ABSTRACT
We have hypothesized that adding dexmedetomidine to bupivacaine in bilateral ultrasound-guided suprazygomatic maxillary nerve block (SMB) would provide prolonged postoperative analgesia following cleft palate (CP) repair. Children posted for CP repair were randomized to receive bilateral ultrasound-guided SMB, with either 0.15 ml/kg 0.25% bupivacaine on each side (B group; n = 40) or 0.5 µg/kg dexmedetomidine plus 0.15 ml/kg 0.25% bupivacaine on each side (BD group; n = 40). Children and Infants Postoperative Pain Scale (CHIPPS) was the primary outcome, the number of children required analgesia, the first time of rescue analgesia, the total nalbuphine consumption, sedation score, and time to feed were recorded. CHIPPS score was comparable between the two groups in the first 6 hours. However, it was significantly less in BD group than B group at 8 h, 12 h, 16 h, 20 h and 24 h postoperatively as well as the number of children required analgesia (7 vs. 26, respectively; P < 0.001) with delay of the first analgesic request (23.6 vs. 14.9 h) and reduced the total nalbuphine consumption (0.3 ± 0.7 vs. 2.1 ± 1.8 mg). Sedation score was higher in group BD at Postoperative Anesthesia Care Unit (P = 0.037). Time to feed was similar between both groups (P = 0.376). In bilateral SMB, the use of 0.5 µg/kg dexmedetomidine as an adjunct to bupivacaine 0.25% was associated with prolonged postoperative analgesia and decreased total analgesic consumption in children assigned for surgical correction of CP.
1. Introduction
Cleft palate (CP) is one of the congenital craniofacial malformations that necessitates an early surgical intervention within the first months of life [Citation1,Citation2]. The aim of surgical procedure is to improve phonation, minimize feeding problems, and reduce complications such as sinusitis and respiratory tract infections [Citation3].
As the surgical correction of CP is a painful procedure, a high dose of intravenous (IV) opioids is highly required which increases the risk of respiratory depression and postoperative airway obstruction [Citation4].
Sensory innervation of the hard and soft palates, upper jaw, upper dental arch, and upper lip is derived from maxillary nerve [Citation5,Citation6]. The suprazygomatic approach of maxillary nerve block is safer than the infrazygomatic one (which has a risk of vascular injury), easy to perform, and provides perioperative analgesia during CP repair [Citation5]. The ultrasound guidance contributed to identify well the neural structures and vascular anatomy, and decrease puncture complications. Moreover, visualize the injected local anesthetics helps prevent its spread out of the pterygopalatine fossa, thus increasing the success rate of the block [Citation7,Citation8].
Dexmedetomidine is a highly selective α2-adrenoceptor agonist. It has sedative and analgesic sparing effects, and reduces anesthetic requirements [Citation9]. The addition of dexmedetomidine to the local anesthetics (LAs) enhances peripheral nerve block, with prolonged anesthesia as well as postoperative analgesia [Citation10].
This study hypothesized that adding dexmedetomidine 0.5 µg/kg to bupivacaine 0.25% in bilateral suprazygomatic maxillary nerve block (SMB) for children undergoing cleft palate repair would prolong the duration of postoperative analgesia and decrease the total analgesic consumption.
2. Materials and methods
Following the approval from the Ethical Committee in Faculty of Medicine, Tanta University (approval number 30914/05/16), registration of the study in the Pan African Clinical Trials Registry was done (PACTR201703002080167). Informed written consent was obtained from the parents or guardians of each child. They were provided with an explanation of the techniques of anesthesia and analgesia. Eighty children aged 6 months to 5 years scheduled for primary cleft palate repair, ASA I–II of both genders were selected and included in this randomized prospective controlled study. The study was conducted between March 2017 to March 2020 in the plastic surgery department.
Exclusion criteria included parent refusal, bleeding disorder, infection at the injection site, allergy to the local anesthetics, failure to perform the SMB, and associated airway anomalies.
Children were randomized into two groups of 40 children each using the computer-generated randomization numbers to assign the participants to each group in the study. Bilateral ultrasound-guided SMB was performed for all children. Group B received 0.15 ml/kg 0.25% bupivacaine on each side (maximum volume 4 ml), whereas group BD received 0.5 µg/kg dexmedetomidine (Precedex®; Hospira Inc., Lake Forest Illinois, USA) plus 0.15 ml/kg 0.25% bupivacaine on each side (maximum volume 4 ml). The drug used for SMB was prepared by an anesthesiologist not involved in the block performance who opened a randomly sealed envelope. The anesthesiologist included in the performance of the SMB, children’s parents and the surgeon were unaware of the solution given.
Premedication was given to all children with 0.5 mg/kg of Midazolam orally 30–60 min before the operation. The two groups were continuously monitored using the pulse oximetry, end-tidal CO2, electrocardiography, non-invasive blood pressure, and non-invasive temperature probe.
Induction of general anesthesia was standardized to all children either by inhalation of sevoflurane 4–6% in 100% oxygen by face mask followed by insertion of IV cannula and administration of 1 µg/kg of fentanyl in children without venous access or with injection of 2 mg/kg of propofol and 1 µg/kg of fentanyl in children with venous access. Atracurium besilate 0.5 mg/kg was administered to enable orotracheal intubation of the proper size of the performed tube, secured to the middle of the lower lip.
Anesthesia was maintained with 2% of sevoflurane in a 50% oxygen/air mixture and the ventilation was controlled mechanically to keep the end tidal-CO2 reading between 30 and 35 mmHg. Ringer’s lactate solution was infused according to the calculated rate and volume. 0.3 mg/kg of dexamethasone (maximum 8 mg) was injected intravenously for the local edema. The child was positioned supine with a neutral head.
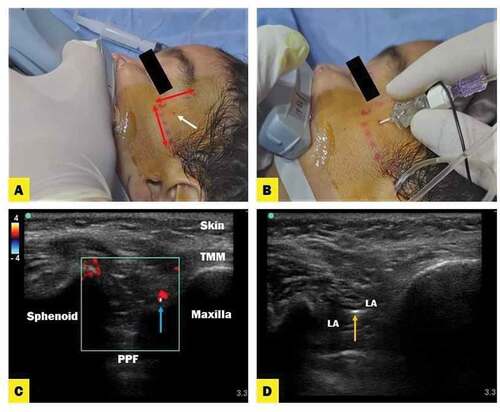
3. Ultrasound-Guided SMB Technique: ()
Before starting the surgery, the bilateral ultrasound-guided SMB was performed under complete aseptic preparation of the skin according to the previously demarcated landmarks [Citation8] using a 25-Gauge 50-mm Sprote insulated needle (Nanoline; Pajunk, Geisingen, Germany). Ultrasound images were obtained using a high-frequency 8–13 MHz linear transducer (SonoScape SSI 6600, China) which was placed in the infrazygomatic area over the maxilla. Using out-of-plan approach, the needle was inserted perpendicular to the skin. The puncture site was located at the frontozygomatic angle formed by the superior edge of the zygomatic arch below, the posterior orbital rim forward and advanced about 20 mm deep to reach the greater wing of sphenoid using color Doppler to localize the neurovascular bundle. After that, the reorientation and advancement of the needle 35–45 mm deep to the pterygopalatine fossa were implemented. The depth and direction of the needle were implemented regardless of the patient’s age [Citation7]. LA with or without dexmedetomidine was injected after a negative aspiration test for blood per side and 15 minutes later, the surgical incision was started.
Figure 1. (A) Anatomical landmark of suprazygomatic maxillary nerve block. (B) The entry point of the needle is located at the frontozygomatic angle bounded by superior edge of the zygomatic arch below and posterior orbital edge forward. (C) Ultrasound images describe suprazygomatic maxillary nerve block; anatomy of PPF (pterygopalatine fossa) and TMM (tempromaxillary muscle) with guidance of Doppler flow (red color) to localize neurovascular bundle. (D) Local anesthetic (LA) spread in PPF
Local infiltration with epinephrine (1/200,000 in 0.9% normal saline) without the local anesthetic was injected to reduce the surgical bleeding.
Fentanyl 1 µg/kg was injected intravenously when baseline heart rate (HR) and/or systolic blood pressure increased by more than 20% during the intraoperative period. Atropine or ephedrine was given if there was bradycardia or hypotension (defined as HR less than 90 beats/min or decreasing systolic blood pressure by more than 20% compared to the preoperative values), respectively. Paracetamol solution (Perfalgan) 15 mg/kg IV was given before the end of the surgery to all patients and repeated every 6 hours. After injection of (atropine 0.02 mg/kg and neostigmine 0.05 mg/kg) and getting adequate signs of recovery extubation was done and patient was transferred to Post Anesthesia Care Unit (PACU).
Evaluation of postoperative pain was done with Children and Infants Postoperative Pain Scale (CHIPPS) score [Citation11] on admission to PACU, at 2, 4, 6, 8, 12, 16, 20, and 24 h postoperative on the pediatric ward which range from 0 (no pain) to 10 (severe pain). Nalbuphine 0.2 mg/kg was given to the patients as rescue analgesia if the CHIPPS score was ≥ 4/10. The first time of rescue analgesia, the number of children required analgesia, and the total doses of nalbuphine within 24 h were recorded. Sedation was assessed postoperatively by sedation score [Citation12] at PACU, 2, and 4 h. Time to feed after tracheal extubation was recorded (it is the time taken for child to tolerate milk feeds after the initial trial of water). Any related complications such as hypotension, bradycardia, postoperative nausea, vomiting, bleeding at the puncture site, local anesthetic toxicity, and ocular lesions were recorded. The assessment and data collection were performed by an anesthesiologist blinded to the group allocation.
Postoperative pain score (CHIPPS) was the primary outcome. The secondary outcomes were the first time requested analgesia, the number of children required analgesia, the total amount of analgesic requirements during first the 24 h, and the time to feed after tracheal extubation.
4. Statistical Analysis
Based on the results of our pilot study, the postoperative pain score in the first 24 h was 4.51 ± 2.74 in bupivacaine group and 2.44 ± 1.78 in the bupivacaine – dexmedetomidine group. Sample size was calculated to be 34 patients needed in each group to detect a statistically significant difference in the postoperative pain scores between both groups at α error of 0.05 and a power of study of 95%. We planned to enroll 40 cases per group to overcome possible dropouts. The sample size was calculated using G٭ Power 3 analysis program (Heinrich Heine University Düsseldorf, Düsseldorf, Germany).
The statistical software IBM SPSS 20.0 (IBM Corp., Armonk, NY) was used for the statistical analysis. Normal distribution of variables was checked with the Kolmogorov–Smirnov or Shapiro–Wilk tests. Numerical data were presented as mean or median (interquartile range) and compared using Student’s independent t-test for data showing normal distribution or Mann–Whitney U test, if otherwise. Categorical variables were presented as patients’ numbers and percentages (%) and were analyzed using the chi-square test or Fisher’s Exact test when appropriate. Kaplan–Meier survival analysis was used to detect the time to the first rescue analgesia. P-values <0.05 were considered significant.
5. Results
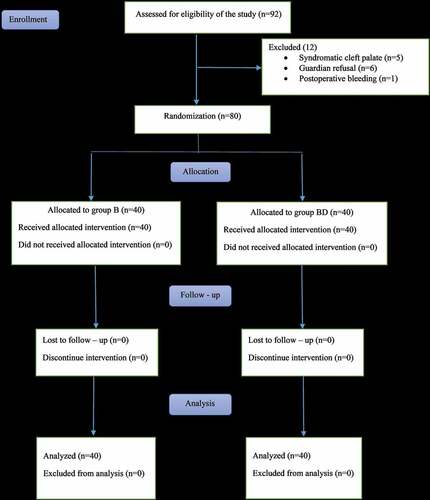
Eighty children were enrolled in this randomized, double-blinded study out of 92 eligible children () assigned for CP repair. Both groups showed no significant difference regarding demographic data and duration of the surgery ().
Table 1. Demographic characteristics and duration of surgery of both studied groups
Figure 2. CONSORT flow diagram of the participants through each stage of the randomized trial. Group B = bupivacaine only; group BD = bupivacaine plus dexmedetomidine
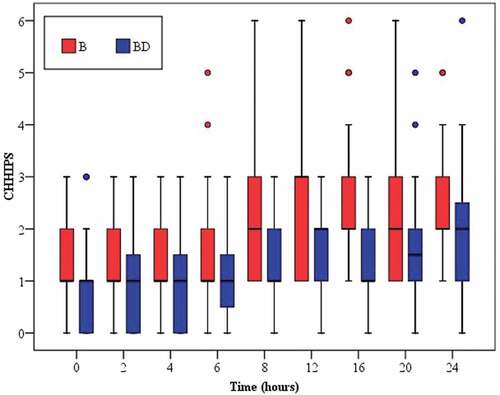
The success rate of the block was adequate in both groups. Regarding the median pain scores (CHIPPS), there were no significant differences between both groups at PACU, 2, 4, and 6 hours postoperatively. However, BD group produced more significant reduction of postoperative pain than B alone group at 8, 12, 16, 20, and 24 hours ().
Table 2. Perioperative rescue of analgesia and time to feed of both studied group
Figure 3. Box plots of CHHIPS score during first 24 h postoperatively in the two studied groups. Group B = bupivacaine only; group BD = bupivacaine plus dexmedetomidine
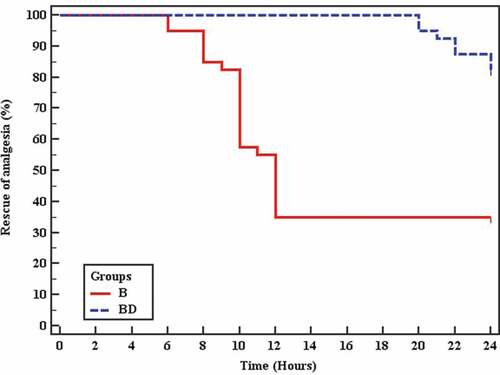
The first request for analgesia was delayed in group BD with a mean time of 23.6 h (range 20–24) in comparison with group B having a mean time of 14.9 h (range 6–12) (P = < 0.001 by log-rank test) ().
Figure 4. Kaplan-Meier survival curve represents the first time of rescue analgesia in the two studied groups. Group B = bupivacaine only; group BD = bupivacaine plus dexmedetomidine
Twenty-six children (65%) in group B required nalbuphine (with a mean 2.1 ± 1.8 mg) throughout the 24 h postoperatively, while only seven children (17.5%) in group BD required nalbuphine (with a mean 0.3 ± 0.7 mg) (P = < 0.001) ().
There is no statistically significant difference between both groups regarding intraoperative fentanyl requirements (P = 0.490). Feeding was started early with a median of 4 h postoperatively (P = 0.376).
The sedation score was significantly higher in group BD than group B (P = 0.037) at PACU with no significant difference at 2 and 4 h postoperatively (P = 0.595, 0.079, respectively,) ().
Table 3. Sedation and complications of both studied group
In group B, three children reported PONV and one child in group BD (P = 0.615). Besides, 8 children developed bradycardia in group BD and 3 children in group B (P = 0.105). Two children developed hypotension in group BD (P = 0.494) ().
No serious complications were reported in both groups such groups as hematoma, infection, or ocular lesion.
6. Discussion
The current study aimed to determine the postoperative analgesic effects of adding dexmedetomidine to bupivacaine in SMB with the aid of ultrasound guidance for CP repair. Our main finding demonstrate that the CHIPPS score was significantly low in children who received dexmedetomidine as an adjunct in SMB that extended up to 24 h. Also, the first time for supplementary analgesia postoperatively was longer in BD group than group B (23.6 versus 14.9 h) with reduced amount of nalbuphine consumption throughout the 24 h postoperatively in BD group compared to B group (0.3 ± 0.7 versus 2.1 ± 1.8). Furthermore, less number of children received rescue analgesia in BD group (17.5%) than B group (65%). The sedation score was significantly higher in dexmedetomidine group at PACU.
Different modalities of analgesia have been used to relieve postoperative pain especially during the first 24 hours after the surgical correction of CP. Opioids are one of the commonly used analgesics. However, they are associated with undesirable side effects such as respiratory depression, nausea, and vomiting [Citation13,Citation14]. Besides, respiratory obstruction and hypoxic episodes those related to decrease in the infant airway flow due to the CP repair [Citation15]. Obstructive sleep apnea-related respiratory symptoms was reported by Prado et al. [Citation16] in children after CP repair.
Pediatric regional anesthesia is being used increasingly as a part of the anesthetic approach combined with general anesthesia to decrease the intraoperative anesthetic requirement with rapid recovery; at the same time, provides postoperative analgesia [Citation17].
Dexmedetomidine is an α2 – adrenoceptor agonist that is 8 times more selective than clonidine [Citation9,Citation18]. The action on the peripheral nerve was found to be likely mediated through the blockade of the hyperpolarization-activated cation current (Ih current), not because of the agonism of the α2-adrenoceptor [Citation19]. Kosugi et al. suggested the delay in the absorption of local anesthetic and/or inhibition of nerve conduction by the vasoconstrictive effect of α2 agonist [Citation20]. It enhances the action in a dose-dependent manner. However, its effectiveness in pediatric patients has not been evaluated and limited to its caudal or intravenous usage [Citation9].
Several previous studies have investigated the effectiveness of SMB in pain control after the cleft palate surgery. Mesnil et al. [Citation21] showed in their prospective study that SMB is a safe and efficient technique with a significant reduction of intra and postoperative opioid consumption. Also, Sole et al. [Citation7] described the performance of ultrasound-guided SMB. They reported low pain scores postoperatively. In addition, Chiono et al. [Citation22] reported a 50% reduction in morphine consumption in their study comparing bilateral SMB with placebo.
Echaniz et al. [Citation3] in their study showed a significant reduction in the intraoperative opioid requirements in SMB compared to infraorbital and palatine nerve block during the cleft surgery with no difference in the postoperative opioid consumption.
Concerning the pediatric use of dexmedetomidine, our results are in agreement with a study conducted by Mostafa et al. [Citation23] who investigated the efficacy of adding dexmedetomidine 0.5 µg/kg to bupivacaine 0.125% in SMB for children undergoing a cleft palate repair. They reported low Children’s Hospital of Eastern Ontario Pain Scale (CHEOPS) scores and no rescue analgesia was given during the first 24 hours postoperatively in the dexmedetomidine group, although they performed SMB blindly. This was different from our study as we used an ultrasound-guided technique that increased the accuracy of the block and therefore the analgesic effect.
Furthermore, Obayah et al. [Citation24] in their study added 1 µg/kg of dexmedetomidine to bupivacaine 0.25% during greater palatine nerve block for children scheduled for CP repair. They reported a low FLACC (Face, Legs, Activity, Cry, Consolability) pain score and extended postoperative analgesia up to 22 h in dexmedetomidine group.
Consistent with our results, Vorobeichik et al. [Citation25] conducted an adult meta-analysis study on the efficacy of perineural dexmedetomidine as an adjunct to LA during brachial plexus block that has been found to prolong the duration of sensory and motor block and reduce postoperative pain score and total analgesic consumption at 24 h. Other studies performed on adults investigated the efficacy of adding dexmedetomidine to LAs in the peripheral nerve block [Citation10,Citation18,Citation26–29] as well as the neuroaxial block [Citation30]. They all concluded that addition of dexmedetomidine provides a better quality of anesthesia and prolongs the duration of postoperative analgesia.
Intraoperative fentanyl requirements were not different between both groups. It may be attributed to pain from temporomandibular joints due to mouth spacers that are not blocked by SMB.
The sedative effect of dexmedetomidine might be explained by central mediated α2-agonist effects due to systemic absorption after perineural administration causing sedation and analgesia. The inhibition of substance P release at the level of dorsal horn root neuron in the nociceptive pathway and activation of α2-agonist in the locus coeruleus were the possible mechanism of actions [Citation18]. Almarakbi and kaki [Citation31] agreed with our study as regards the sedative effect of dexmedetomidine in the first hour postoperatively when added to bupivacaine in the transversus abdominis plane block. But Karan et al. [Citation32] disagreed with our result as they showed no significant difference.
No difference between both groups was recorded regarding hypotension or bradycardia, which can be explained by the administration of a low dose of dexmedetomidine [Citation32,Citation33].
The resumption of feeding was started early within 4 hours in both groups and this is possibly explained by the postoperative analgesia provided by SMB. This is very important as infants and young children cannot tolerate long periods of fasting. Gunawardana and Ratnayaka [Citation34] suggested that the early resumption of milk feeding may have a calming through the endogenous opioid system.
Limitation in this study that we used only a single small dose of dexmedetomidine [Citation27,Citation31] as an adjuvants to LAs, so further studies are required to study the dose–response effects and determine the optimum dose. Also, the small single-center study and therefore more studies need to be performed.
7. Conclusion
In bilateral Suprazygomatic Maxillary Nerve block, the use of 0.5 µg/kg dexmedetomidine as an adjunct to bupivacaine 0.25% has been shown to be associated with prolonged postoperative analgesia up to 24 h and decrease the total analgesic consumption in the children assigned for the surgical correction of cleft palate.
Disclosure statement
The authors declare no competing interests.
References
- Takemura H, Yasumoto K, Toi T, et al. Correlation of cleft type with incidence of perioperative respiratory complications in infants with cleft lip and palate. Paediatr Anaesth. 2002;12(7):585–588.
- Prigge L, van Schoor AN, Bosman MC, et al. Clinical anatomy of the maxillary nerve block in pediatric patients. Paediatr Anaesth. 2014;24(11):1120–1126.
- Echaniz G, De Miguel M, Merritt G. et al. Bilateral suprazygomatic maxillary nerve blocks vs infraorbital and palatine nerve blocks in cleft lip and palate repair: a double-blind, randomized study. Eur J Anaesthiol. 2019;36(1):40–47.
- Abu Elyazed MM, Mostafa SF. Bilateral suprazygomatic maxillary nerve block versus palatal block for cleft palate repair in children: a randomized controlled trial. Egypt J Anaesth. 2018;34(3):83–88.
- Mostafa MF, Herdan R, Elshazly M. Comparative study of levobupivacaine and bupivacaine for bilateral maxillary nerve block during pediatric primary cleft palate surgery: a randomized double-blind controlled study. Korean Journal of Anesthesiology. 2018;71(2):135–140.
- Singh B. Anatomic considerations in relation to the maxillary nerve block. Reg Anesth Pain Med. 2001;26(6):507–511.
- Sola C, Raux O, Savath L, et al. Ultrasound guidance characteristics and efficiency of suprazygomatic maxillary nerve blocks in infants: a descriptive prospective study. Paediatr Anaesth. 2012;22(9):841–846.
- Captier G, Dadure C, Leboucq N, et al. Anatomic study using three-dimensional computed tomographic scan measurement for truncal maxillary nerve blocks via the suprazygomatic route in infants. J Craniofac Surg. 2009;20(1):224–228.
- Mahmoud M, Mason KP. Dexmedetomidine: review, update, and future considerations of paediatric perioperative and periprocedural applications and limitations. Br J Anaesth. 2015;115(2):171–182.
- Hussain N, Grzywacz VP, Ferreri CA, et al. Investigating the efficacy of dexmedetomidine as an adjuvant to local anesthesia in brachial plexus block: a systematic review and meta-analysis of 18 randomized controlled trials. Reg Anesth Pain Med. 2017;42(2):184–196.
- Bütter W, Finke W. Analysis of behavioural and physiological parameters for the assessment of postoperative analgesic demand in newborns, infants and young children: a comprehensive report on seven consecutive studies. Paediatr Anaesth. 2000;10(3):303–318.
- Culebras X, Van Gessel E, Hoffmeyer P, et al. Clonidine combined with a long acting local anesthetic does not prolong postoperative analgesia after brachial plexus block but does induce hemodynamic changes. Anesth Analg. 2001;92(1):199–204.
- Choi SH, Lee WK, Lee SJ, et al. Parent-controlled analgesia in children undergoing cleft palate repair. J Korean Med Sci. 2008;23(1):122–125.
- Michael B, Azza E, Karim G, et al. Comparative Study of Bilateral Greater Palatine Nerve Block and Bilateral Suprazygomatic Maxillary Nerve Block for Intraoperative Analgesia in Children Undergoing Palatoplasty. Med J Cairo Univ 2016;84:257–261.
- Kulkarni KR, Patil MR, Shirke AM, et al. Perioperative respiratory complications in cleft lip and palate repairs: an audit of 1000 cases under ′Smile Train Project′. Indian Journal of Anaesthesia. 2013;57(6):562–568.
- Prado PC. de Bragança Lopes Fernandes M, dos Santos Trettene A, Graziela Noronha Silva Salgueiro A, Kiemle Trindade-Suedam I, Trindade IE . Surgical closure of the cleft palate has a transient obstructive effect on the upper airway in children. Cleft Palate Craniofac J. 2018;55(1):112–118.
- Kendall MC, Alves LJC, Suh EI, et al. Regional anesthesia to ameliorate postoperative analgesia outcomes in pediatric surgical patients: an updated systematic review of randomized controlled trials. Local Reg Anesth. 2018;11:91–109.
- Marhofer D, Kettner SC, Marhofer P, et al. Dexmedetomidine as an adjuvant to ropivacaine prolongs peripheral nerve block: a volunteer study. Br J Anaesth. 2013;110(3):438–442.
- Brummett CM, Hong EK, Janda AM, et al. Perineural dexmedetomidine added to ropivacaine for sciatic nerve block in rats prolongs the duration of analgesia by blocking the hyperpolarization-activated cation current. Anesthesiology. 2011;115(4):836–843.
- Kosugi T, Mizuta K, Fujita T, et al. High concentrations of dexmedetomidine inhibit compound action potentials in frog sciatic nerves without α2 adrenoceptor activation. Br J Pharmacol. 2010;160(7):1662–1676.
- Mesnil M, Dadure C, Captier G, et al. A new approach for peri‐operative analgesia of cleft palate repair in infants: the bilateral suprazygomatic maxillary nerve block. Paediatr Anaesth. 2010;20(4):343–349.
- Chiono J, Raux O, Bringuier S, et al. Bilateral suprazygomatic maxillary nerve block for cleft palate repair in children: a prospective, randomized, double-blind study versus placebo. Anesthesiology. 2014;120(6):1362–1369.
- Mostafa MF, Aal FAA, Ali IH, et al. Dexmedetomidine during suprazygomatic maxillary nerve block for pediatric cleft palate repair, randomized double-blind controlled study. The Korean Journal of Pain. 2020;33(1):81–89.
- Obayah GM, Refaie A, Aboushanab O, et al. Addition of dexmedetomidine to bupivacaine for greater palatine nerve block prolongs postoperative analgesia after cleft palate repair. Eur J Anaesth. 2010;27(3):280–284.
- Vorobeichik L, Brull R, Abdallah FW. Evidence basis for using perineural dexmedetomidine to enhance the quality of brachial plexus nerve blocks: a systematic review and meta-analysis of randomized controlled trials. Br J Anaesth. 2017;118(2):167–181.
- Lee MJ, Koo DJ, Choi YS, et al. Dexamethasone or dexmedetomidine as local anesthetic adjuvants for ultrasound-guided axillary brachial plexus blocks with nerve stimulation. The Korean Journal of Pain. 2016;29(1):29–33.
- Abdallah FW, Dwyer T, Chan VWS, et al. IV and perineural dexmedetomidine similarly prolong the duration of analgesia after interscalene brachial plexus block: a randomized, three-arm, triple-masked, placebo-controlled trial. Anesthesiology. 2016;124(3):683–695.
- Rashmi HD, Komala HK. Effect of dexmedetomidine as an adjuvant to 0.75% ropivacaine in interscalene brachial plexus block using nerve stimulator: a prospective, Randomized Double-blind study. Anesthesia: Essays and Researches. 2017;11(1):134–139.
- Packiasabapathy SK, Kashyap L, Arora MK, et al. Effect of dexmedetomidine as an adjuvant to bupivacaine in femoral nerve block for perioperative analgesia in patients undergoing total knee replacement arthroplasty: a dose–response study. Saudi J Anaesth. 2017;11(3):293–298.
- Abdallah FW, Brull R. Facilitatory effects of perineural dexmedetomidine on neuraxial and peripheral nerve block: a systematic review and meta-analysis. Br J Anaesth. 2013;110(6):915–925.
- Kaki WA, Almarakbi W. Addition of dexmedetomidine to bupivacaine in transversus abdominis plane block potentiates post-operative pain relief among abdominal hysterectomy patients: a prospective randomized controlled trial. Saudi J Anaesth. 2014;8(2):161–166.
- Karan D, Swaro S, Mahapatra PR, et al. Effect of dexmedetomidine as an adjuvant to ropivacaine in ilioinguinal-iliohypogastric nerve blocks for inguinal hernia repair in pediatric patients: a randomized, double-blind, control trial. Anesth Essays Res. 2018;12(4):924–927.
- Abdulatif M, Fawzy M, Nassar H, et al. The effects of perineural dexmedetomidine on the pharmacodynamic profile of femoral nerve block: a dose-finding randomised, controlled, double-blind study. Anaesthesia. 2016;71(10):1177–1185.
- Gunawardana RH, Ratnayaka JI. Postoperative crying in infants. Anaesthesia. 2000;55(2):197.
