?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
There are often two phases in the desorption of polycyclic aromatic hydrocarbons (PAHs): an initial phase of rapid desorption and a subsequent phase of much slower release. By assessing the rapidly desorbing fraction of PAHs, a direct measure of the microbially degradable component of PAH contamination can be obtained and achievable bioremediation performances can be predicted. In this study, microbial biosurfactant produced by a Pseudomonas aeruginosa strain, identified as a lipopeptide by attenuated total reflectance Fourier transform infrared spectroscopy, was investigated for its efficacy in enhancing PAH desorption and mobilization in a spiked soil system. The desorption of pyrene and phenanthrene from the artificially spiked soil was enhanced 3.5–4.0 times at 700 mg L−1 lipopeptide amendment than at 150 mg L−1 amendment or in the unamended soil. The amount desorbed was generally in direct proportion to the amount of lipopeptide present. Mathematical modelling using a first-order two-compartment model was applied to simulate the process of desorption from the soil in the presence of different concentrations of lipopeptide and to predict the effect of the biosurfactant on the rapidly desorbing fraction. With the increase of supplementation of lipopeptide from 150 to 700 mg L−1, the rapidly desorbing fraction, which is generally considered to be the bioavailable fraction, increased from 18% to 73% and from 6% to 51% for phenanthrene and pyrene, respectively. This shows the potential application of the biosurfactant in increasing the bioavailable fraction and enhancing the bioremediation of PAH contaminated media.
Introduction
Polycyclic aromatic hydrocarbons (PAHs) are considered hazardous for human health due to their known or suspected genotoxic, mutagenic and carcinogenic potential.[Citation1] They are ubiquitous pollutants and are generated mainly from anthropogenic activities such as the burning of fossil fuels, the use of wood preservatives such as creosote and the generation of wastes from coal gasification plants and other industrial activities.[Citation2,Citation3] As PAHs are highly hydrophobic, they interact strongly with organic matter in the soil, which is a major pool for hydrophobic contaminants.[Citation4,Citation5]
PAHs are persistent organic pollutants, which is mainly due to their molecular stability and hydrophobicity.[Citation6,Citation7] Bioremediation is generally considered as a promising option for the complete removal and destruction of contaminants.[Citation7,Citation8] However, bioremediation can be limited by the bioavailability of soil-bound PAHs due to their low aqueous solubility, high hydrophobicity and strong sorption to soil, which is exacerbated by the long ageing of contaminants in field-contaminated soils.[Citation9] As a consequence, the bioremediation of PAHs in soil–water systems depends strongly on their desorption rates from the soil surface and the subsequent incorporation of the pollutant into the bulk aqueous phase,[Citation10] since it is the aqueous phase where most microorganisms take PAHs from.[Citation11]
One method to enhance the PAH desorption rate into the aqueous phase is to add surfactants. Surfactants are known to improve the efficiency of desorption and bioavailability of hydrophobic organic compounds (HOCs) through enhancing their solubility in aqueous systems.[Citation12–14] It has been suggested that the underlying mechanisms of surfactant-enhanced removal of PAHs from soil include two steps: mobilization and solubilization.[Citation15,Citation16] The mobilization mechanism occurs at concentrations below the surfactant critical micelle concentration (CMC).[Citation17] Phenomena associated with this mechanism include reduction of surface and interfacial tension, reduction of capillary force, wettability and reduction of contact angle.[Citation16] In turn, above the surfactant CMC, solubilization takes place, i.e., incorporation of these molecules into a micelle (for review see [Citation18]). Surfactants have been found to enhance microbial remediation of PAH-contaminated soils.[Citation19,Citation20]
In recent years, microbially produced biosurfactants have found a new area of application in environmental remediation processes. Biosurfactants possess distinct advantages over synthetic ones, including biodegradability and biocompatibility, multifunctional characteristics, stable activity under extreme environmental conditions (e.g., high or low temperature, pH and salinity), and thus can be more effective in remediation of contaminated soil.[Citation21,Citation22] Bacteria of various genera such as Pseudomonas, Bacillus, Acinetobacter, Arthrobacter and Rhodococcus are able to produce biosurfactants during hydrocarbon degradation.[Citation21,Citation22]
As a contaminated soil ages PAHs tend to move into the deeper recesses of soil particles, soil aggregates and the organic matter sorbed to soil particle surfaces.[Citation23–25] As a result, the process of desorption is commonly considered as a rapid initial release of PAHs that are close to the surface and a very slow release of PAHs that are more deeply sorbed.[Citation24,Citation25] A similar ‘biphasic’ profile has been observed for biodegradation. Although considerable amounts of sorbed PAHs will eventually leach out over years, this time frame is usually too long for shorter term remediation techniques, such as land-farming treatment (months), to be effective. If strategic modifications of bioremediation techniques can be made to increase desorption rates over the shorter treatment term, then the added amount of degradation may mean meeting cleanup goals in a reasonable time.[Citation17,Citation26] It has been hypothesized that a direct measure of the microbially degradable component of HOC contamination can be achieved by assessing the rapidly desorbing fraction of PAHs.[Citation24] The similarity between contaminant desorption kinetics, often calculated using a first-order two- or three-compartment mathematical model, and microbial degradation, has been the focus of several investigations.[Citation24,Citation27] The rapidly desorbing fraction can be extracted e.g., with Tenax beads,[Citation28] cyclodextrin,[Citation27], biosurfactant [Citation29] and solvents.[Citation30]
In this study, the efficacy of lipopeptide biosurfactant produced by Pseudomonas aeruginosa (P. aeruginosa) strain was investigated in increasing the desorption rate of PAHs from artificially spiked soils, using a first-order two-compartment mathematical model. Phenanthrene (PHE), a three-ring PAH, and pyrene (PYR), a four-ring PAH, were used as model compounds to analyse the effect of the biosurfactant on the desorption of the hydrocarbons with emphasis on its capability in increasing the rapidly desorbing fraction.
Materials and methods
Biosurfactant production and surface-active properties
P. aeruginosa Lbp5 was selectively isolated from petroleum-contaminated soil. The strain was selected for its ability to produce extracellular biosurfactant and for being able to reduce surface tension of the growth medium below 35 mN m−1, the details of biosurfactant producing strain isolation and biosurfactant production have been described elsewhere.[Citation31] Growth conditions favourable for the production of biosurfactants require limiting addition of inorganic nutrients, including phosphate, nitrogen, iron and carbon excess.[Citation32] For this purpose, a limitation of phosphate (phosphate-free set-up) was performed. The growth phase was separated from the production phase to overcome the inhibition of glycolipid production by inorganic phosphate. A two-step process was developed according to Ramana and Karanth.[Citation33]
P. aeruginosa Lbp5 was purified and maintained in nutrient broth. The inoculum was incubated for 24 h at 30 °C and 120 r min−1 on a rotary shaker. The 24 h old culture was transferred to a 250 mL Erlenmeyer flask containing 50 mL of mineral salt medium (MSM) and incubated for 48 h. The composition of MSM [Citation34] was as follows: 3.68 g L−1 NH4NO3, 0.4 g L−1 MgSO4⋅7 H2O, 0.4 g L−1 CaCl2⋅2H2O, 7.59 g L−1 Na2HPO4⋅2 H2O, 4.43 g L−1 KH2PO4 and 2 mL L−1 of trace element solution. The trace element solution consisted of: 20.1 g L−1 ethylenediaminetetraacetic acid disodium salt, 16.7 g L−1 FeCl3⋅6H2O, 0.18 g L−1 CoCl2⋅6H2O, 0.18 g L−1 ZnSO4⋅7H2O, 0.16 g L−1 CuSO4⋅5 H2O and 0.10 g L−1 MnSO4⋅H2O.
Preparation of resting cells: P. aeruginosa Lbp5 was grown for 48 h in a 2 L flask, with growth medium containing 5 g L−1 of glucose as a carbon source. The cells were harvested by centrifugation at 10,000 r min−1 for 10 min and washed twice with 0.9% NaCl solution. Finally, 5% (w/v) of cells were suspended in the above MSM without Na2HPO4⋅2H2O and 4.43 g L−1 KH2PO4.
Growth limited (phosphate-free) medium was of same composition as the growth medium, except that no phosphate source was added. Lipopeptide production was carried out in a 2 L flask containing1000 mL of phosphate-free MSM, 5% (w/v) of the inoculum and 50 g L−1 of glycerol as a carbon and energy source and was incubated for 7 days. All the experiments were carried out at 30 °C and a shaker speed of 120 r min−1. All media were adjusted to pH 7.
Extraction of biosurfactant
The culture broth was centrifuged at 13,000 r min−1 for 20 min at 4 °C. The cell-free supernatant was adjusted to pH 2.0 with 6 mol/L HCl and then incubated at 4 °C overnight. Afterwards, the precipitate was collected by centrifugation (13,000 r min−1, 20 min, 4 °C). The precipitate was extracted twice with an equal volume of chloroform:methanol (2:1) by shaking vigorously each time and allowing the two layers to separate in a separating funnel. The organic phase was transferred to a round-bottom flask connected to a rotary evaporator at 40 °C to remove the solvent, yielding a yellow-coloured biosurfactant product.
This partially purified preparation was used for Fourier transform infrared (FTIR) spectroscopy characterization. The surface tension of the aqueous solution was measured by using a du Noüy ring-type tensiometer (KRÜSS GmbH, Hamburg, Germany). The surface tension measurement was carried out at 25 °C after dipping the platinum ring in the solution for a while in order to attain equilibrium conditions. All measurements were made on cell-free broth obtained by centrifuging the cultures at 13,000 r min−1 for 10 min. The biosurfactant concentration is expressed in terms of critical micelle dilution (CMD) estimated by measuring the surface tension for varying dilutions (10–100-fold) of the sample. The dilution at which the surface tension begins to increase is termed the CMD, which is the factor by which the effective biosurfactant concentration exceeds the CMC.[Citation35] The CMC was determined by measuring the surface tension for a series of decreasing biosurfactant concentrations. A stock solution of the crude biosurfactant (2000 mg L−1) was prepared in water and serial dilutions were made in decreasing concentrations. The concentration at which the surface tension begins to increase was determined, which is called the CMC.
The chemical structures of the components in the crude biosurfactant sample were determined by using FTIR spectroscopy (Perkin Elmer 1600 FTIR) equipped with an attenuated total reflectance (ATR) crystal accessory (Perkin Elmer, Connecticut, USA). The IR scan was performed over a 400–4000 cm−1 wavenumber range with a resolution of 2 cm−1. The reflectance spectra were recorded and averaged over 32 scans, using the total internal reflectance configuration with a Harrick™ Mvp-pro cell consisting of a diamond crystal.
Chemicals
PHE (purity > 98%), PYR (purity > 98%), acetone, acetonitrile and hexane (high-performance liquid chromatography (HPLC) grade), were all purchased from Sigma–Aldrich Chemical Company (Aldrich, USA). Stock solutions (4 mg mL−1) of PHE and PYR were prepared by dissolving the precisely weighed compound in acetonitrile in a sealed volumetric flask and were stored at 4 °C in the dark. Different concentrations of working solution and HPLC calibration standards were prepared by diluting the stock solution, using either acetone or acetonitrile.
Contaminated soil
Soil was collected from a pristine supply and was sieved to <2 mm size. Texture of the soil was 26% sand, 33% clay and 41% silt; the water holding capacity was 30% and the total organic carbon was 22 g Kg −1.
One-hundred grams of sterile dry soil were placed in a 1 L bottle and spiked with 80 mg of PHE and 80 mg of PYR dissolved in approximately 100 mL of acetone to achieve soil contamination of 800 mg kg −1 of PHE and 800 mg kg −1 PYR each. The soil was shaken vigorously for 5 min to promote homogeneous distribution of the PAHs in the soil. The amount of acetone added was sufficient to completely saturate the soil. The acetone in the mixture was allowed to evaporate for one week at 30 °C under a fume hood, and the contaminated soils were aged for 6 months at room temperature before the experiment starts to reach equilibrium.
Batch desorption study
Batch experiments were conducted in triplicate to determine the desorption percentage of PHE and PYR in 100 mL Erlenmeyer flasks. A mass of 10 g of contaminated soil sample was weighed into each flask containing 50 mL of MSM 20% (w/v) with a different amount of lipopeptide. All aqueous solutions for soil tests contained 0.01 mol/L NaCl to maintain a constant ionic strength and 0.01% (w/w) NaN3 to inhibit microbial growth. The samples were shaken on a rotary shaker at 150 r min−1 in darkness at 32 °C. Triplicate samples were collected every 24 h by centrifugation for 10 min at 10,000 r min−1. The supernatant was drained off and the soil samples were air-dried at room temperature. Five grams of the air-dried and homogenized soil sample were weighed directly in a flask where 30 mL of solvent hexane/acetone (1:1 v/v) were added and ultrasonicated twice (frequency 50–60 Hz, Bransonic 2200, Danbury, CT, USA) at 45 °C for 60 min.[Citation36] The extracts were pooled and vacuum filtered (Whatman no.1 filter paper), the solvent was evaporated under a fume hood of dry nitrogen and the residual PAHs were recovered in 5 mL of acetonitrile (exchanged to mobile phase medium) and HPLC analysis was performed.
The PAHs desorption percentage was computed from the difference of the initial and final concentrations of the soil.
The desorption Percentage was determined as
where C is the PAH concentration in the eluting agents (mg L−1), V is the initial volume of the eluting agent (L), Ci is the original PAH concentration in the polluted soil (mg kg−1) and m is the initial weight of the polluted soil (kg).
Desorption data modelling
A two-compartment first-order rate constant model was used to fit the desorption data.[Citation24,Citation28]
(1)
(1) where St (mg kg −1) is the PAHs content in the soil at time t (h) and S0 (mg kg −1) at the start of the experiment; Frap and Fslow are the rapidly and slowly desorbing fractions and krap and kslow (h−1) are the rate constants of rapid and slow desorption compartments, assuming that kslow≪ krap. It was assumed that the two defined fractions covered the entire amount of PAHs (no other compartment), which leads to
(2)
(2) The values of Frap, Fslow, krap and kslow were determined by minimizing the cumulative squared residuals between experimental and calculated values of (St /S0) in EquationEquation (1
(1)
(1) ) using the software Microsoft Excel 2010 (SOLVER option).
Analytical methods
The surface tension of mixtures with addition of different concentrations of lipopeptide was determined by using a du Noüy ring-type tensiometer (KRÜSS GmbH, Hamburg, Germany).
The emulsification index (E24) of the cell-free supernatant was determined by adding 2 mL of a hydrocarbon (hexane) to the same amount of supernatant, mixing with a vortex for 5 min, and leaving the mixture to stand for 24 h. The E24 index is given as percentage of the height of the emulsified layer divided by the total height of the liquid column.[Citation37]
The amount of PAHs extracted was syringe filtered (0.45 µm polytetrafluoroethylene) and analysed by an HPLC system with a slightly modified EPA Method 8310 [Citation38] using a linear gradient of acetonitrile and ultra-pure-water (UPW) mobile phase over 30 min at a flow rate of 1 mL min−1. The elution conditions were: 0–1 min, 70% acetonitrile (ACN):30% UPW isocratic; 1–10 min, linear gradient 70% ACN:30% UPW –100% ACN; 10–20 min, 100% ACN isocratic; 20–25 min, linear gradient 100% ACN – 70% ACN:30% UPW and finally, 25–30 min 70% ACN:30% UPW isocratic back to the initial condition and reconditioning of the column. For HPLC analysis, a Waters 2695 separation module equipped with a photo diode array detector was used. The PAHs were separated with a reverse phase Waters PAH C18 column (4.6 mm × 25 cm with 5 µm packing) at a column temperature of 25 °C at 254 nm. Each PAH was identified by its retention time and absorption spectrum and quantified by its absorbance compared with the external calibration curve prepared with the standards. The detection limit of the HPLC system was 0.01 mg L−1. Quantitation was performed by external standard calibration with a five-point calibration curves in the range of 0.1–100 mg L−1.
Results and discussion
Biosurfactant properties
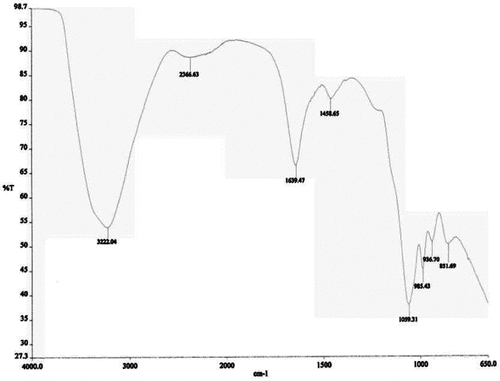
During the seven-day incubation of a 2 L flask containing 1000 mL growth limited (phosphate-free) medium, 5% (w/v) of the inoculum and 50 g L−1 of glycerol, the surface tension of the whole broth dropped rapidly from around 72 mN m−1 to about 35 mN m−1 in the first three days. The biosurfactant concentration in the cell-free broth was 30× CMD at a surface tension of ∼35 mN m−1. The lipopeptide had a CMC of 150 ± 5 mg L−1 corresponding to the minimum surface tension of ∼35 mN m−1, and showed an emulsification index of 75 ± 2.0 with hexane. The FTIR analysis () showed deformation vibrations at 1458 cm−1, which reflect aliphatic chains (–CH3, –CH2–) of the fraction. The sharp peak around 1639 cm−1 (stretching mode of the CO–N bond) is due to an amide group. This characteristically indicates the presence of a fatty acid chain of lipopeptide biosurfactant. Bands at 3235 cm−1 (NH stretching mode) are characteristic of peptides. This is the characteristic of carbon-containing compounds with amino groups. Sharp peaks in the range of 1100–1040 cm−1 indicate the presence of amine groups, which shows that peptide-containing moieties were present in the compound. This characterization shows that the biosurfactant is of a lipopeptide nature.
Desorption kinetics
The amount of PAHs desorbed from the soil increased as the concentration of biosurfactant in the solution and the equilibration time increased ( and ). Biosurfactant concentration is commonly considered as a critical factor for the removal of HOCs from soil. In the soil samples equilibrated with a solution containing lipopeptide at 700 mg L−1, 71% of the sorbed PHE and 48% of PYR were released after five days of equilibration from the contaminated soil of 800 mg kg−1 contamination level. This rapid desorption phase was followed by a second phase characterized by a slower rate, which remained constant until the end of the experiment (day 8). At concentrations above the CMC, hydrophobic pollutants can readily partition into the hydrophobic core at the centre of the micelle, thus increasing the HOC aqueous concentration through micellar solubilization and promoting the desorption of HOCs from the soil into the aqueous phase.[Citation39] Low desorption was observed when no or a relatively low concentration of lipopeptide was present in the soil–water system due to the high octanol/water partition coefficient of PHE and PYR (logKow of 4.57 and 5.18, respectively) and the fact that a portion of surfactant monomers in the aqueous phase was lost as a result of surfactant sorption onto soil.[Citation40,Citation41] Consequently, much higher chemical or biosurfactant concentrations are required to promote pseudo-solubilization of hydrophobic contaminants present in soil compared to requirements for solubilization in aqueous media alone.[Citation42] In fact, it has been demonstrated that the surfactant concentration required for soil biotreatment may have to be increased by an order of magnitude as compared to the amount of surfactant required for biotreatment in an aqueous system.[Citation42,Citation43] The amount of surfactant required to desorb HOCs in soil/sediment–water systems, which is considerably greater than the CMC in water, is described as critical desorption concentration, above which the desorption process was sharply accelerated with increasing surfactant concentration.[Citation39,Citation41,Citation44] In the 700 and 400 mg L−1 supplemented systems, PYR and PHE desorbed remarkably during the rapid desorption stage, while there was no significant desorption at 150 mg L−1, suggesting that the concentration is too low to promote pseudo-solubilization of the PAHs. The results in this study are similar to previous reports [Citation15,Citation22,Citation45,Citation46] that increasing the concentration of biosurfactant could enhance the removal of PAHs and total petroleum hydrocarbons from contaminated soil. On the contrary, the toxic effect of some biosurfactants needs to be considered when the biosurfactant is used to facilitate biodegradation of PAH pollutants with the indigenous microbial population in the soil, as excessive biosurfactant addition would adversely affect the microorganisms.[Citation5] However, for the purpose of washing hydrocarbon-contaminated soil, removing HOC pollutants, for oil recovery or further ex situ treatment, the amount of biosurfactant used could be much higher.[Citation15,Citation45]
Figure 2. Percentage of phenanthrene (PHE) desorbed in the presence of different concentrations of lipopeptide. Data are mean values from three independent experiments. Error bars represent standard error of the means.
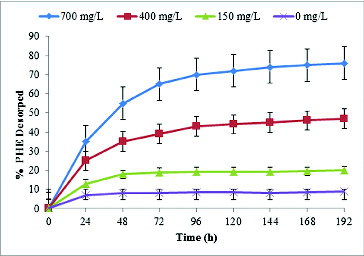
Figure 3. Percentage of pyrene (PYR) desorbed in the presence of different concentrations of lipopeptide. Data are mean values from three independent experiments. Error bars represent standard error of the means.
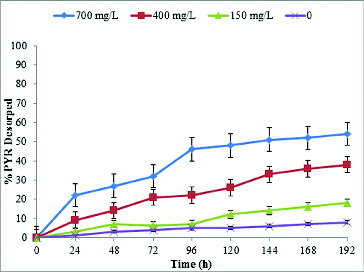
The extent and rate of sorption and desorption correlate with the organic matter content and texture of the soil and the hydrophobicity of the PAHs. The lower desorption rate of PYR (48%) compared to PHE (71%) can be explained by the more hydrophobic nature of PYR, which can be reflected by the higher octanol/water partition coefficient (Log Kow) of PYR. These results are in accordance with similar ones [Citation47,Citation48] showing greater affinities for a specific sorbent for more hydrophobic PAHs.
The obtained desorption kinetic curves were similar in shape to those reported in previous studies, using other extraction techniques, either with model sorbents [Citation24] or sediments [Citation29,Citation49]. All desorption kinetics curves were indeed observed to include an initial rapid desorption phase followed by slow desorption rates ( and ). The amount of PAHs desorbed from the soil increased as the concentration of biosurfactant in the solution and the equilibration time increased, as previously reported [Citation47,Citation50].
Desorption kinetics modelling
Mathematical fitting of desorption kinetics curves can give information about the rapidly desorbing fraction, which is generally considered to be the bioavailable fraction.[Citation24,Citation51] In our study, 192 h desorption kinetics curves were modelled for each PAH at each lipopeptide supplementation dosage, using the two-compartment model. For all PAHs, the experimental results fitted with the two-compartment model satisfactorily ((A) and (B)). Fitting the data to EquationEquation (1)(1)
(1) gave sums of squared deviations ranging from 0.00761 to 0.00013, indicating satisfactory fitness. The values obtained for the rapidly and slowly desorbing fractions (Frap, Fslow) and their rate constants (krap, kslow) are presented in . As expected, the desorption rate constants for the two-compartment model followed the progression of krap > kslow and were generally in the order of
, respectively. These results are in accordance with values reported in other studies for PAH-spiked soils and sediments.[Citation52,Citation53] The data for krap in suggest a slight decrease in desorption on increasing the molecular weight of PAHs.
Figure 4. Two-compartment model fits to PHE (A) and PYR (B) desorption kinetics data in the presence of 150 (■), 400 (▴) and 700 mg L−1 (♦) of lipopeptide. Data points are mean values from three independent experiments. Error bars represent standard errors of the means.
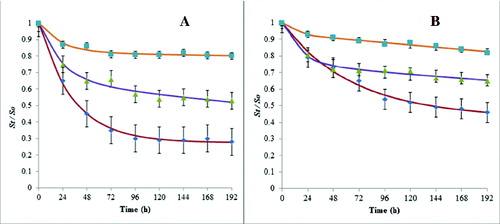
Table 1. Best-fit parameters of the two-compartment model for the different lipopeptide supplementations for each PAH.
Desorption rates
The desorption of PYR differed among the three samples at different lipopeptide supplementation levels (). The 8-day desorption percentage of PYR ranged from 51.2% to 6.4% with desorption proceeding at a greater extent in the 700 mg L−1amended variant than in the 400 and 150 mg L−1 amended ones. The increase in lipopeptide concentration for the spiked soil resulted in a similar enhancement of PYR desorption. The two-compartment model was used to analyse the data in and and the best-fit parameters of the model [EquationEquation (1(1)
(1) )] are summarized in . The rapid/slow desorption fractions were 51.2/48.8%, 24.5/75.5% and 6.4/93.6% for the 700, 400 and 150 mg L−1 supplementations, respectively. A less rapid fraction occurred for the 150 and 400 mg L−1 amendments compared to the 700 mg L−1 amendment.
The 8-day desorption percentage of PHE in the three samples of different lipopeptide supplementation levels () ranged from 72.8% to 18.1%. The same trend for a greater extent of desorption taking place in the 700 mg L−1 amended sample than in the 400 and 150 mg L−1amended ones. When the two-compartment model was used to analyse the data (), the rapid/slow desorption fractions were 72.8/27.2%, 39/61% and 18.2/81.8% for the 700, 400 and 150 mg L−1 supplementations, respectively. A less rapid fraction was observed for the 150 and 400 mg L−1 amendments as compared to the 700 mg L−1 amendment.
The values of kslow were two to three orders of magnitude lower than the krap values for all PAHs in different samples, which is consistent with other studies that apply the two-compartment desorption model [Citation42,Citation48,Citation54]. These results could be considered to well validate the biphasic behaviour of organic compounds desorption and to confirm the supposition of the model. In addition, the extractability of the PAHs in the studied soils decreased generally with increasing the molecular weight of the contaminating compound. The rapidly desorbing fraction decreased with the increasing hydrophobicity of PAHs and a positive relationship was found between the Fslow and the hydrophobicity of PAHs. A similar lipophilicity trend has also been observed for chlorobenzenes, other PAHs and polychlorinated biphenyls.[Citation55] This behaviour is the result of the increase in the hydrophobicity of PAHs as their molecular weight increases (four-ring PAHs have octanol/water partition coefficients (logKow) in the range of 5.20–5.80, in comparison to 3.94–4.60 for three-ring PAHs.[Citation56] This increased hydrophobicity indicates a greater tendency to remain adsorbed to organic matter in the soil.[Citation57]
Desorption of the fast-desorbing fraction occurred within four to six days, which is comparative with observations from other desorption studies of two to four days, [Citation24,Citation42,Citation58] where Tenax polymeric adsorbent beads were used in the desorption experiments. A number of assays have been developed to measure PAH bioavailability involving non-exhaustive extractions with low-molecular-weight primary alcohols such as propanol and butanol,[Citation59] and solid-phase adsorbents such as XAD resin or Tenax.[Citation5] Solid-phase extraction is one of the most common estimation methods, in which polymeric adsorbent resins (such as XAD resin or Tenax) function as an infinite sink, maintaining a steep concentration gradient between the aqueous and solid phases for maximum desorption.[Citation48] In general, the methods result in the extraction of a portion of the total amount of pollutant. It is assumed that the quantity of contaminant extracted by a non-exhaustive extraction technique or Tenax beads gives a measurement of the available pollutant pool.[Citation5] The rapidly desorbing (bioavailable) fraction has been used successfully to predict the extent of PAH degradation in field-contaminated sediments.[Citation48] In this study, the addition of increasing concentrations of lipopetide helped to increase the PAH desorption and expand the rapidly desorbing fraction. Accordingly, from the increasing rapidly desorbing fraction, which is the microbially degradable component of PAH contamination, we can predict an increase in the achievable bioremediation performance.
Conclusions
This study showed that biosurfactant produced by P. aeruginosa strain Lbp5 was effective in enhancing the desorption of sorbed PAHs and increasing the rate of mass transfer to the aqueous phase. This method, which allows the measurement of the rapidly desorbing fraction, could prove more relevant when predicting achievable bioremediation performances and designing intervention strategies to further increase the rapid desorption fraction. The study showed that the amount of PAHs desorbed in the rapid phase was in direct proportion to the biosurfactant present.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Xue W, Warshawsky D. Metabolic activation of polycyclic and heterocyclic aromatic hydrocarbons and DNA damage: a review. Toxicol Appl Pharmacol. 2005;206(1):73–93.
- Chadhain SMN, Norman RS, Pesce KV, Kukor JJ, Zylstra GJ. Microbial dioxygenase gene population shifts during polycyclic aromatic hydrocarbon biodegradation. Appl Environ Microbiol. 2006;72(6):4078–4087.
- Thenmozhi R. Characterization of microorganisms degrading used engine oil [dissertation]. Thiruchirappalli: Bharathidasan University; 2013. Available from: http://www.hdl.handle.net/10603/9642.
- Ouvrard S, Leglize P, Morel JL. PAH phytoremediation: rhizodegradation or rhizoattenuation? Int J Phytoremediation. 2014;16(1):46–61.
- Barnier C, Ouvrard S, Robin C, Morel JL. Desorption kinetics of PAHs from aged industrial soils for availability assessment. Sci Total Environ. 2014;470:639–645.
- Kanaly RA, Harayama S. Biodegradation of high-molecular-weight polycyclic aromatic hydrocarbons by bacteria. J Bacteriol. 2000;182(8):2059–2067.
- Elliot R, Singhal N, Swift S. Surfactants and bacterial bioremediation of polycyclic aromatic hydrocarbon contaminated soil—unlocking the targets. Crit Rev Env Sci Technol. 2010;41(1):78–124.
- Castaldini F. Bioremediation of PAHs - limitations and soultions [dissertation]. Bologna: Università di Bologna; 2008. Available from: http://www.amslaurea.unibo.it/130/.
- Zhu H, Aitken MD. Surfactant-enhanced desorption and biodegradation of polycyclic aromatic hydrocarbons in contaminated soil. Environ Sci Technol. 2010;44(19):7260–7265.
- Fortin N, Beaumier D, Lee K, Greer CW. Soil washing improves the recovery of total community DNA from polluted and high organic content sediments. J Microbiol Methods. 2004;56(2):181–191.
- Li H, Chen J, Jiang L. Elevated critical micelle concentration in soil–water system and its implication on PAH removal and surfactant selecting. Environ Earth Sci. 2014;71(9):3991–3998.
- Alcántara MT, Gómez J, Pazos M, Sanromán M. PAHs soil decontamination in two steps: desorption and electrochemical treatment. J Hazard Mater. 2009;166(1):462–468.
- Yu H, Huang G H, An C J, Wei J. Combined effects of DOM extracted from site soil/compost and biosurfactant on the sorption and desorption of PAHs in a soil–water system. J Hazard Mater. 2011;190(1):883–890.
- Yang XH, Garnier P, Wang SZ, Bergheaud V, Huang XF, Qiu RL. PAHs sorption and desorption on soil influenced by pine needle litter-derived dissolved organic matter. Pedosphere. 2014;24(5):575–584.
- Urum K, Pekdemir T, Gopur M. Optimum conditions for washing of crude oil-contaminated soil with biosurfactant solutions. Process Saf Environ Prot. 2003;81(3):203–209.
- Muherei MA, Junin R. Effect of electrolyte on synergism of anionic-nonionic surfactant mixture. J Appl Sci. 2007;7:1362–1371.
- Vreysen S, Maes A. Remediation of a diesel contaminated, sandy-loam soil using low concentrated surfactant solutions. J Soils Sediments. 2005;5(4):240–244.
- Pacwa-Płociniczak M, Płaza GA, Piotrowska-Seget Z, Cameotra SS. Environmental applications of biosurfactants: recent advances. Int J Mol Sci. 2011;12(1);633–654.
- Pei XH, Zhan XH, Wang SM, Lin YS, Zhou LX. Effects of a biosurfactant and a synthetic surfactant on phenanthrene degradation by a Sphingomonas strain. Pedosphere. 2010;20(6):771–779.
- Wang C, Liu H, Li J, Sun H. Degradation of PAHs in soil by Lasiodiplodia theobromae and enhanced benzo [a] pyrene degradation by the addition of Tween-80. Environ Sci Pollut Res. 2014;21(18):10614–10625.
- Mulligan CN, Yong RN, Gibbs BF. Surfactant-enhanced remediation of contaminated soil: a review. Eng Geol. 2001;60(1):371–380.
- Kuyukina MS, Ivshina IB, Makarov SO, Litvinenko L V, Cunningham CJ, Philp JC. Effect of biosurfactants on crude oil desorption and mobilization in a soil system. Environ Int. 2005;31(2):155–161.
- Pignatello JJ, Xing B. Mechanisms of slow sorption of organic chemicals to natural particles. Environ Sci Technol. 1995;30(1):1–11.
- Cornelissen G, van Noort PC, Govers H A. Mechanism of slow desorption of organic compounds from sediments: a study using model sorbents. Environ Sci Technol. 1998;32(20):3124–3131.
- Prichard H, Jones-Meehan J, Nestler C, Hansen LD, Straube W, Jones W, Talley JW. Polycyclic aromatic hydrocarbons (PAHs): improved land treatment with bioaugmentation. In: Talley J, editor. Bioremediation of recalcitrant compounds. Boca Raton, FL: CRC Press; 2006. p. 215–300.
- Bajpai R, Felt DR, Nestler CC, Wani A, Spain JC. Federal Integrated Biotreatment Research Consortium (FIBRC): flask to field initiative (no. ERDC/EL-TR-02-37). Vicksburg (MS): U.S. Army Engineer Research and Development Center; 2002.
- Cuypers C, Pancras T, Grotenhuis T, Rulkens W. The estimation of PAH bioavailability in contaminated sediments using hydroxypropyl-β-cyclodextrin and Triton X-100 extraction techniques. Chemosphere. 2002;46(8):1235–1245.
- Poot A, Jonker MTO, Gillissen F, Koelmans AA. Explaining PAH desorption from sediments using Rock Eval analysis. Environ Pollut. 2014;193:247–253.
- Congiu E, Ortega-Calvo JJ. Role of desorption kinetics in the rhamnolipid-enhanced biodegradation of polycyclic aromatic hydrocarbons. Environ Sci Technol. 2014;48(18):10869–10877.
- Liste HH, Alexander M. Butanol extraction to predict bioavailability of PAHs in soil. Chemosphere. 2002;46(7):1011–1017.
- Bezza FA, Chirwa EM. Optimization strategy of polycyclic aromatic hydrocarbon contaminated media bioremediation through biosurfactant addition. Chem Eng Trans. 2014;39:1597–1602.
- Vasileva-Tonkova E, Gousterova A, Neshev G. Ecologically safe method for improved feather wastes biodegradation. Int Biodeterioration Biodegradation. 2009;63(8):1008–1012.
- Ramana KV, Karanth NG. Factors affecting biosurfactant production using Pseudomonas aeruginosa CFTR-6 under submerged conditions. J Chem Technol Biotechnol. 1989;45(4):249–257.
- Trummler K, Effenberger F, Syldatk C. An integrated microbial/enzymatic process for production of rhamnolipids and L-(+)-rhamnose from rapeseed oil with Pseudomonas sp. DSM 2874. Eur J Lipid Sci Technol. 2003;105(10):563–571.
- Ghurye GL, Vipulanandan C, Willson RC. A practical approach to biosurfactant production using nonaseptic fermentation of mixed cultures. Biotechnol Bioeng. 1994;44(5):661–666.
- United States Environmental Protection Agency (US EPA). Ultrasonic extraction, method USEPA 3550B. Washington (DC): US EPA; 1996.
- Cooper DG, Goldenberg BG. Surface-active agents from two Bacillus species. Appl Environ Microbiol.1987;53(2):224–229.
- United States Environmental Protection Agency (US EPA). Method 8310, polynuclear aromatic hydrocarbons. Washington (DC): US EPA; 1986.
- Cheng KY, Zhao ZY, Wong JWC. Solubilization and desorption of PAHs in soil aqueous system by biosurfactants produced from Pseudomonas aeruginosa P-CG3 under thermophilic condition. Environ Technol. 2004;25(10):1159–1165.
- Zhang G, Liu X, Sun K, Zhao Y, Lin C. Sorption of tetracycline to sediments and soils: assessing the roles of pH, the presence of cadmium and properties of sediments and soils. Front Environ Sci Eng. 2010;4(4):421–429.
- Greenberg MS, Burton GA, Landrum PF, Leppänen MT, Kukkonen JV. Desorption kinetics of fluoranthene and trifluralin from Lake Huron and Lake Erie, USA, sediments. Environ Toxicol Chem. 2005;24(1):31–39.
- Ward OP. Microbial biosurfactants and biodegradation. In: Sen R, editor. Biosurfactants. Advances in experimental medicine and biology. Vol. 672. New York (NY): Springer Science+Business Media; 2010. p. 65–74.
- Sarubbo LA, de Luna JM, Rufino RD, Farias CBB, Santos VA. Production of biosurfactants for application in the removal of hydrophobic contaminants originated by the petroleum industry. Chem Eng Trans. 2012;27:7–12
- Zheng G, Wong JW. Application of microemulsion to remediate organochlorine pesticides contaminated soils. In Proceedings of the Annual International Conference on Soils, Sediments, Water and Energy; 2010. Vol. 15, Article 1. Available from: http://scholarworks.umass.edu/soilsproceedings/vol15/iss1/1.
- Schwab K, Brack W. Large volume TENAX® extraction of the bioaccessible fraction of sediment-associated organic compounds for a subsequent effect-directed analysis. J Soils Sediments. 2007;7(3):178–186.
- Van Noort P, Cornelissen G, ten Hulscher TE, Vrind BA, Rigterink H, Belfroid A. Slow and very slow desorption of organic compounds from sediment: influence of sorbate planarity. Water Res. 2003;37(10):2317–2322.
- Brinck J, Jönsson B, Tiberg F. Influence of long-chain alcohols on the adsorption of nonionic surfactants to silica. Langmuir. 1999;15(22):7719–7724.
- Richardson SD, Aitken MD. Desorption and bioavailability of polycyclic aromatic hydrocarbons in contaminated soil subjected to long-term in situ biostimulation. Environ Toxicol Chem. 2011; 30(12):2674–2681.
- Cornelissen G, van Noort P, Govers HA. Desorption kinetics of chlorobenzenes, polycyclic aromatic hydrocarbons, and polychlorinated biphenyls: sediment extraction with Tenax® and effects of contact time and solute hydrophobicity. Environ Toxicol Chem. 1997;16(7):1351–1357.
- Sverdrup LE, Nielsen T, Krogh PH. Soil ecotoxicity of polycyclic aromatic hydrocarbons in relation to soil sorption, lipophilicity, and water solubility. Environ Sci Technol. 2002;36(11):2429–2435.
- Loehr RC, Lamar MR, Poppendieck DG. A protocol to estimate the release of anthropogenic hydrocarbons from contaminated soils. Environ Toxicol Chem. 2003;22(9):2202–2208.
- Zhou W, Zhu L. Efficiency of surfactant-enhanced desorption for contaminated soils depending on the component characteristics of soil-surfactant–PAHs system. Environ Pollut. 2007;147(1):66–73.
- Yang K, Zhu L, Xing B. Enhanced soil washing of phenanthrene by mixed solutions of TX100 and SDBS. Environ Sci Technol. 2006;40(13):4274–4280.
- Zheng G, Selvam A, Wong JW. Enhanced solubilisation and desorption of organochlorine pesticides (OCPs) from soil by oil-swollen micelles formed with a nonionic surfactant. Environ Sci Technol. 2012;46(21):12062–12068.
- Lai CC, Huang YC, Wei YH, Chang JS. Biosurfactant-enhanced removal of total petroleum hydrocarbons from contaminated soil. J Hazard Mater. 2009;167(1):609–614.
- An CJ, Huang GH, Wei J, Yu H. Effect of short-chain organic acids on the enhanced desorption of phenanthrene by rhamnolipid biosurfactant in soil–water environment. Water Res. 2011:45(17),5501–5510.
- Sánchez-Trujillo MA, Morillo E, Villaverde J, Lacorte S. Comparative effects of several cyclodextrins on the extraction of PAHs from an aged contaminated soil. Environ Pollut. 2013;178(1):52–58.
- Franzetti A, Gandolfi I, Bestetti G, Banat IM. Biosurfactant and bioremediation, successes and failures. In: Plaza G, editor. Trends in bioremediation and phytoremediation. Kerala: Research Signpost; 2011. p. 145–156.
- Mahmoudi N, Slater GF, Juhasz AL. Assessing limitations for PAH biodegradation in long-term contaminated soils using bioaccessibility assays. Water Air Soil Pollut. 2013;224(2):1–11.