Abstract
This paper is the first to describe the silk produced by Segestriidae spiders. Field and specimen data together with webs secreted by Namibian arid-adapted Ariadna spiders were collected from different research work stations. The silks were solubilized with hexafluoroisopropanol and characterized by Fourier transform infrared (FT-IR), matrix-assisted laser desorption/ionization time-of-flight mass spectrometry (MALDI-TOF MS) and differential scanning calorimetry (DSC) techniques, in order to get data on the silk composition. FT-IR analysis confirms that proteins are the main component of the extracted materials. MALDI-TOF mass spectra of silks of three different sites (R, M and K, with three varied environmental conditions: coastal foggy area, hot central desert area and savannah) reveal the presence of low-molecular-weight (< 10,000 g/mol) proteins mostly based on glycine and alanine amino acids. Besides many peptides with identical or similar composition, other low-molar mass proteins with different compositions were also revealed in these silks. The DSC curves show that the studied silks have different melting temperature ranges. This behaviour may be due to the proteins having different molecular weight and/or different amino acid composition. The presence of small peptides with different amino acid composition could be correlated to the different habitats where the spiders live. Hypotheses linking different amino acid compositions with environmental features are suggested.
Introduction
Spider webs have always impressed people’s imaginations and spider silk has always attracted much scientific interest due to its elasticity (Gosline et al. Citation1986) and mechanical resistance (Denny Citation1976). The existence of spiders dates back over 400 million years. There are now 44,032 spider species belonging to 3905 genera and 112 families (Platnick Citation2013), spread worldwide on every continent except for Antarctica. Their extraordinary success is surely linked to their ability to produce silk: the evolution of spiders is correlated to the evolution of silk. Spiders utilize their silk for a lot of applications: for webs, protection of offspring, prey wrapping and so on (Andersen Citation1970). Moreover, spider silk is used for a variety of human purposes such as bulletproof clothing, ropes, nets, seatbelts, bandages and surgical thread, since it is completely biodegradable and incredibly strong (Hsia et al. Citation2011).
The investigation of silk properties led to the discovery that the silk of all araneomorph spiders consists of proteins from major ampullate glands (MaSp1), containing varying amounts of crystalline domains. The true orb spiders (Orbiculariae) can add a novel protein (MaSp2) (Hinman & Lewis Citation1992), which gives the silk the extensibility and toughness necessary for the building of aerial webs thanks to the significant presence of proline (Blackledge et al. Citation2012).
Until now, in-depth studies have concerned the orb web spiders belonging to the genera Nephila (Andersen Citation1970; Hinman & Lewis Citation1992; Frische et al. Citation1998; Higgins & Rankin Citation1999; Vollrath & Knight Citation1999; Augsten et al. Citation2000; Schulz Citation2001; Gaines & Marcotte Citation2008; Lefèvre et al. Citation2008), Araneus (Andersen Citation1970; Beckwitt & Arcidiacono Citation1994) and Argiope (Craig et al. Citation2000; Blackledge & Hayashi Citation2006; Opell et al. Citation2009).
However, very little research has been carried out on the structure and mechanical properties of the silk of Haplogynae (Hajer et al. Citation2013). Nevertheless, nonorbicularian spiders are hypothesized to have a plesiomorphic silk expression of MaSp1 proteins only (Tai et al. Citation2004; Garb et al. Citation2006; Blackledge et al. Citation2009, Citation2012). Segestriidae are haplogyne araneomorphs related to the Dysderidae and are known as tube-dwelling spiders; they live permanently inside tube-shaped retreats made in crevices, walls, rocks, fallen tree trunks, bark and soil (Dippenaar-Schoeman & Jocqué Citation1997). They are present on all continents, except Antarctica, and in very different habitats ranging from humid forest to arid or semi-arid environments, such as desert and savannah (Platnick Citation2013). In the Namibian gravel plains, some of us (Costa et al. Citation1993) discovered various populations belonging to undescribed Ariadna species (Conti et al. Citation2004). These spiders dig in the soil an individual silk-lined burrow with a circular entrance surrounded by a ring of small stones, and sometimes also lichen bits (Costa et al. Citation1995). The silk threads placed under the stones enable the predator, waiting at the bottom of its burrow, to detect prey brushing against them (Henschel Citation1995). The features of the burrow rings vary according to population and habitat (Costa et al. Citation2000).
The pattern of construction of the burrow is complex and energetically expensive. In the laboratory, it was verified that an adult female may spend a whole night in nonstop work to reconstruct its burrow if it has been destroyed (Conti & Costa Citation2004). Not surprisingly, then, spiders seal the mouth of the burrow with a small stone in case of sudden rain or sand storms, to avoid the collapse of their home (Costa & Conti Citation2013).
The silk composition of the Namibian Ariadna spp. seems to be of special interest. In fact, these spiders, adapted to live in dry habitats and staying permanently in their burrows, have to face many difficult problems, such as the maintenance of adequate moisture conditions, defense against excessive heat due to soil overheating, and protection of eggs and offspring. In addition, the ability to attract prey or potential partners while lurking at the bottom of the burrow also requires proper strategies. We therefore have carried out a series of studies on the chemical and thermal properties of silks secreted by different Namibian arid-adapted Ariadna spiders, with two aims: to find any differences among spider web structures, and to try to link these differences, if any, to habitat features.
Materials and methods
Sampling and statistical analysis
Sampling was carried out during March and April 2012 in the western part of Namibia, largely occupied by the Namib Desert, located around the Tropic of Capricorn. Like all desert areas, Namib is characterized by high temperature and scanty, irregular rainfall that does increase from the coast inland (Viles Citation2005). It, however, has an alternative source of moisture: the fog, which is very high along the coast, decreases inland and represents an important resource of water that is fundamental for the survival of plants and animals (Costa Citation1995; Shanyengana et al. Citation2002). Great dune systems, extensive gravel plains, rugged inselbergs and dry riverbeds are the main components of the Namib landscape. Occasional gravel plains are also present in semi-arid areas of the contiguous Great Escarpment.
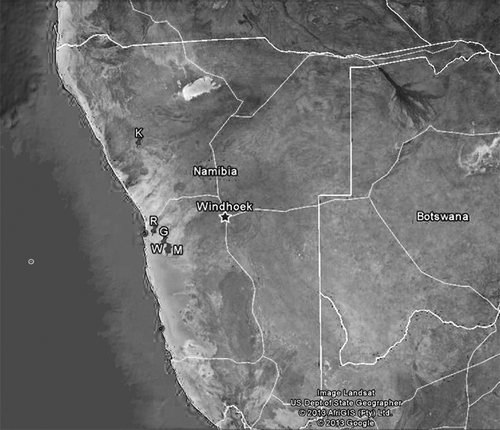
Four gravelly sites (R, G, M, W) within the Namib, and one site (K) external to the desert, in a contiguous savannah (), were our research stations.
R (23°0.0′32.7″S, 14°43.0′38.0″E): marginal area of the desert, rich in lichens, about 20 km from the Atlantic coast, with a thick fog daily and where wind can reach up to 80 km/h, frequently causing sandstorms (Seely Citation1987). The spider burrow rings include 6–15 quartz stones mixed with pieces of lichens and arranged in one to four strata to shape a typical turret.
G (23°19.0′38.4″S, 15°2.0′23.3″E), M (23°32.0′53.2″S, 15°8.0′23.8″E) and W (23°36.0′32.9″S, 15°10.0′2.7″E): in the central part of the Namib desert, 56, 70 and 72 km from the Atlantic coast, respectively. In these areas, precipitation is pulsed and unpredictable (Agnew Citation1997; Jürgens et al. Citation1997) and fog is less dense than in the coastal areas. Therefore, the mean annual humidity is lower, while the mean annual temperature is higher (Seely Citation1987). The G and M spider burrow rings include most commonly seven quartz stones, similar in size, shape and colour and arranged in only one layer; but M rings are less regular than G ones. The W spider burrows are dug on the slopes of a dry bed; the ring stones are numerous, irregular and arranged in up to four strata.
K (20°25.0′53.1″S, 14°20.0′44.9″E): dry savannah area of the Great Escarpment, external to the Namib desert, about 115 km from the Atlantic coast, with daily very high temperatures. The spider burrow rings include numerous small stones, placed in a single layer and differing in size, shape and structure.
At each of these stations, we sampled 20 Ariadna burrows and collected inhabitants, webs and samples of soil. For each burrow we recorded: entrance diameter (measured with a Vernier caliper, measurement error 0.05 mm), depth and temperature at the bottom (with a digital portable probe thermometer Salmoiraghi); moreover, we measured the body length (using the same caliper) and mass of each spider (using a Sartorius high-precision balance).
The soil samples were subjected to grain size analysis to type the five areas according to the Wentworth grain-size classification (Wentworth Citation1922).
To verify the existence of any differences among the five research sites, we performed a one-way analysis of variance (ANOVA), considering diameter, depth and bottom temperature of burrows and the length/mass ratio of the spiders. Post hoc comparisons among sites were conducted using Bonferroni tests. Discriminant analysis was also used in order to define multivariate difference between a priori groups.
Analytical methods
The chemical composition of silks was studied by Fourier transform infrared (FT-IR) and matrix-assisted laser desorption/ionization time-of-flight mass spectrometry (MALDI-TOF MS) techniques. MALDI-TOF MS proved to be a fast and sensitive technique for the analysis of mixtures consisting of proteins, polypeptides (Domon and Aebersold Citation2006; Bich & Zenobi Citation2009; Chen et al. Citation2013; Weidmann et al. Citation2013) or synthetic polymers (Montaudo et al. Citation2002, Citation2006). It is able to calculate the mass of individual molecules in a mixture of homologues, thus permitting the structural identification of single macromolecules. Montaudo et al. (Citation2002, Citation2006) pointed out that the MALDI-TOF MS technique provides mass-resolved spectra up to 50–70 kDa, allowing to the identification of the repeat units, the end chains and also species present in minor amounts. Thermal properties were investigated by differential scanning calorimetry (DSC).
Silks were solubilized with hexafluoroisopropanol (HFIP, 1 mg/mL) at room temperature (25°C), and 50% relative humidity (RH), which is a good solvent for proteins (Kluge et al. Citation2008), to separate them from the inorganic materials constituted of part of soil where spiders live. The solvent was evaporated at room temperature under nitrogen (N2) flow, and the solid organic materials were characterized by FT-IR and MALDI-TOF MS techniques, to obtain information on their chemical composition.
FT-IR spectroscopy
The FT-IR absorption spectra were recorded on a Perkin Elmer model Spectrum 100 at 2 cm−1 resolution. Spectra were acquired in the solid state in a potassium bromide (KBr) transparent disc prepared by mixing each powder silk with KBr (1 mg/100 mg) and then pressing the solid mixture at 10 atm.
MALDI-TOF MS
The MALDI-TOF mass spectra were recorded in linear delayed extraction mode, using a Voyager-DE STR (PerSeptive Biosystem) mass spectrometer, equipped with a nitrogen laser (λ = 337 nm, pulse width = 3 ns), working in a positive ion mode. The accelerating voltage was 20 kV, and grid voltage and delay time (delayed extraction, time lag) were optimized for each sample to achieve the higher mass resolution (M/DM). Laser irradiance was maintained slightly above threshold. The 2-(4’-Hydroxybenzeneazo) benzoic acid (HABA) (0.1 Min HFIP) and sinapinic acid (SA) (0.1 M in HFIP) were used as matrices. The better spectra were recorded using the traditional dried-droplet sample preparation method and SA, a favoured matrix for biomolecules such as proteins (Chen et al. Citation2013). A mass resolution of about 1000–1200 was obtained in the mass range from m/z 1000 to m/z 8000. All spectra were calibrated using an external calibration file made by MALDI analysis of an equimolar mixture of polypeptides with different molecular weights (i.e. P14R MW (molecular weight) = 1533; ACTH (Adeno Cortico Tropic Hormone) Fragment 18–39 MW = 2464.2; insuline MW = 5729.6; cytochrome MW = 12362; albumine MW = 66429g/mol, purchased from Aldrich-Italy), using an SA matrix and applying the same parameters used for the analysis of silks. The mass peaks corresponding to the macromolecular species were measured with an accuracy of ± 0.5 Da.
Differential scanning calorimetry (DSC)
The thermal properties of all silks were investigated in a nitrogen atmosphere (60mL/min) with a TA Instrument Q100 DSC, calibrated with melt purity indium standard (156.6°C and 28.45 J/g). Samples with a mass of 1–1.5 mg were used. Before any experiment, the baseline was recorded using an empty aluminium pan (reference and sample). Each sample was analyzed following the same runs: (a) heating at 10°C/min from –90° to 180°C; (b) cooling at 50°C/min from 180° to –90°C; (c) heating at 10°C/min from –90° to 180°C; (d) cooling at 1°C/min from 180° to 100°C and at 5°C/min within 100°C and –90°C; (c) heating at 10°C/min from –90° to 200°C. Four repeated scans were performed to verify the reproducibility of thermal transitions. The melting point temperatures (Tm) were taken as the maximum of the specific endothermic peaks.
Results
shows the results of the soil granulometric analysis. In all study sites, clay is absent; the maximum percentage of gravel and the lowest values of sand were found at the W station; the maximum percentage of silt as well as the minimum percentage of gravel were at the K station, while the maximum percentage of sand is present at the M station; finally, R and G stations present similar values as regards the granulometric aspect.
Table I. Granulometric soil analysis of the research stations. Values are expressed as percentages.
In , we report the descriptive parameters of field data. Burrow diameter, temperature at the bottom of burrows and length/mass ratio of spiders do not differ significantly among sites. The main difference concerns the burrow depth, as ANOVA confirms (F = 77.88, P << 0.001), with the maximum value found in K and the minimum found in R. The Bonferroni post hoc comparisons reveal that this difference is due to the R, W and K stations, while there was no difference between G and M stations.
Table II. Arithmetic means and standard deviation of recorded parameters in different fieldwork stations.
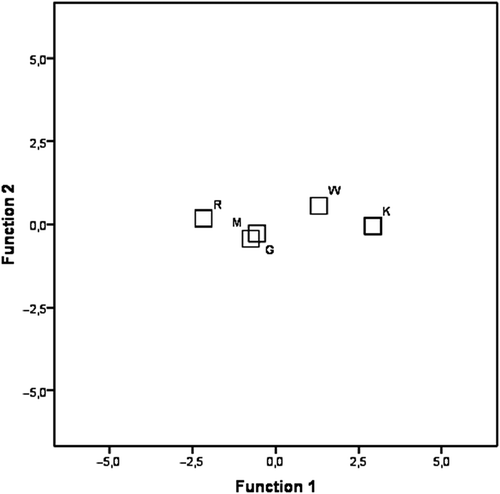
In the discriminant analysis, the two canonical variables represent 96.1% and 2% respectively of the total variance; the coefficients of canonical correlation (0.895 and 0.278) and Wilk’s lambda (0.179 and 0.855) indicate the greater importance of the first canonical variable. The first axis is correlated to the variables “burrow depth” (correlation 0.969) and isolates the W and K populations from the R, M and G ones ().
Figure 2. Discriminant analysis. Plot of the first two canonical variables and centroids of the five populations.
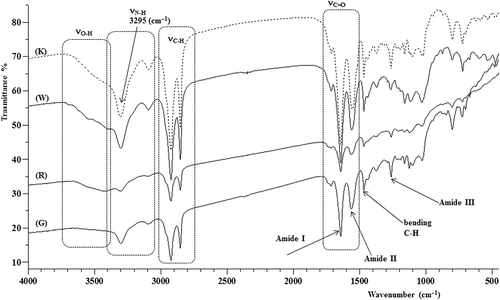
All samples give very similar infrared spectra, as shown in that displays those of samples K, W, R and G (M is not displayed because it overlaps the G spectrum).
The mass spectra of M, R and K silks show mass resolved peaks in the mass range lower than m/z 9000. The mass spectrum of silk M is displayed in . It shows, in the mass range m/z 2000–8500, a series of mass-resolved repeating peaks having a peak spacing of 198 Da, 199 Da, 215 Da, 225 Da, 231 Da or 245 Da. Some low-intensity peaks spaced at 242 Da indicate that some proteins could contain asparagine-lysine and/or asparagine-glutamine amino acid sequences along the chains. A similar result was obtained for the K silk. Its MALDI spectrum, reported in , similar to the mass spectrum of the M silk, shows in the mass range m/z 1200–8500 a series of intense peaks spaced at 198 Da or 199 Da. However, it is less complex with respect to that of the M silk discussed previously, because it shows a minor series of repeating peaks. Nevertheless, it presents a new family of peaks spaced at 184 Da (i.e. peaks at m/z 2044, 2228, 2412 and 2596 and at m/z 2069, 2243, 2427 and 2611, in the inset of ).
Figure 4. Matrix-assisted laser desorption/ionization time-of-flight (MALDI-TOF) mass spectrum of the M silk. Peaks spaced at 198 Da, 199 Da and 215 Da are labeled with the symbol *, whereas those spaced at 231 Da and 245 Da are marked with the symbol •.
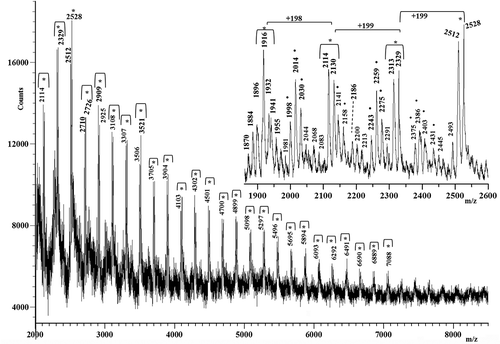
Figure 5. Matrix-assisted laser desorption/ionization time-of-flight (MALDI-TOF) mass spectrum of the K silk.
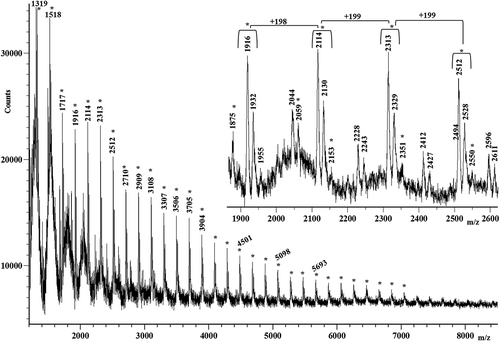
MALDI-TOF MS analysis reveals the presence of low-molecular weight proteins also for the R silk, as shows. Similar to the mass spectra of the M silk and K silk, the mass spectrum of the R silk shows a homologous family of peaks spaced at 198 Da and 199 Da, in the mass range m/z 2000–4000. It also shows other peaks spaced at 256 Da, which may be due to polypeptides containing glycine–alanine–glutamine sequences. However, amino acid sequences corresponding to 256 Da could also be due to glutamine–glutamine, lysine–lysine or glutamine–lysine repeats. The last amino acid sequences may be present in the low-molecular-weight polypeptides composing the silks studied.
Figure 6. Matrix-assisted laser desorption/ionization time-of-flight (MALDI-TOF) mass spectrum of the R silk.
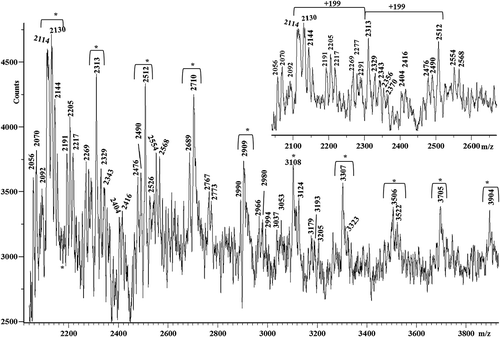
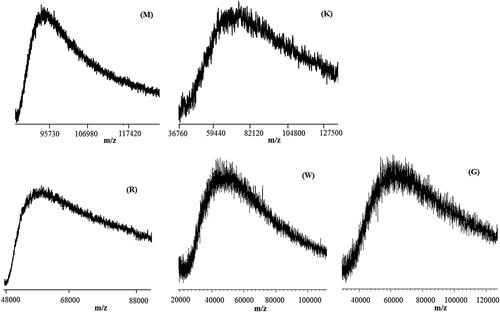
The mass spectra of the three silks R, M and K, besides the mass-resolved peaks discussed, also showed an unresolved peak at a high molecular weight, as highlighted in , which could be due to the high-molecular weight proteins. Mass spectra of silks G and W show only unresolved peaks centred around m/z 40,000–60,000, and therefore we cannot get information on their chemical composition.
Figure 7. Matrix-assisted laser desorption/ionization time-of-flight (MALDI-TOF) mass spectra in the higher mass range of M, K, R, W and G silks.
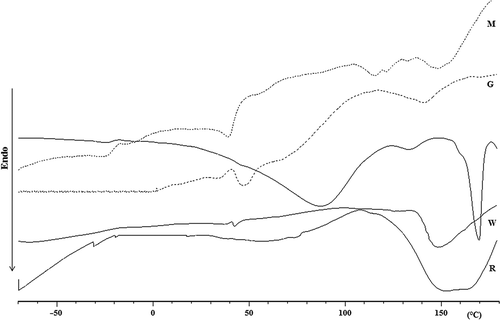
The thermal properties of all extracted silks were investigated by DSC, and in , the thermograms recorded in the first heating run are portrayed. We observe that all samples present a broad endothermic peak in a temperature range from 30 to 110°C, which may be associated with solvent (HFIP) and/or water evaporation; in fact, these peaks disappear or are negligible in the second heating run. The W silk shows a broad endothermic melting peak from 135 to 180°C, whereas the broad endothermic melting peak of the G silk is observable in the temperature range 118–163°C. The R silk shows a broad endothermic peak between 110 and 180°C with two maxima at about 150°C (Tm1) and 165°C (Tm2), due to the first and second structural melt that can be associated with the melting of proteins with different molecular weights. The M silk shows a first melting peak between 105 and 130°C and a second melting peak between 147 and 160°C. Similarly, the DSC curve of the K silk shows a first low intense melting peak from 125 to 143°C, and a second intense melting peak between 152 and 176°C. The total normalized enthalpy of melting (ΔHm) calculated for the melting processes observed in the first heating run for K, R, W, M and G silks is 68, 58, 23, 7.4 and 1.6 J/g, respectively. In the second heating run, melting processes were observed only for K and R silks with a ΔHm of 28 J/g (within 150 and 176°C) and 8 J/g (within 105 and 150°C), respectively. In the third heating step, the melting process was observed only for the K silk, with a maximum at 175°C and a ΔHm of 56 J/g. The Tm of the K silk is almost unaltered during the different heating runs and, since is similar to that of the polyalanine based peptide (about 173°C) (Ghosh et al. Citation2003), we believe that its polypeptides components contain long polyalanine sequences along the chains. These sequences allow the formation of the crystalline domains during the cooling runs in the DSC analysis. The ΔHm values determined in the first heating step indicate that the crystalline domains prevail in the K and R silks with respect to those of the other studied samples. This behaviour confirms that different amino acid sequence distributions lead to different crystalline and amorphous domains.
Discussion
This work is the first survey of the chemical structure of the silk produced by the Namibian Ariadna spiders (and also the first survey of this kind carried out in the whole superfamily Dysderoidea).
The FT-IR analysis reveals that the silks taken from the five research sites have a similar protein composition, as the signals of the asymmetric stretching of the amide carbonyl (1640 cm−1) and the amide NH (3295 cm−1) confirm. On the basis of the data in the literature (Moore & Krimm Citation1976; Jackson and Mantsch Citation1995; Bramanti et al. Citation2005; Papadopoulos et al. Citation2007; Lorusso et al. Citation2011) and, in particular, according to published FT-IR spectra of glycine- and alanine-based polypeptides, we affirm that the HFIP extracts the protein constituting the spider silk. In fact, the absorption bands i the 1600–1700 cm−1 and 1480–1600 cm−1 regions assigned to the characteristic vibrational modes of the amide (-CONH) groups fit well with the so-called amide II and amide I absorptions of proteins, respectively. The absorption band centred at about 1650 cm−1 is primarily due to the C = O (carbonyl) stretching vibration (νC=O) of amide I, while the band picked at 1580 cm−1 referred to as amide II is due to the coupling of the N-H in-plane bending and C-N stretching modes. The less intense peak at about 1260 cm−1 was assigned to amide III and is due to the C-N stretching coupled to the in-plane N-H bending mode. Typical absorption bands due to the stretching of the N-H bond (νN-H) appear at about 3295 and 3100 cm−1 and are referred as amide A and B, respectively (Moore & Krimm Citation1976; Jackson & Mantsch Citation1995; Bramanti et al. Citation2005; Papadopoulos et al. Citation2007; Lorusso et al. Citation2011). A large band due to the stretching of O-H moieties appears between 3400 and 3700 cm−1. By comparing the recorded FTIR spectra with the literature data (Moore & Krimm Citation1976; Blondelle et al. Citation1997; Papadopoulos et al. Citation2007), we can hypothesize that extracted spider silk could be constituted of proteins composed prevalently by alanine and glycine amino acids. However, the FT-IR analysis does not give complete information on the composition of the silks studied.
Taking into account the FT-IR and MALDI-MS data and the published studies on the chemical characterization of spider silks (Craig et al. Citation2000; Papadopoulos et al. Citation2007; Vasanthavada et al. Citation2007; Kluge et al. Citation2008; Romer & Scheibel Citation2008; Blamires et al. Citation2012), we suggest that mass difference of 198 Da corresponds to the proline-threonine (P-T) peptide sequences; that of 199 Da corresponds to the alanine-alanine-glycine (A-A-G) amino acids sequences; that of 215 Da corresponds glycine-alanine-serine (G-A-S) repeats; that of 225 Da corresponds to the glycine-alanine-proline (G-A-P) repeats; that of 231 Da is due to the serine-serine-glycine (S-S-G) triad sequence; that of 245 Da belongs to the serine-serine-alanine (S-S-A) repeats. The mass differences of the most common amino acid sequences are summarized in . On the basis of these findings, we have tentatively assigned the most intense peak series to the corresponding amino acid sequences that should compose the low-molecular-weight proteins of the silk extracted. summarizes the most important chemical compositional assignments corresponding to the mass peaks in the mass spectra of silks M, K and R (–).
Table III. Mass differences of the most common amino acid sequences.
Table IV. Assignments of the most important peaks observed in the low mass range of the matrix-assisted laser desorption/ionization time-of-flight (MALDI-TOF) mass spectra of the silks M, R and K. In parentheses are reported the variable amino acids sequences of each polypeptide.
Comparing the MALDI mass spectra and the data in , it emerges that the most intense peaks belong to the polypeptides rich in the alanine-alanine-glycine amino acid sequences (species P1 and P2 in ).
The presence of a new family of peaks spaced at 184 Da in the K silk suggests that some polypeptides composing the silk could contain the alanine-leucine (A–L) or the alanine-isoleucine (A–I) sequences along the chains. Unfortunately, these two sequences cannot be distinguished by MALDI-TOF MS because they are isobars (m/z 184).
The MALDI-MS data (– and ) reveal that the silk proteins produced by Ariadna spiders as well as those in other spider families (especially Araneidae) have some common low-molecular-weight proteins rich in alanine (A) and glycine (G) amino acids as the primary constituents. The remaining amino acid components are mostly threonine (T), proline (P), serine (S) and glutamine (Q). Nevertheless, the presence of other amino acids such as arginine (R), lysine (K), leucine (L) and/or isoleucine (I) cannot be excluded. On the basis of the data in the literature (Simmons et al. Citation1996; Hsia et al. Citation2011), it appears that polyalanine-rich regions form crystal domains and are responsible for the strength of the silk; on the contrary, polyglycine-rich regions form amorphous regions and characterize the elasticity of the silk fiber. This chemical characterization is broadly consistent with the hypothesis of the exclusive presence of MaSp1 protein in the haplogyne spiders, with proline contents more or less low (Gatesy et al. Citation2001; Gaines & Marcotte Citation2008). The different Ariadna silks analyzed in this paper show different proline contents, the Rooikop silk having the highest amount while the Khorixas silk has the lowest.
In accordance with the MALDI data, the first melting peak of the DSC analysis could be associated with the melting of low-molecular-weight proteins, and the second one should be due to the melting of the high-molecular-weight polypeptides. The different melting temperatures of silks can be associated with the composition and molecular weight of the proteins composing the silks. This is also confirmed by different enthalpy of fusion of the studied silks, neglecting the sites W and G for which we did not get low mass protein spectra. The highest value, found in the K silk, could be linked to the presence of polyalanine groups that results in a considerable amount of the crystalline component; on the contrary, the lowest value is in the M silk, indicating a prevalence of amorphous components with a relative consistence of serine amino acid. The enthalpy of fusion of the R silk is between those of the K and M silks; however, a crystalline component maintains a significant presence.
Variations in low-molecular-weight proteins observed in the mass spectra and in the melting temperature of silks might be correlated with the different habitats where spiders live. R, M and K spider populations reflect three varied environmental conditions: a coastal foggy area, hot central desert area and savannah, respectively. Their needs are therefore different and we expect that the chemical and thermal properties of their webs couple to habitat features. Indeed, we can assert that silk composition has a greater or lesser complexity in the different analyzed webs.
In the savannah site, where K is, external to the desert, we find a simpler chemical composition of silk protein. On the other hand, DSC analysis shows an intense, narrow melting peak at about 170°C that suggests that some crystalline domains are due to the polyalanine sequences composing the K silk. We could suppose that this simplified structure may be a sign of a primitive step on the way to desert colonization by Ariadna spp. This hypothesis is also supported by both the corresponding simple structure of the stone ring and the prevalence of silt in the soil composition, which is, in turn, evidence of an ancient erosion process.
The polyalanine blocks (crystal domains) that characterize the K silk give a greater strength to the web that could be linked to habitat characteristics. The semi-arid conditions of the savannah with high temperature values compel spiders to construct their homes deep in the soil to guarantee optimal microclimatic conditions for adults, eggs and spiderlings. The high depth of the burrows requires, in turn, a tough web able to face both collapse and the rainy season.
In both the M and R sites (which even the discriminant analysis clearly distances from K), the composition of web silk appears to be far more complex; we hypothesize that this increased complexity could have evolved in response to different habitat features. The increased presence of proline in the silks at these stations could be considered an evolutionary step to a progressive colonization of the desert by the Ariadna spiders, and then a step to their major specialization.
R station, located at the beginning of the desert and not too far from the Atlantic coast, with a gravelly substrate, is characterized by both the fog (and the moisture it brings to the desert) and the widespread growth of ground-dwelling lichens; its soil undergoes daily variations ranging from high humidity during the morning and at night to high aridity in the daytime peak hours. A substantial amount of gravel in the soil composition makes spider burrowing more difficult; on the other hand, climatic conditions (moderate temperature and high humidity) drive spiders to dig short burrows with webs composed of strong silks to prevent their home from becoming damaged. This silk stiffness is also supported by the broad endothermic peak of DSC analysis. R silks include a large amount of proline, a hydrophobic amino acid (Savage & Gosline Citation2008); its presence is justified by the high-humidity conditions of the habitat. R spiders do not need to have hydrophilic amino acids, as their habitat near the coastal area and rich in lichens ensures the spiders’ water intake.
M spiders inhabit the desert interior and experience the hyperarid climatic condition of the Namib (temperatures up to 45°C; very low precipitation and humidity) where the substrate is mostly sandy. In such conditions, it is certainly convenient for the spiders to have a burrow sufficiently deep to ensure a suitable microhabitat. MALDI spectra indicate the presence of asparagine–lysine and/or asparagine–glutamine sequences and a large presence of serine. These hydrophilic amino acids are able to retain moisture thanks to the presence of hydrogen bonds, and this could be a significant adaptation by spiders to allow them to inhabit desert areas: in this way, these animals can get the amount of water necessary for their survival.
In order to obtain more information on the chemical composition (% mol of each amino acid component) and the primary chemical structure (i.e. amino acid sequences) of the polypeptides composing the silks, total and partial acid hydrolysis, and also tryptic digestion, will be carried out and then the products will be investigated by HPLC (High-performance liquid chromatography), MALDI-TOF MS, MALDI-TOF MS/MS (tandem mass spectrometry) and ESI-TOF (electrospray ionization-time of flight).
Despite being the first chemical analysis of the silk of Namibian spiders belonging to the genus Ariadna, our study has led to significant results. Moreover, to the best of our knowledge, the present work represents the first case of characterization of the neat polypeptides composing the spider silks, by MALDI-TOF MS analysis.
Acknowledgements
We would like to thank the Ministry of Environment and Tourism, Namibia, for a permit to work in the Namib-Naukluft Park and to collect some specimens of Ariadna. We also are indebted to Professor Diego Puglisi at University of Catania for his assistance with the granulometric analysis. Many thank to Dr. Concetto Puglisi as manager of the Istituto per i Polimeri, Composti e Biomateriali--Unità Organizzativa di Supporto (IPCB--UOS) of the National Council of Research (CNR) of Italy and also for their availability for the critical analysis of data. We especially thank the anonymous reviewer and co-editor for providing constructive critiques of the manuscript. Financial support was provided by the Ministry of University and Scientific Research (MIUR) of Italy and CNR-Italy.
References
- Agnew ADQ. 1997. Swiches, pulses and grazing in arid vegetation. Journal of Arid Environments 37:609–617. doi:10.1006/jare.1997.0304.
- Andersen SO. 1970. Amino acid composition of spider silks. Comparative Biochemistry and Physiology 35:705–711. doi:10.1016/0010-406X(70)90988-6.
- Augsten K, Mühlig P, Herrmann C. 2000. Glycoproteins and skin-core structure in Nephila clavipes spider silk observed by light and electron microscopy. Scanning 22:12–15. doi:10.1002/sca.4950220103.
- Beckwitt R, Arcidiacono S. 1994. Sequence conservation in the C-terminal region of spider silk proteins (spidroin) from Nephila clavipes (Tetragnathidae) and Araneus bicentenarius (Araneidae). Journal of Biological Chemistry 269:6661–6663.
- Bich C, Zenobi R. 2009. Mass spectrometry of large complexes. Current Opinion in Structural Biology 19:632–639. doi:10.1016/j.sbi.2009.08.004.
- Blackledge TA, Hayashi CY. 2006. Silken toolkits: Biomechanics of silk fibers spun by the orb web spider Argiope argentata (Fabricius 1775). Journal of Experimental Biology 209:2452–2461. doi:10.1242/jeb.02275.
- Blackledge TA, Kuntner M, Marhabaie M, Leeper TC, Agnarsson I. 2012. Biomaterial evolution parallels behavioral innovation in the origin of orb-like spiders webs. Scientific Reports 2:833.
- Blackledge TA, Scharff N, Coddington JA, Szüts T, Wenzel JW, Hayashi CY, Agnarsson I. 2009. Reconstructing web evolution and spider diversification in the molecular era. Proceedings of the National Academy of Sciences 106:5229–5234.
- Blamires SJ, Wu C-L, Tso I-M. 2012. Variation in protein intake induces variation in spider silk expression. Plos One 7:e31626–9. doi:10.1371/journal.pone.0031626.
- Blondelle SE, Forood B, Houghten RA, Pérez-Payá E. 1997. Polyalanine-based peptides as models for self-associated β-pleated-sheet complexes. Biochemistry 36:8393–8400. doi:10.1021/bi963015b.
- Bramanti E, Catalano D, Forte C, Giovanneschi M, Masetti M, Veracini C A. 2005. Solid state 13C NMR and FT-IR spectroscopy of the cocoon silk of two common spiders. Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy 62:105–111. doi:10.1016/j.saa.2004.12.008.
- Chen F, Gerber S, Heuser K, Korkhov VM, Lizak C, Mireku S, Locher KP, Zenobi R. 2013. High-mass matrix-assisted laser desorption ionization-mass spectrometry of integral membrane proteins and their complexes. Analytical Chemistry 85:3483–3488. doi:10.1021/ac4000943.
- Conti E, Costa G. 2004. La costruzione della tana in Ariadna sp., ragno segestriide vivente in pianure ghiaiose del Namib Desert. 65° Congresso Unione Zoologica Italiana:30.
- Conti E, Costa G, Montesanto G, Patané MG, Torre F, Lombardo BM. 2004. Analisi del differenziamento genetico in popolazioni namibiane di ragni Segestriidi del genere Ariadna, mediante MLEE e RAPD-PCR (Arachnida, Araneae). 64° Congresso Unione Zoologica Italiana:108. Taormina, Giardini Naxos (ME), 21–25 September 2004.
- Costa G. 1995. Behavioural adaptations of desert animals. Berlin, Heidelberg: Springer Verlag. doi:10.1007/978-3-642-79356-1.
- Costa G, Conti E. 2013. Opening and closing of burrows by the Namibian spider Ariadna sp. (Araneae: Segestriidae) in a year of heavy rainfall. Journal of Arachnology 41:215–218. doi:10.1636/Hi13-04.1.
- Costa G, Petralia A, Conti E. 2000. Population dynamics of stone-ring spiders of the genus Ariadna Audoin (Araneae: Segestriidae), in western Namibia. Cimbebasia 16:223–229.
- Costa G, Petralia A, Conti E, Hänel C. 1995. A ‘mathematical’ spider living on gravel plains of the Namib Desert. Journal of Arid Environments 29:485–494. doi:10.1016/S0140-1963(95)80020-4.
- Costa G, Petralia A, Conti E, Hänel C, Seely MK. 1993. Seven stone spiders on the gravel plains of the Namib Desert. Bollettino dell’Accademia Gioenia di Scienze Naturali in Catania 26:77–83.
- Craig CL, Riekel C, Herberstein ME, Weber RS, Kaplan D, Pierce NE. 2000. Evidence for diet effects on the composition of silk proteins produced by spiders. Molecular Biology and Evolution 17:1904–1913. doi:10.1093/oxfordjournals.molbev.a026292.
- Denny MW. 1976. The physical properties of spider’s silk and their role in the design of orb-webs. Journal of Experimental Biology 65:483–506.
- Dippenaar-Schoeman AS, Jocqué R. 1997. African spiders. An identification manual. Pretoria: ARC-Plant Protection Research Institute Handbook No.9.
- Domon B, Aebersold R. 2006. Mass spectrometry and protein analysis. Science 312:212–217. doi:10.1126/science.1124619.
- Frische S, Maunsbach AB, Vollrath F. 1998. Elongate cavities and skin-core structure in Nephila spider silk observed by electron microscopy. Journal of Microscopy 189:64–70. doi:10.1046/j.1365-2818.1998.00285.x.
- Gaines WA, Marcotte Jr WR. 2008. Identification and characterization of multiple Spidroin 1 genes encoding major ampullate silk proteins in Nephila clavipes. Insect Molecular Biology 17:465–474. doi:10.1111/j.1365-2583.2008.00828.x.
- Garb JE, Di Mauro T, Vo V, Hayashi CY. 2006. Silk genes support the single origin of orb-webs. Science 312:1762.
- Gatesy J, Hayashi C, Motriuk D, Woods J, Lewis R. 2001. Extreme diversity, conservation, and convergence of spider silk fibroin sequences. Science 291:2603–2605.
- Ghosh T, Garde S, García AE. 2003. Role of backbone hydration and salt-bridge formation in stability of α-helix in solution. Biophysical Journal 85:3187–3193. doi:10.1016/S0006-3495(03)74736-5.
- Gosline JM, Demont ME, Denny M. 1986. The structure and properties of spider silks. Endeavour 10:37–43. doi:10.1016/0160-9327(86)90049-9.
- Hajer J, Malý J, Řeháková D. 2013. Silk fibers and silk-producing organs of Harpactea rubicunda (C. L. Koch 1838) (Araneae, Dysderidae). Microscopy Research and Technique 76:28–35. doi:10.1002/jemt.22131.
- Henschel JR. 1995. Tool use by spiders: Stone selection and placement by Corolla Spiders Ariadna (Segestriidae) of the Namib Desert. Ethology 101:187–199. doi:10.1111/j.1439-0310.1995.tb00357.x.
- Higgins L, Rankin MA. 1999. Nutritional requirements for web synthesis in the tetragnathid spider Nephila clavipes. Physiological Entomology 24:263–270. doi:10.1046/j.1365-3032.1999.00135.x.
- Hinman MB, Lewis RV. 1992. Isolation of a clone encoding a second dragline silk fibroin. Nephila clavipes dragline silk is a two protein fiber. Journal of Biological Chemistry 267:19320–19324.
- Hsia Y, Gnesa E, Jeffery F, Tang S, Craig Vierra C. 2011. Spider silk composites and applications. In: Cuppoletti J, editor. Metal, ceramic and polymeric composites for various uses. InTech. pp. 303–324. Available: http://www.intechopen.com/books/metal-ceramic-and-polymeric-composites-for-various-uses/spider-silk-composites-and-applications.
- Jackson M, Mantsch HH. 1995. The use and misuse of FTIR spectroscopy in the determination of protein structure. Critical Reviews in Biochemistry and Molecular Biology 30:95–120. doi:10.3109/10409239509085140.
- Jürgens N, Burke A, Seely MK, Jacobsen KM. 1997. The Namib Desert. In: Cowling RM, Richardson D, editors. Vegetation of Southern Africa. Cambridge: University Press. pp. 189–214.
- Kluge JA, Rabotyagova O, Leisk GG, Kaplan DL. 2008. Spider silks and their applications. Trends in Biotechnology 26:244–251. doi:10.1016/j.tibtech.2008.02.006.
- Lefèvre T, Boudreault S, Cloutier C, Pézolet M. 2008. Conformational and orientational transformation of silk proteins in the major ampullate gland of Nephila clavipes spiders. Biomacromolecules 9:2399–2407. doi:10.1021/bm800390j.
- Lorusso M, Pepe A, Ibris N, Bochicchio B. 2011. Molecular and supramolecular studies on polyglycine and poly-L-proline. Soft Matter 7:6327–6336. doi:10.1039/c1sm05726j.
- Montaudo G, Montaudo MS, Samperi F. 2002. Matrix-assisted laser desorption ionization/mass spectrometry of polymers MALDI-MS. In: Montaudo G, Lattimer RP, editors. Mass spectrometry of polymers. Boca Ratón: CRC Press. pp. 419–521.
- Montaudo G, Samperi F, Montaudo MS. 2006. Characterization of synthetic polymers by MALDI-MS. Progress in Polymer Science 31:277–357. doi:10.1016/j.progpolymsci.2005.12.001.
- Moore WH, Krimm S. 1976. Vibrational analysis of peptides, polypeptides, and proteins. II. β-Poly(L-alanine) and β-poly(L-alanylglycine). Biopolymers 15:2465–2483. doi:10.1002/bip.1976.360151211.
- Opell BD, Lipkey GK, Hendricks ML, Vito ST. 2009. Daily and seasonal changes in the stickiness of viscous capture threads in Argiope aurantia and Argiope trifasciata orb webs. Journal of Experimental Zoology Part A: Ecological Genetics and Physiology 311A:217–225. doi:10.1002/jez.526.
- Papadopoulos P, Sölter J, Kremer F. 2007. Structure property relationships in major ampullate spider silk as deduced from polarized FTIR spectroscopy. The European Physical Journal E 24:193–199. doi:10.1140/epje/i2007-10229-9.
- Platnick NI. 2013. The world spider catalog, version 14.0. American Museum of Natural History. Available: http://research.amnh.org/entomology/spiders/catalog/index.html DOI: 10.5531/db.iz.0001. Jul 2013.
- Romer L, Scheibel T. 2008. The elaborate structure of spider silk. Landes Bioscience 2:154–161.
- Savage KN, Gosline JM. 2008. The role of proline in the elastic mechanism of hydrated spider silks. Journal of Experimental Biology 211:1948–1957. doi:10.1242/jeb.014225.
- Schulz S. 2001. Composition of the silk lipids of the spider Nephila clavipes. Lipids 36:637–647. doi:10.1007/s11745-001-0768-7.
- Seely MK. 1987. The Namib. Natural history of an ancient desert. Windhoek, Namibia: Shell Oil, S.W.A., Ltd.
- Shanyengana ES, Henschel JR, Seely MK, Sanderson RD. 2002. Exploring fog as a supplementary water source in Namibia. Atmospheric Research 64:251–259. doi:10.1016/S0169-8095(02)00096-0.
- Simmons AH, Michal CA, Jelinski LW. 1996. Molecular orientation and two-component nature of the crystalline fraction of spider dragline silk. Science 271:84–87. doi:10.1126/science.271.5245.84.
- Tai P-L, Hwang G-Y, Tso I-M. 2004. Inter-specific sequence conservation and intra-individual sequence variation in a spider silk gene. International Journal of Biological Macromolecules 34:295–301.
- Vasanthavada K, Hu X, Falick AM, La Mattina C, Moore AMF, Jones PR, Yee R, Reza R, Tuton T, Vierra C. 2007. Aciniform spidroin, a constituent of egg case sacs and wrapping silk fibers from the black widow spider Latrodectus hesperus. The Journal of Biological Chemistry 282:35088–35097. doi:10.1074/jbc.M705791200.
- Viles HA. 2005. Microclimate and weathering in the central Namib Desert, Namibia. Geomorphology 67:189–209. doi:10.1016/j.geomorph.2004.04.006.
- Vollrath F, Knight DP. 1999. Structure and function of the silk production pathway in the spider Nephila edulis. International Journal of Biological Macromolecules 24:243–249. doi:10.1016/S0141-8130(98)00095-6.
- Weidmann S, Mikutis G, Barylyuk K, Zenobi R. 2013. Mass discrimination in high-mass MALDI-MS. Journal of the American Society for Mass Spectrometry 24:1396–1404. doi:10.1007/s13361-013-0686-x.
- Wentworth K. 1922. A scale of grade and class terms for clastic sediments. The Journal of Geology 30:377–392. doi:10.1086/622910.