Abstract
In this study, the performance of two newly developed personal bioaerosol samplers was evaluated. The two test samplers are cyclone-based personal samplers that incorporate a recirculating liquid film. The performance evaluations focused on the physical efficiencies that a personal bioaerosol sampler could provide, including aspiration, collection, and capture efficiencies. The evaluation tests were carried out in a wind tunnel, and the test personal samplers were mounted on the chest of a full-size manikin placed in the test chamber of the wind tunnel. Monodisperse fluorescent aerosols ranging from 0.5 to 20 μm were used to challenge the samplers. Two wind speeds of 0.5 and 2.0 m/sec were employed as the test wind speeds in this study. The test results indicated that the aspiration efficiency of the two test samplers closely agreed with the ACGIH inhalable convention within the size range of the test aerosols. The aspiration efficiency was found to be independent of the sampling orientation. The collection efficiency acquired from these two samplers showed that the 50% cutoff diameters were both around 0.6 μm. However, the wall loss of these two test samplers increased as the aerosol size increased, and the wall loss of PAS-4 was considerably higher than that of PAS-5, especially in the aerosol size larger than 5 μm, which resulted in PAS-4 having a relatively lower capture efficiency than PAS-5. Overall, the PAS-5 is considered a better personal bioaerosol sampler than the PAS-4.
Detection of personal exposure to airborne microorganisms (bioaerosols) is essential for developing adequate environmental and occupational air monitoring programs. Recently, two liquid-cyclone-based personal bioaerosol samplers were developed for this purpose. This study is to evaluate the performance of these two newly developed samplers in terms of their physical sampling efficiencies, such as the aspiration, collection, and capture efficiencies. The results acquired can offer insight into the efficacy of the newly designed samplers on achieving the goal of providing reliable and accurate personal bioaerosol exposure assessment.
Introduction
Airborne microorganisms (bioaerosols) such as viruses, allergens, bacteria, and fungal spores are almost ubiquitous and highly diverse in the environment. They can be found in different residential environments such as homes, day cares, and schools (CitationGorny et al., 1999; CitationMeklin et al., 2002; CitationReponen et al., 1994), as well as various occupational settings including food processing, agriculture, health care, and biotechnology (CitationHunter, 1992; CitationKotimaa, 1990; CitationOlenchock, 1988; CitationVerreault et al., 2011). Exposure to bioaerosols in these environments may pose health risks to the general and occupational population. The adverse health effects include local respiratory system complications such as asthma and mucous membrane irritation, and systemic effects such as mycotoxicosis and infectious disease (CitationLacey and Dutkiewicz, 1994). Therefore, it is necessary to employ suitable methods to accurately monitor the concentration of the airborne microorganisms in the environment to ensure sanitary living or occupational surroundings for the relevant public (CitationFakhri, 1998).
Personal aerosol sampling is considered the most appropriate method with which to conduct health-related exposure assessments (CitationVincent, 2007). Thus, sampling bioaerosols using personal bioaerosol samplers should be a preferred method for assessing personal exposure to airborne microorganisms. However, bioaerosols, unlike common inert aerosols, are very unique due to their viable characteristics. Thus, bioaerosol sampling requires a special approach to maintain the viability of the collected bioaerosols. To date, it is widely agreed that wet-type bioaerosol samplers (using liquid as the collection medium) can provide a relatively higher bioaerosol survival rate than dry-type bioaerosol samplers (using filters or impaction plates as the collection medium) (CitationWilleke et al., 1998). Collecting airborne microorganism into a liquid medium such as physiological saline has the benefits for bioaerosol sampling not only in reducing the stress and desiccation imposed upon the bioaerosols during the sampling process (CitationJensen et al., 1992), but also by offering convenience to the consequent microbiological analysis such as the traditional cell culture growth analysis and the advanced polymerase chain reaction assay (CitationLin et al., 2000). Therefore, researchers worldwide have designed various wet-type bioaerosol samplers to facilitate optimal bioaerosol sampling, and many of them have been sold on the market or presented to the academia, such as AGI-30, GCS, BioSampler, NIOSH one-stage, NIOSH two-stage, CIP-10M, and the predecessors of the test samplers in this study (CitationChen et al., 2004; CitationGorner et al., 2006; CitationGrinshpun et al., 1994; CitationLindsley et al., 2006; CitationTolchinsky et al., 2010; CitationUpton et al., 1994).
In our previous studies, a prototype of a new personal bioaerosol sampler was designed and manufactured based on the rationale of a swirling cyclone incorporating a recirculating liquid film (CitationSigaev et al., 2006). Bioaerosol sampling into liquid using swirling air in a cyclone features high microbial viability for the collected bioaerosols because the sampling air that entered the cyclone contacts the recirculated liquid film in a tangential way, which allows the bioaerosols to be gently captured by the collection liquid (CitationLin et al., 1999; CitationTolchinsky et al., 2010). The performance evaluation of the prototype demonstrated that this cyclone-based wet-type personal bioaerosol sampler has a high aerosol collection efficiency with cutoff diameter, d50 , equal to 0.7–0.75 μm (cutoff diameter: most of the particles greater than d50 are collected and most of the particles less than d50 pass through the sampler) and can permit fair bioaerosol survival (CitationTolchinsky et al., 2010). Recently, two improved versions were developed by adding more features on the previous prototype. The preliminary evaluation tests carried out in a mixing chamber showed that the improvements made on the samplers have enhanced their overall ability of bioaerosol sampling. The results of the physical efficiency tests showed that the collection efficiencies of both samplers were >85% for aerosols larger than 1.2 μm. The results of the biological efficiency tests indicated that the two test samplers could provide acceptable bioaerosol survival rate for the airborne microorganisms collected (CitationTolchinsky et al., 2011). The experimental data obtained demonstrated a promising result that these two samplers are capable of high-efficiency bioaerosol sampling.
To further evaluate the physical sampling efficiency of these two newly developed personal bioaerosol samplers in realistic sampling conditions, the main purpose of this study was to focus on testing the aspiration efficiency, collection efficiency, and the capture efficiency of these two samplers under a controlled moving air environment. It is widely recognized that for any newly developed personal aerosol sampler, examining the aspiration efficiency is an important test of its overall performance evaluation because the aspiration efficiency can show the ability of the test sampler to collect representative data from the sampling environment. The collection efficiency and capture efficiency acquired from the tests were also used as indices for evaluating the design features such as the recirculated liquid in both samplers. The evaluation tests were conducted in a large wind tunnel facility (CitationCheng et al., 2004), and the personal bioaerosol samplers were mounted on an adult manikin to mimic the sampling scenario of a moving person wearing a personal sampler. The aspiration efficiency acquired was compared with the ACGIH inhalable convention (CitationACGIH 1985), and the effects of the sampling orientation, wind speed, and aerosol size on the aspiration efficiency were investigated as well.
Materials and Methods
Test Samplers
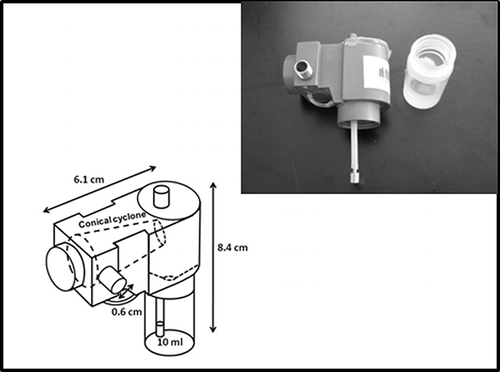
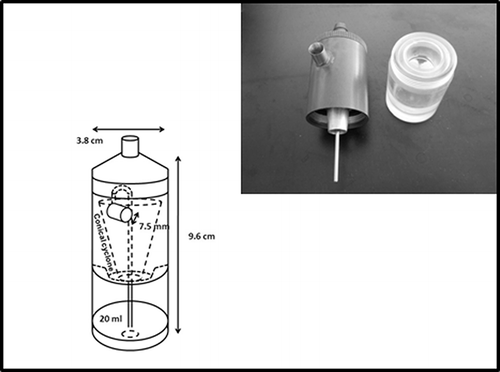
The two test personal bioaerosol samplers, PAS-4 () and PAS-5 (), were developed by the Research Center for Toxicology and Hygienic Regulation of Biopreparations (RCT & HRB) at Serpukchov, Russia. These two samplers were designed based on the rationale of a cyclone sampler incorporating a recirculating liquid film. The operation of a cyclone sampler with recirculating liquid is as follows: When the sampler is running, the high sampling air velocity will create a low-pressure region at the opening of the liquid ejector (located behind the inlet). This pressure drop will bring up the liquid from the liquid cartridge through a liquid feed tube. The liquid that has been brought up will flow out at the anterior section of the cyclone and then follow the swirling air in the cyclone toward the posterior section of the cyclone. The outflow liquid generates a liquid film on the inner surface of the cyclone. Bioaerosols aspirated into the sampler with the air will be gently captured by the liquid film in the cyclone due to the centrifugal force. Then the liquid film containing the bioaerosols will drain back into the liquid cartridge and continue to recirculate in the sampler unless the liquid completely evaporates.
The PAS-4 and PAS-5 are modified versions of their predecessor, PAS-2. The PAS-4 is 8.4 cm in height, 6.1 cm in width, and has its conical cyclone in a horizontal position (). The sampling inlet diameter of the PAS-4 is 0.6 cm (inward narrowing to 1.5 mm) and the capacity of its screw-top removable liquid cartridge is 10 ml, which can allow 115 min of continuous operation under 10 l/min sampling flow rate. The PAS-5 is 9.6 cm in height, 3.8 cm in width and has the conical cyclone oriented vertically in the sampler (). The PAS-5 has a 0.75 cm diameter inlet (also inward narrowing to 1.5 mm) and can be continuously operated for about 200 min with 13 ml deionized water in its 20-ml-capacity screw-top liquid cartridge under the sampling flow rate of 10 L/min. Details of the sampler dimensions and design parameters as well as the improvements and modifications made on these two newly developed personal bioaerosol samplers have been fully described in our recent publication (CitationTolchinsky et al., 2011).
Wind Tunnel
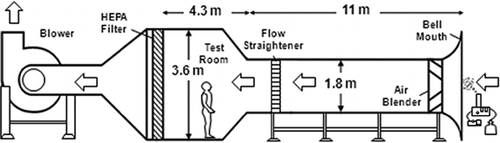
The scientific basis and criteria of evaluating personal aerosol samplers using a wind tunnel have been well established and have been widely applied in numerous occupational-health-related sampler tests (CitationAizenberg et al., 2000; CitationKennedy et al., 2001; CitationKenny et al., 1997; CitationMark and Vincent 1986). shows the schematic diagram of the Lovelace Respiratory Research Institute (LRRI) large wind tunnel facility employed in this research. This wind tunnel facility has been used in many sampler performance studies for different stationary and personal aerosol samplers (CitationCheng et al., 2004; CitationIrshad et al., 2006; CitationZhou and Cheng, 2010), and the results acquired have shown that this wind tunnel can provide reliable experimental data for general aerosol sampler evaluation studies. The LRRI large wind tunnel consists of an 11-m circular duct (1.8 m in diameter), an array of industrial gas mixing blenders (Blender Products, Inc. Denver, CO), a honeycomb flow straightener, a 4.3 m (L) × 3.7 m (W) × 3.6 m (H) test chamber, a outflow HEPA filter bank, and a 50-hp motor (Marathon Electric, Wausau, WI). The flow arrangement of the wind tunnel is an open-loop design so that the incoming air and exhausted air are drawn from and return to the same room. The wind tunnel can generate uniform wind speeds from 0.5 to 6.6 m/sec. The aerosol generation system is positioned outside the wind tunnel right next to the bell mouth air inlet. The generated aerosols first pass through the mixing blender to become well dispersed due to the large-scale turbulence created by the blender. Then, by traveling through the 11-m wind tunnel duct, the spatial uniformity of the air and aerosol mixture can reach an ideal status. The mixture then passes a honeycomb flow straightener to dampen the turbulence before entering the test chamber for experiments. The test chamber of the LRRI wind tunnel is large enough to place any type of stationary sampler or a full-size manikin wearing personal samplers for performance tests. At the back wall of the test chamber, a panel of 35 HEPA filters is mounted to ensure that particulate-free air is released back into the room. The blower used for this wind tunnel has a capacity of 1100 m3/min at 1.25 kPa static pressure (IAP, Inc., Phillips, WI). In this study, two wind speeds, 0.5 and 2.0 m/sec, were used to test the samplers. These wind speeds have been used in many wind tunnel studies for evaluating newly developed aerosol samplers (CitationAizenberg et al., 2000; CitationKennedy et al., 2001).
Test Aerosols
The particle diameter of the test aerosol ranged from 0.5 to 20 μm. This size range covers most of the common bacteria and fungal spores in the environment (CitationGorny et al., 1999; CitationHinds, 1999). Fluorescent polystyrene latex (PSL) microspheres and fluorescein-tagged oleic acid particles were utilized as the test aerosols. The PSL microspheres (Thermo Fisher Scientific, Inc., Waltham, MA) used were from 0.5 to 5 μm and were aerosolized by four medication nebulizers (Up-Mist, Hospitak Inc., Farmingdale, NY). The fluorescein-tagged oleic acid aerosols ranged from 10 to 20 μm, and were generated by a vibrating orifice aerosol generator (VOAG) (model 3050, TSI, Inc., Shoreview, MN). As mentioned earlier, the test monodisperse aerosols were released in front of the wind tunnel inlet. The aerosols were passed through a Kr-85 charge neutralizer to reach Boltzmann equilibrium before being introduced into the air stream. An aerodynamic particle sizer (model 3310A, TSI, Inc., Shoreview, MN) was used for measuring the size distribution of the aerosols generated to assure that the test aerosols were monodisperse.
Experimental Setup
The personal bioaerosol samplers were mounted on the chest of a full-size adult manikin placed inside the test chamber of the wind tunnel. Two isokinetic probes were placed side-by-side with the manikin. The isokinetic probes were positioned to the front left and front right at least 1 m away from the manikin (). The 47-mm cellulose filters (TISCH Environmental, Inc., Cleves, OH) were used by the isokinetic probes to collect reference aerosol concentrations in the test chamber. Together with the test samplers mounted on the manikin, a plastic Institute of Occupational Medicine (IOM) personal inhalable aerosol sampler (SKC, Inc., Eighty Four, PA) was used as a reference sampler and operated with a 2-L/min sampling flow rate for obtaining reference data for the purpose of data comparison. The IOM aerosol sampler is widely regarded as an appropriate reference sampler, which can provide reliable aspiration efficiency for sampling the inhalable particulate matter (IPM) fraction (CitationKennedy et al., 2001; CitationMark and Vincent, 1986). The outlet of the test personal bioaerosol sampler was installed with a filter holder having a 25-mm cellulose filter (TISCH Environmental, Inc., Cleves, OH) to collect aerosols that penetrate the entire sampler. All the personal samplers and the isokinetic probes were connected to vacuum pumps, and the flow rates designated to each sampler and probe were monitored by flow meters and rotameters. The flow rates to the two personal bioaerosol samplers were set at 10 L/min, and the flow rates of the isokinetic probes were adjusted to maintain the probe inlet air velocities matched to the wind velocity in the wind tunnel. For each test run, 8 ml of deionized water was added into the liquid cartridges of the test personal bioaerosol samplers as the collection liquid.
Experimental Procedure
In this study, three different sampling orientations with sampler inlet facing 0°, 90°, and 180° to the wind were tested to cover possible scenarios of using personal aerosol samplers (CitationAizenberg et al., 2000). All experimental conditions were repeated a minimum of three times, and each test run was set to be 30 min. At the end of each test run, the filters in the test personal bioaerosol samplers, the isokinetic probes, and the IOM sampler were removed and soaked in the prepared solution. The internal walls of the test samplers, the isokinetic probe, and the cassette top of the IOM sampler were also flushed by the solution to recover the wall deposits. The prepared solution in the experiments using PSL microspheres was 22 ml mixed isopropyl alcohol and ethyl acetate (1:1) used for extracting the fluorescent dye on the test aerosols. For experiments using fluorescein-tagged oleic acid aerosols, the prepared solution was 22 ml diluted isopropyl alcohol (50% water) used for dissolving the oleic acid droplets. All the relevant solutions were put in individual vials for at least 3 hr before being measured by a fluorometer (model 450, Sequoia-Turner Corp., Mountain View, CA) for obtaining the fluorescence intensity in the solutions. Different narrow band-pass (NB) and short-cut (SC) filters were used for the fluorometer depending upon the type of particles. For the PSL microspheres, the filters were NB 450 and SC500 filters. For the fluorescein-tagged oleic acid droplets, the filters used were NB 490 and SC515. In this way, the relative aerosol concentration (C) in each solution could be estimated by
The aspiration efficiency (A) is defined as the efficiency that aerosols in the ambient air are transported into the body of the sampler (CitationVincent 2007). The aspiration efficiency in this study can be calculated by
In this study, the collection efficiency (CE) is defined as the ratio of the aerosols remaining in the sampler (which includes those collected by the collection liquid and deposited on the inner wall of the sampler) to the total aerosols entering the sampler,
The capture efficiency (CP) is defined in this study as the ratio of the aerosols captured by the recirculating liquid to those entering the sampler. The capture efficiency indicates the only portion of the bioaerosols entering the sampler that is useful for the subsequent microbiological analysis. Thus, it is an essential factor to be evaluated especially for the two newly developed personal bioaerosol samplers. The capture efficiency can be calculated by
Results and Discussion
Aspiration Efficiency
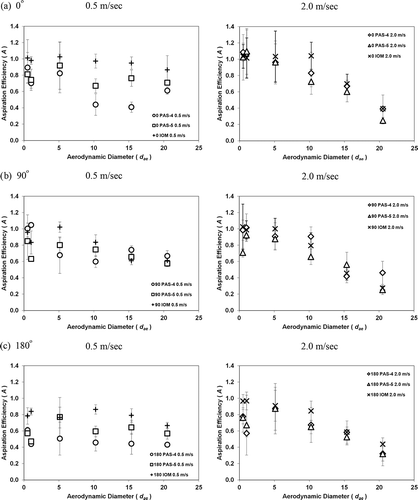
shows the experimental data of the aspiration efficiency (A) of PAS-4 and PAS-5 as a function of the aerodynamic diameter (dae ) of the test aerosols in different sampling orientations and wind speeds. Also shown in are the aspiration efficiencies of the reference sampler IOM. As can be seen, the aspiration efficiencies of PAS-4 and PAS-5 were lower than those of IOM in general for all sampling orientations and wind speeds used. The discrepancy seems prominent in the small aerosol size range from 0.5 to 5 μm. This result predicts that the two test samplers, PAS-4 and PAS-5, will perform with lower overall aspiration efficiency than IOM. In addition, in , it is worth noting that the aspiration efficiencies obtained by the two test samplers in different sampling orientations were not notably different (except the aspiration efficiencies of PAS-4 in a wind speed of 0.5 m/sec). No specific sampling orientation permitted higher aspiration efficiency than the other sampling orientations. This result implies that the sampling orientation has a very limited influence on the aspiration efficiency of these two personal bioaerosol samplers. The study conducted by Upton and his colleagues (CitationUpton et al., 1994), as well as the research conducted by CitationKar and Gautam (1995), obtained similar results when evaluating the performance of cyclone based samplers in the wind tunnel. In the study of Upton and his colleagues, the test sampler was also a wet-type cyclone bioaerosol sampler (Glass Cyclone Sampler, GCS) that used a sampling principle similar to that of the two test samplers in this study. The experimental results acquired demonstrated that the sampling efficiency (defined as being similar to the aspiration efficiency in this study) is independent of the sampler orientation. On the other hand, Kar and Gautam studied the aspiration efficiency of a 10-mm Dorr–Oliver cyclone sampler in a wind tunnel, and they found that the effect of the sampling orientation only caused very minor changes in aspiration efficiency in two out of the three wind speeds used in the study. These results found in the literature agree with what was shown in this study for cyclone-based samplers. Therefore, practically, when applying the test samplers PAS-4 and PAS-5 for real bioaerosol sampling in working environments, these two samplers are able to provide orientation-independent aspiration efficiency for bioaerosol sampling under investigated wind speeds. This feature will offer a more accurate personal exposure assessment, especially for those wearers who need to hold their position with respect to the wind direction for a long period of time. The exact reason for the generally low aspiration efficiency acquired by PAS-4 in low wind speed compared with the other sampling conditions is not immediately clear. Experimental errors were suspected but not verified. Numerical simulations might be needed to investigate the reason and mechanism causing that result.
Figure 5. The aspiration efficiency as a function of the aerodynamic diameter of the test aerosols in different sampling orientations with the sampler inlet facing (a) 0° against the wind, (b) 90° to the wind, and (c) 180° to the wind.
presents the orientation-averaged aspiration efficiency (A) as a function of the aerodynamic diameter (dae ) for the two test personal bioaerosol samplers in two wind speeds. The orientation-averaged aspiration efficiency was calculated by the orientation based aspiration efficiencies
Figure 6. The orientation-averaged aspiration efficiency as a function of the aerodynamic diameter for the two test personal bioaerosol samplers (PAS-4 and PAS-5) and the reference sampler (IOM): (a) U = 0.5 m/sec, and (b) U = 2.0 m/sec. Error bar represents the standard deviation of the mean.
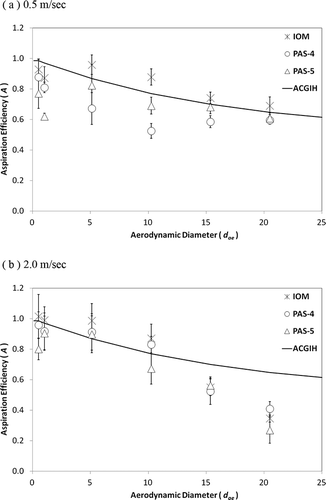
It is believed that the health-related ACGIH inhalable convention for inert aerosol sampling is also suitable to be applied to bioaerosol sampling. Many bioaerosols are considered hazardous once they are inhaled and deposited in the human respiratory tract, regardless of the location of deposition. Therefore, this particle size-selective inhalable convention can be utilized as the criterion for evaluating the aspiration efficiencies of the two test samplers. As can be seen in , the orientation-averaged aspiration efficiencies of PAS-4 and PAS-5 have patterns similar to the IOM sampler. The aspiration efficiency decreased as the aerodynamic diameters of the aerosols increase. For the wind speed of 0.5 m/sec, the aspiration efficiencies of PAS-4 and PAS-5 both slightly underestimated the inhalable convention and were generally lower than that of the IOM sampler (). On the other hand, for the wind speed of 2.0 m/sec (), the aspiration efficiencies of both test samplers closely agreed with the inhalable convention within the size range of the test aerosols, and were comparable to the results of the IOM. Although shows that the aspiration efficiency of PAS-4 is slightly lower than that of PAS-5, and shows an opposite situation, the aspiration efficiencies between PAS-4 and PAS-5 were not found to be statistically different at p < 0.05 using Student's t-test. In general, it would be fair to say that both the PAS-4 and PAS-5 are able to meet the ACGIH criterion in the size range from 0.5 to 20 μm. Therefore, it can be concluded that the performance of these two newly developed personal bioaerosol samplers in terms of the aspiration efficiency is considered to be satisfactory.
Collection Efficiency
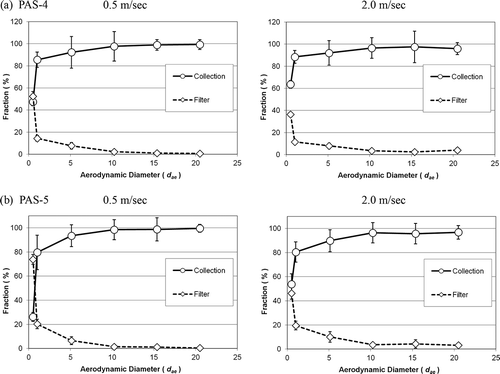
shows the orientation-averaged collection efficiency (CE) for the two test samplers. The collection efficiency presented was obtained by similar orientation-averaged calculation as shown in Equationeq (5),
Figure 7. The orientation-averaged collection efficiency of the two test bioaerosol samplers at different wind speeds: (a) PAS-4 and (b) PAS-5. Error bar represents the standard deviation of the mean.
Capture Efficiency
shows the fractions of the test aerosols that entered the two personal bioaerosol samplers and were then recovered from the remaining collection liquid, inner wall flushing, and the backup filter. Attention should be focused on the fraction of aerosols in the collection liquid because it is the capture efficiency of the test sampler and, as pointed out earlier, it is the only portion of the collected bioaerosols that can be used for further microbiological analysis. The capture efficiency (CP) shown in is also obtained from orientation-averaged calculation
Figure 8. The orientation-averaged capture efficiency, wall loss, and filter collection of the test personal bioaerosol samplers at different wind speeds: (a) PAS-4 and (b) PAS-5. (Errors represent the standard deviation of the mean.)
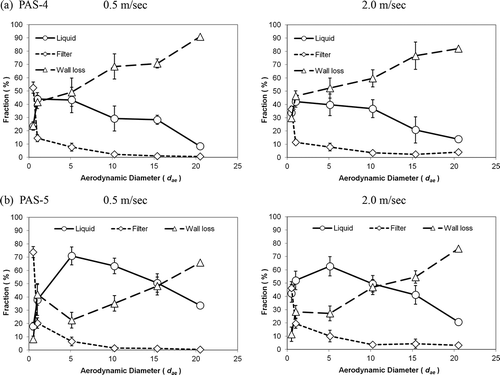
Based on all the performance evaluations shown here with respect to the physical sampling efficiencies, it can be concluded that PAS-4 and PAS-5 have similar aspiration efficiencies and collection efficiencies, but PAS-5 presents relatively higher capture efficiency and a considerably lower wall loss than PAS-4. This shows that PAS-5 is a superior personal bioaerosol sampler to PAS-4 because it allows more bioaerosols to be captured in the collection liquid, which will greatly benefit the consequent microbiological analysis to provide representative collected samples.
Conclusions
This study carried out a series of wind tunnel tests to evaluate the performance of two newly developed personal bioaerosol samplers (PAS-4 and PAS-5) designed based on a cyclone incorporating a recirculating liquid film. The tests were focused on the physical efficiency that a bioaerosol sampler could provide to the bioaerosol sampling, which includes the aspiration efficiency, collection efficiency, and capture efficiency. The results of the performance evaluation showed that the aspiration efficiency obtained from these two samplers closely agreed with the ACGIH inhalable convention within the size range of the test aerosols. The collection efficiency acquired from the two samplers showed that the d50 for both was around 0.6 μm. The wall loss of PAS-4 was considerably higher than that of PAS-5, especially for the aerosol size larger than 5 μm, which caused PAS-4 to have a relatively lower capture efficiency than PAS-5. Therefore, in general, PAS-5 is considered a superior personal bioaerosol sampler in terms of physical efficiencies to PAS-4. The experimental data acquired from this study completed the physical efficiency assessment of the two newly developed personal bioaerosol samplers and also demonstrated the essential characteristics that the two samplers possess. Future studies will concentrate on further modifying the PAS-5 and will conduct associated evaluation tests in a small wind tunnel using model bioaerosols (BG or MS2) to fully evaluate its ability to perform bioaerosol sampling.
Acknowledgments
The authors are grateful to J. Sun for technical assistance, and to S. Shinnick for reviewing this paper. This project is sponsored by NIOSH grant R01 OH008913 and R01 OH009801.
References
- ACGIH . 1985 . Particle Size-Selective Sampling in the Workplace. Report of the ACGIH Technical Committee on Air Sampling Procedures , Cincinnati , OH : American Conference of Governmental Industrial Hygienists .
- Aizenberg , V. , Grinshpun , S.A. , Willeke , K. , Smith , J. and Baron , P.A. 2000 . Performance characteristics of the button personal inhalable aerosol sampler . Am. Ind. Hyg. Assoc. J. , 61 : 398 – 404 .
- Chen , B.T. , Feather , G.A. , Maynard , A. and Rao , C.Y. 2004 . Development of a personal sampler for collecting fungal spores . Aerosol Sci. Technol. , 38 : 926 – 937 .
- Cheng , Y.S. , Irshad , H. , McFarland , A.R. , Su , W.C. , Zhou , Y. and Barringer , D. 2004 . An aerosol wind tunnel for evaluation of massive-flow air samplers and calibration of Snow White sampler . Aerosol Sci. Technol. , 38 : 1099 – 1107 .
- Fakhri , Z.I. 1998 . “ Biological hazards ” . In Encyclopedia of Occupational Health and Safety , 4th , Edited by: Stellman , J. M. 38.2 Geneva : International Labour Office .
- Gorner , P. , Fabries , J.F. , Duquenne , P. , Witschger , O. and Wrobel , R. 2006 . Bioaerosol sampling by a personal rotation cup sampler CIP 10-M . J. Environ. Monit. , 8 : 43 – 48 .
- Gorny , R.L. , Dutkiewicz , J. and Krysinska-Traczyk , E. 1999 . Size distribution of bacterial and fungal bioaerosols in indoor air . Ann. Agric. Environ. Med. , 6 : 105 – 113 .
- Grinshpun , S.A. , Chang , C.W. , Nevalainen , A. and Willeke , K. 1994 . Inlet characteristics of bioaerosol samplers . J. Aerosol Sci. , 25 ( 8 ) : 1503 – 1522 .
- Hinds , W.C. 1999 . Aerosol technology: Properties, behavior, and measurement of airborne particles , New York , NY : John Wiley & Sons .
- Hunter , P.R. 1992 . Occupational infections of healthcare workers. Part II: Herpesviruses, influenza, and miscellaneous viral infections . Microbiol. Eur. , 1 ( 2 ) : 14 – 17 .
- Irshad , H. , Su , W.C. , Cheng , Y.S. and Medici , F. 2006 . Testing of high-volume sampler inlets for the sampling of atmospheric radionuclides . Health Phys , 91 ( 3 ) : 188 – 199 .
- Jensen , P.A. , Todd , W.F. , Davis , G.N. and Scarpino , P.V. 1992 . Evaluation of eight bioaerosol samplers challenged with aerosols of free bacteria . Am. Ind. Hyg. Assoc. J. , 53 ( 10 ) : 660 – 667 .
- Kar , K. and Gautam , M. 1995 . Orientation bias of the isolated 10-mm nylon cyclone at low stream velocity . Am. Ind. Hyg. Assoc. J. , 56 ( 11 ) : 1090 – 1098 .
- Kennedy , N.J. , Tatyan , K. and Hinds , W.C. 2001 . Comparison of a simplified and full-size mannequin for the evaluation of inhalable sampler performance . Aerosol Sci. Technol. , 35 : 564 – 568 .
- Kenny , L.C. , Aitken , R. , Chalmers , C. , Fabries , J.F. , Gonzalez-Fernandez , E. , Kromhout , H. , Liden , G. , Mark , D. , Riediger , G. and Prodi , V. 1997 . A collaborative European study of personal inhalable aerosol sampler performance . Ann. Occup. Hyg. , 41 ( 2 ) : 135 – 153 .
- Kotimaa , M. 1990 . Occupational exposure to spores in the handling of wood chips . Grana , 29 : 153 – 156 .
- Lacey , J. and Dutkiewicz , J. 1994 . Bioaerosol and occupational lung disease . J. Aerosol. Sci. , 25 : 1371 – 1404 .
- Lin , X. , Reponen , T.A. , Willeke , K. , Grinshpun , S.A. , Foarde , K.K. and Ensor , D.S. 1999 . Long-term sampling of airborn bacteria and fungi into a non-evaporating liquid . Atmos. Environ. , 33 : 4291 – 4298 .
- Lin , X. , Reponen , T. , Willeke , K. , Wang , Z. , Grinshpun , S.A. and Trunov , M. 2000 . Survival of airborn microorganisms during swirling aerosol collection . Aerosol Sci. Technol. , 32 : 184 – 196 .
- Lindsley , W.G. , Schmechel , D. and Chen , B.T. 2006 . A two-stage cyclone using microcentrifuge tubes for personal bioaerosol sampling . J. Environ. Monit. , 8 : 1136 – 1142 .
- Mark , D. and Vincent , J.H. 1986 . A new personal sampler for airborne total dust in workplaces . Ann. Occup. Hyg. , 30 ( 1 ) : 89 – 102 .
- Meklin , T. , Reponen , T. , Toivola , M. , Koponen , V. , Husman , T. , Hyvarinen , A. and Nevalainen , A. 2002 . Size distributions of airborne microbes in moisture-damaged and reference school buildings of two construction types . Atmos. Environ. , 36 : 6031 – 6039 .
- Olenchock , S.A. 1988 . Quantitation of airborne endotoxin levels in various occupational environments . Scand. J. Work Envir. Health , 14 ( S ) : 72 – 73 .
- Reponen , T. , Hyvarinen , A. , Ruuskanen , J. , Raunemaa , T. and Nevalainen , A. 1994 . Comparison of concentrations and size distributions of fungal spores in buildings with and without mould problems . J. Aerosol Sci. , 25 ( 8 ) : 1595 – 1603 .
- Sigaev , G.I. , Tolchinsky , A.D. , Sigaev , V.I. , Soloviev , K.G. , Varfolomeev , A.N. and Chen , B.T. 2006 . Development of a cyclone-based aerosol sampler with re-circulating liquid film: Theory and experiment . Aerosol Sci. Technol. , 40 : 293 – 308 .
- Tolchinsky , A.D. , Sigaev , V.I. , Sigaev , G.I. , Varfolomeev , A.N. , Zvyagina , E.V. , Brasel , T. and Cheng , Y.S. 2010 . Development of a personal bioaerosol sampler based on a conical cyclone with re-circulating liquid film . J. Occup. Environ. Hyg. , 7 ( 3 ) : 156 – 162 .
- Tolchinsky , A.D. , Sigaev , V.I. , Varfolomeev , A.N. , Uspenskaya , S.N. , Cheng , Y.S. and Su , W.C. 2011 . Performance evaluation of two personal bioaerosol samplers . J. Environ. Sci. Health Part A , 46 : 1690 – 1698 .
- Upton , S.L. , Mark , D. , Douglass , E.J. , Hall , D.J. and Griffiths , W.D. 1994 . A wind tunnel evaluation of the physical sampling efficiencies of three bioaerosol samplers . J. Aerosol Sci. , 25 ( 8 ) : 1493 – 1501 .
- Verreault , D. , Gendron , L. , Rousseau , G.M. , Veillette , M. , Massé , D. , Lindsley , W.G. , Moineau , S. and Duchaine , C. 2011 . Detection of airborne lactococcal bacteriophages in cheese manufacturing plants . Appl. Environ. Microbiol. , 77 ( 2 ) : 491 – 497 .
- Vincent , J.H. 2007 . Aerosol Sampling: Science, Standards, Instrumentation and Applications , West Sussex , , UK : John Wiley & Son Ltd .
- Willeke , K. , Lin , X. and Grinshpun , S.A. 1998 . Improved aerosol collection by combined impaction and centrifugal motion . Aerosol Sci. Technol. , 28 : 439 – 456 .
- Zhou , Y. and Cheng , Y.S. 2010 . Evaluation of IOM personal sampler at different flow rates . J. Occup. Environ. Hyg. , 7 ( 2 ) : 88 – 93 .