharmacol. Pharmaceut. Sci 2022, 2022, 1–17. DOI: 10.1155/2022/5916013.
- Jarwan, B.; Tawalbeh, J.; Malkawi, R.; S M, B. Assessment of Phenol and Antioxidant Content of Olive Varieties and Their Potential Health Benefits for Colon Health. Sci. World J. 2023, 2023, 1–8. DOI: 10.1155/2023/9165902.
- Siddiqui, N.; Rauf, A.; Latif, A.; Mahmood, Z. Spectrophotometric Determination of the Total Phenolic Content, Spectral and Fluorescence Study of the Herbal Unani Drug Gul-E-Zoofa (Nepeta Bracteata Benth). J Taibah Univers. Med. Sci. 2017, 12(4), 360. DOI: 10.1016/j.jtumed.2016.11.006.
- Gil, M. I.; Tomas-Barberan, F. A.; Hess-Pierce, B.; Holcroft, D. M.; Kader, A. A. Antioxidant Activity of Pomegranate Juice and Its Relationship with Phenolic Composition and Processing. J. Agric. Food. Chem. 2000, 48(10), 4581–4589. DOI: 10.1021/jf000404a.
- Li, Y.; Guo, C.; Yang, J.; Wei, J.; Xu, J.; Cheng, S. Evaluation of Antioxidant Properties of Pomegranate Peel Extract in Comparison with Pomegranate Pulp Extract. Food Chem. 2006, 96(2), 254–260. DOI: 10.1016/j.foodchem.2005.02.033.
- Zare, H., et al. Reporting of Adverse Effects of Pomegranate in Clinical Studies: A Systematic Review. J. Complement Integr. Med. 2023, 5, 10–25.
- Moga, A.; Dimienescu, O. G.; Bălan, A.; Dima, L.; Toma, S. I.; Bîgiu, N. F.; Blidaru, A. Pharmacological and Therapeutic Properties of Punica Granatum Phytochemicals: Possible Roles in Breast Cancer Marius. Molecul. 2021, 26(4), 1054. DOI: 10.3390/molecules26041054.
- Reddy, M. K.; Gupta, S. K.; Jacob, M. R.; Khan, S. I.; Ferreira, D. Antioxidant, Antimalarial and Antimicrobial Activities of Tannin-Rich Fractions, Ellagitannins and Phenolic Acids from Punica Granatum L. Planta. med. 2007, 73(5), 461–467. DOI: 10.1055/s-2007-967167.
- Sweidan, N.; Rayyan, W. A.; Mahmoud, I.; Ali, L.; Chávez-González, M. L. Phytochemical Analysis, Antioxidant, and Antimicrobial Activities of Jordanian Pomegranate Peels. PLoS One. 2023, 18(11), e0295129. DOI: 10.1371/journal.pone.0295129.
- Shiban, M. S.; Al-Otaibi, M. M.; Al-Zoreky, N. S. Antioxidant Activity of Pomegranate (Punica Granatum L.) Fruit Peels. FNS. 2012, 3, 991–996. DOI: 10.4236/fns.2012.37131. 7
- Panza, O.; Conte, A.; Del Nobile, M. A. Pomegranate By-Products as Natural Preservative to Prolong the Shelf Life of Breaded Cod Stick. Molecul. 2021, 26(8), 2385. DOI: 10.3390/molecules26082385.
- Islam, F.; Nath, N.; Zehravi, M.; Khan, J.; Jashim, S. B.-T.; Charde, M. S.; Chakole, R. D.; Kumar, K. P.; Babu, A. K.; Nainu, F., et al. Exploring the Role of Natural Bioactive Molecules in Genitourinary Cancers: How Far Has Research Progressed? Nat. Prod. Bioprospect. 2023, 13(1), 1–19.
- Gigliobianco, M. R.; Cortese, M.; Nannini, S.; Di Nicolantonio, L.; Peregrina, D. V.; Lupidi, G.; Vitali, L. A.; Bocchietto, E.; Di Martino, P.; Censi, R., et al. Chemical, Antioxidant, and Antimicrobial Properties of the Peel and Male Flower By-Products of Four Varieties of Punica Granatum L. Cultivated in the Marche Region for Their Use in Cosmetic Products. Antioxid. 2022, 11(4), 768.
- Malek Mahdavi, A.; Javadivala, Z. Systematic Review of the Effects of Pomegranate (Punica Granatum) on Osteoarthritis. Health Promot Perspect. 2021, 11(4), 411–425. DOI: 10.34172/hpp.2021.51.