ABSTRACT
Enzymatic browning is one of the main obstacles encountered in the establishment of suspension systems of licorice cells. Browning of cells may result in decreased viability, poor growth and even death. The present study investigated the mechanism of browning reactions and the effective controlling methods. The results showed that the cell viability and membrane permeabilization obviously changed when the cells were transferred to liquid medium. The transformation caused rapid increase in the levels of polyphenol oxidase activity and in the production of polyphenols. Osmotic and hydrodynamic stresses arising from liquid culture were regarded as the major causes of enzymatic browning. Ascorbic acid and L-cysteine were found to be the most significant anti-browning agents that could decrease the degree of browning with 55.8% and 52.2%, respectively, at the end of the suspension culture's lag phase. When cultured with a cycle of 21 days, the maximum biomass of the cells cultured with ascorbic acid and L-cysteine increased with 31.1% and 26.5%, respectively, when compared to the control. These findings may be essential for the development of licorice cell cultures devoted to browning prevention and cell viability maintaining.
Abbreviations
| PPO | = | polyphenol oxidase |
| 6-BA | = | 6-benzyladenine |
| 2,4-D | = | 2,4-dichlorophenoxyacetic acid |
| NAA | = | naphthaleneacetic acid |
| TTC | = | 2,3,5-triphenyltetrazolium chloride |
| MS | = | Murashige and Skoog |
| SDS | = | sodium dodecyl sulfate |
| wt | = | weight |
| OD | = | optical density |
| EDTA-Na2 | = | disodium ethylenediaminetetraacetate dihydrate |
| LSD | = | Fisher's least significant difference |
| GAE | = | gallic acid equivalent |
| VC | = | ascorbic acid |
Introduction
Licorice (Glycyrrhiza inflata Batalin), a perennial plant native to Iran, has been used medicinally for over 2000 years in China, where herbalists generally prescribe it as a component in formulations.[Citation1,Citation2] It has numerous pharmacological effects, including antioxidant, antiulcer, anti-inflammatory and antiviral properties. In addition, it is also widely used as flavouring additive in food industry, due to its sweet taste. In China, licorice is called Gan-Cao, which means ‘sweet weed.’ Glycyrrhizin, the main ingredient in licorice, is a sweetening agent with 50 times higher sweetness than sucrose and is often used in confectionary and tobacco products. The natural sources of G. inflata are gradually exhausted. Plant cell cultures are promising potential alternative sources for the production of high value secondary metabolites of industrial importance.[Citation3] In our research, it was found that the major problem in building a stable suspension culture system of licorice cells was the high mortality rate, due to lethal browning, when the cells were initially transferred from solid to liquid medium. In a severe case, all the cells in the flask died because of some harmful substances produced from brown cells, such as o-quinones and other dark-coloured compounds, which would gradually infect normal cells.[Citation4,Citation5] This is why it is especially important to control the browning on time.
The browning reaction requires the presence of oxygen, phenolic compounds and polyphenol oxidases (PPO). The process is usually initiated by the enzymatic oxidation of monophenols into o-diphenols and o-diphenols into o-quinones. Thus phenolic compounds, together with the activity of PPO, are responsible for the enzymatic browning.[Citation6,Citation7] For most plant tissues, PPO is believed to be located in plastid membranes, including the amyloplast,[Citation8,Citation9] whereas phenols are assumed to be located in the vacuole. PPO activation occurs only when these compartments are disrupted after tissue wounding.[Citation10] The browning in plant tissues is the result of physical damage. The naturally occurring monophenolic compounds interact with the atmospheric oxygen and the endogenous PPO enzymes are hydroxylated to o-diphenols.[Citation11]
The purpose of this study was to investigate the mechanism of enzymatic browning reactions in licorice cell suspension cultures. Based on the mechanism, an effective inhibition strategy was applied to inhibit the occurrence of browning. These findings may be essential for future developments of a large scale culture of licorice cells devoted to prevention of browning and maintaining cell viability.
Materials and methods
Materials and chemicals
The original seeds of G. inflata were obtained from Sinkiang, China. Naphthaleneacetic acid (NAA), 2,4-dichlorophenoxyacetic acid (2,4-D), 6-benzyladenine (6-BA), 2,3,5-triphenyltetrazolium chloride (TTC) and Evans blue were purchased from Sigma, USA. Water was obtained from a Milli-Q water purification system (Millipore, Bedford, MA, USA). All other chemicals were of analytical grade.
Cells culture
Cell suspension culture was initiated via the agitation of 6 g callus induced from young hypocotyls and cotyledons of G. inflata in 250 mL Erlenmeyer flasks, containing 100 mL Murashige and Skoog (MS) culture liquid medium, supplemented with 30 g/L sucrose (Sigma, USA), 0.5 mg/L NAA and 0.5 mg/L 6-BA, at 120 rpm for 3 weeks. Previous studies have shown that the cells culture is an aerobic process.[Citation12] On the other hand, the cells would be slowly dying in anaerobic conditions, so the Erlenmeyer flasks were sealed with a membrane with filter holes to allow sterile air into the flasks.
Cell membrane permeability tests
The cell membrane permeability was analysed on day 8 by staining the cells with Evans blue.[Citation13] The culture's suspension (10 mL) was centrifuged (Eppendorf, Germany) at 10,000 rpm for 10 min and was washed three times with 50 mmol/L phosphate buffer (pH 5.8); then the cells were collected and stained for 5 min with a 0.15% (w/v) solution of Evans blue. The stained cells (50 mg) were collected and resuspended in 5 mL solution consisting of 1.0% (w/v) sodium dodecyl sulphate (SDS) and 50% (v/v) methanol for 30 min at 50 °C. The supernatant was collected and its absorbance was measured (Purkinje General, China) at 600 nm at room temperature. Cells cultured in solid medium were used as a control.
Cell viability tests
The cell viability was assayed with TTC for 18 days.[Citation11,Citation13] Fresh cells (200 mg) were collected in 8 mL TTC solution (0.8% TTC in 0.05 mol/L phosphate buffer, pH 7.4, and 0.5 mL/L Tween 20 (Sigma, USA)) and the mixture was infiltrated for 5 min. Then the cells were rinsed with distilled water, placed in separate quartz tubes containing 3 mL of 95% ethanol and submerged for 24 h at 25 °C in the dark. The absorbance of samples per gram of biomass (abs/g fresh weight (Fw)) was measured at 485 nm. The cells cultured in solid medium were used as a control.
PPO extraction and activity assay
The licorice cells were collected on culture day 6 by obtaining fresh weights after filtration through a Buchner funnel. Washed with sterile water cells (1 g, fresh weight (Fw)) were homogenized with 0.1 mol/L phosphate buffer, pH 6.5 in ice water. Cell homogenate was centrifuged at 14,000 rpm for 30 min at 4 °C. The precipitate was dissolved in 5 mL of 10 mmol/L phosphate buffer (pH 6.5) and dialyzed against the same buffer at 4 °C for 24 h.[Citation7,Citation14,Citation15] The PPO enzymatic activity was measured by using catechol (Sigma, USA) as an exogenous substrate. The reaction medium contained enzymatic extract and 100 mmol/L catechol in 50 mmol/L phosphate buffer and was incubated at 37 °C for 120 min. PPO activity was measured by increased absorbance at 400 nm using a spectrophotometer. One unit of PPO activity was defined as the amount of enzyme that caused a change in absorbance of 0.001 optical density (OD) per min. The extracellular PPO activity was directly obtained at 25 °C for 60 s by measuring the absorbance of the extracellular culture medium at 400 nm. The cells cultured in the solid medium were used as a control.
Polyphenol measurements
The concentration of polyphenols was used as a quantitative index of enzymatic browning in plant cell cultures, since the brown or black pigments of enzymatic browning are caused mainly by polymeric phenols. In addition, the polyphenols content also gave information about phenol release from the cultures.[Citation11] For the extraction of polyphenols, the fresh cells were homogenized in the culture medium on culture day 5.[Citation16] The homogenate was spun at 13,000 rpm to obtain a cell-free extract. Polyphenols concentration in the cell-free extract (intracellular) and the cell-free medium (extracellular) was calculated by measuring the absorbance at 420 nm. The standard curve measurement was accomplished by using gallic acid, according to the gallic acid standard curve. The acid's equivalent concentration was calculated (mg gallic acid equivalent (GAE)/g cell). This was the total polyphenols content.
Analysis of relative browning degree in different culture conditions
To determine if the lethal browning is related to osmotic stress induced by the MS liquid medium and hydrodynamic events, induced by the oscillating waves, the degree of relative browning of the suspension cultures was analysed in different initial sucrose concentrations and in series of initial agitation speeds. The sucrose concentrations were between 10 and 40 g/L (sucrose concentration is important factor causing osmotic stress) at agitation speed of 120 rpm, and the agitation speed was between 80 and 160 rpm at an initial sucrose concentration of 30 g/L. The cell suspension solution was centrifuged at 10,000g for 20 min and the absorbance of the supernatant was determined at 420 nm to evaluate the degree of relative browning.
Effect of modulating agents on PPO activity
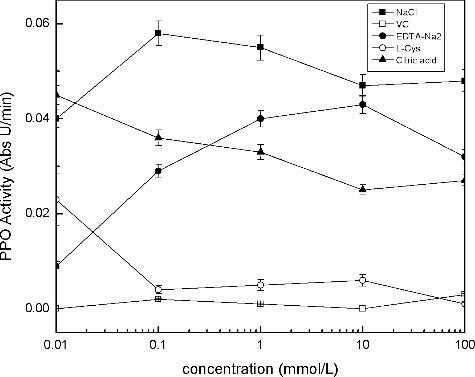
Licorice cell PPO activity was tested in the presence of varied concentrations (0.01–100 mmol/L) of sodium chloride, L-cysteine, ascorbic acid, disodium ethylenediaminetetraacetate dihydrate (EDTA-Na2) and citric acid. PPO activity was determined using 50 mmol/L catechol as a substrate.[Citation7]
Examination of the inhibitory effects of anti-browning agents
In order to test the inhibitory effects of anti-browning agents, 3 g of fresh cells were inoculated into 250 mL sterile Erlenmeyer flasks containing 80 mL liquid medium and various screened anti-browning agents (0.1 mmol/L L-cysteine and 0.1 mmol/L ascorbic acid) were added in the medium. No inhibitor was added for the control. On day 6 during the 21 d cycle, the cell suspension solution was centrifuged at 10,000g for 20 min and the absorbance of the supernatant was determined at 420 nm to evaluate the degree of relative browning. In addition, the growth of cells was investigated after adding the anti-browning agents. The samples from flasks were washed with distilled water and filtered. The cells were weighed to obtain the fresh weight. The obtained fresh weight values were the mean of three replicates.
Statistical analysis
All the experiments were carried out in three replicates. Analyses of variance and Fisher's least significant difference (LSD) analysis (p < 0.05) were carried out to determine the statistical differences between the treatments and the control (SPSS 17.0).
Results and discussion
Changes of membrane permeability observed with Evans blue staining
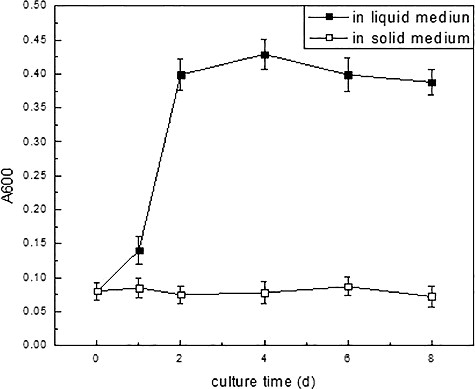
The change in the cell membrane permeability in liquid medium was reflected by the Evans blue assay (). Evans blue uptake rates increased by about 4.5-fold of the initial value on day 4. The results indicated that the membrane integrity broke when the cell was transferred from solid to liquid medium. Integrated cell membrane is impermeable for macromolecules, such as Evans blue.[Citation13,Citation17] When cell membrane undergoes an injury, however, macromolecules can penetrate it. Thus, the amount of Evans blue entering and binding to the cell reflects the degree of cell membrane integrity loss.[Citation18] The results showed that the initial suspension culture destroyed the integrity of the cell membrane.
Effects of suspension culture on cell viability
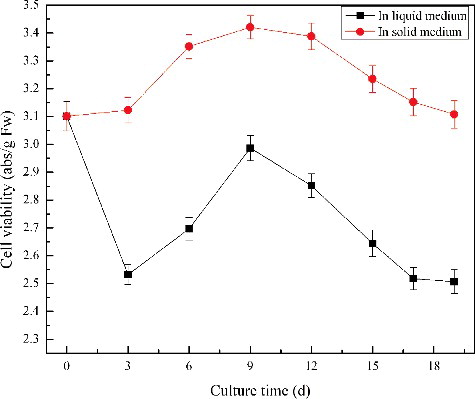
Cell viability was quantified by mitochondrial reduction of TTC (). The results showed that the cell viability was affected and decreased with 19.3% 3 days after the cells were transferred to liquid medium. The decline of cell viability was due to cells' browning and death, inclusions leaking and accumulation of cell debris.
Changes of PPO activity and polyphenols production
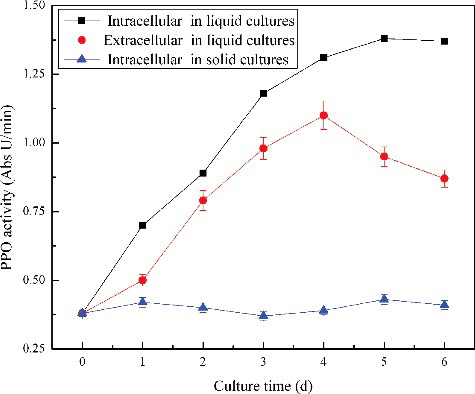
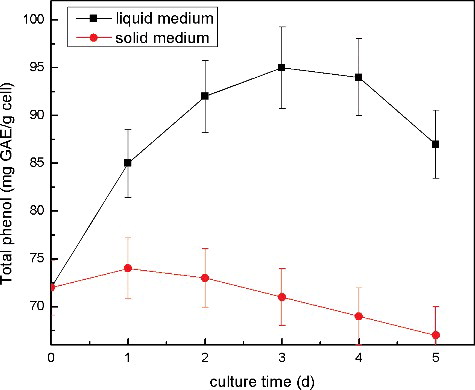
After the licorice cells were transferred to liquid medium, it could be seen that some cells gradually turned darker. The intracellular activity of PPO significantly increased in suspension cultures on day 4, when compared to that of the control (). The total (intracellular + extracellular) polyphenols increased, accompanied by the increasing of the PPO activity in the suspension cultures (). The increase of both PPO activity and polyphenols proved that enzymatic browning occurred in the suspension cultures. Enzymatic browning is a result from the PPO catalysed oxidation of phenolic compounds and the polymerization of the oxidation products, so that the reaction rate mainly depends on the PPO activity. The PPO oxidation of phenolics requires the disruption or permeabilization of the tonoplast membrane surrounding the vacuoles for the release of phenolics into the cytosol. Therefore, the browning reactions that occurred in the cell suspension cultures suggested that the cell damage and membrane permeabilization occurred in the cells.
Relative browning degree in different culture conditions
The analysis showed that the relative browning degree of suspension cultures increased with the increase of the initial sucrose concentration in suspension cultures after day 1 (). Sucrose concentration above 30 g/L resulted in fast browning and death of the suspension cells. This result indicated that a sucrose concentration of 30 g/L produced a high osmotic pressure environment for licorice cells. The difference of intracellular and extracellular osmotic pressure results in an irreversible permeabilization of the tonoplast surrounding the cell vacuole. The occurrence of irreversible permeabilization of the plasma membrane could be seen from the increased loss of intracellular liquid into the culture medium. Release of intracellular liquid indicated a decompartmentation of the cells leading to interactions between phenolic substrates and PPO. In addition, the relative browning degree of suspension cultures also increased with the increase of the agitation speed, when it was greater than 100 rpm (). Successful culture of plant cell suspensions requires mixing of the cells with the medium and facilitating homogenous nutrient uptake. However, this subjects the cells to hydrodynamic shear forces that can cause many physiological and morphological changes in cell size and shape, aggregation, cell wall composition, cell membrane integrity, etc.[Citation16] These results showed that the cell damage that caused browning reaction was the main result from osmotic and hydrodynamic stress of liquid culture environment.
Table 1. Degree of suspension cultures' relative browning under series of initial sucrose concentrations (OD420 nm × 10−2).
Table 2. Degree of suspension cultures' relative browning under a series of initial agitation speeds (OD420 nm × 10−2).
Effect of modulating agents
According to the literature,[Citation19] anti-browning agents can be classified as reducing agents, acidulants, chelating agents, complexing agents and enzyme inhibitors. In this study, PPO activity can be regulated by the action of series of inhibitors (). Among the tested anti-browning agents, sodium chloride promoted PPO activity at concentrations of 0.01 and 100 mmol/L, EDTA-Na2 and citric acid had no significant effect on the inhibition of PPO activity at any concentration. L-cysteine prevented browning at concentrations of 0.1 mmol/L. As a sulfhydryl-containing amino acid, L-cysteine prevented brown pigment formation by reacting with the o-quinones intermediates to form stable colourless compounds. Cysteine–quinone adducts were proved to be competitive inhibitors of polyphenol oxidase.[Citation19] Direct inhibition of polyphenol oxidase by cysteine through the formation of stable complexes with copper has also been proposed.[Citation19] Ascorbic acid was a stronger inhibitor of PPO activity, causing almost a complete enzyme inactivation even at low concentrations. It is the most frequently used chemical product for the chemical reduction of o-quinones back into o-diphenolic compounds for browning control.[Citation20] Thus, subsequent assays only focused on some effective inhibitor including ascorbic acid and L-cysteine.
Inhibitory effects of anti-browning agents
The inhibitory effects of the two inhibitors, L-cysteine and ascorbic acid, on the browning of the suspension culture cells are shown in . The relative browning degree of suspension cultures was determined with the two anti-browning agents at the lag phase, because the browning mainly occurred at this stage. When ascorbic acid was used as an inhibitor, the degree of browning reduced with 55.8% and the cells' biomass (fresh wt) increased with 35.2% at the 6th day, when compared with the control. When cultured with a cycle of 21 d, the maximum biomass of the cells increased with 31.1%, when compared to the control. It could be seen that the inhibitory effect of ascorbic acid was more obvious at the beginning of the culture period. This was maybe because nearly all of the ascorbic acid was converted into dehydroascorbic acid at later time.[Citation21] In general, ascorbic acid turned out to be effective to control the browning. L-cysteine was another effective inhibitor of the browning. The degree of browning decreased with 52.2% and the cells biomass increased with 12.5%, when compared with the control at the 6th day. The maximum biomass of the cells increased with 26.5% when cultured with a cycle of 21 d.
Table 3. Inhibitory effects of two inhibitors (ascorbic acid and L-cysteine).
Conclusions
In conclusion, browning occurred only when a physical stress that was sufficient to cause membrane damage was applied to the suspend cells, containing the required enzymes and substrates. The damage is related to osmotic and hydrodynamic stresses from the liquid culture environment. PPO is responsible for the enzymatic browning reaction in plant cell suspension cultures. In the present study, ascorbic acid and L-cysteine showed to be potent enzymatic inhibitors for the suspension culture of licorice cells. The browning was effectively inhibited when 0.1 mmol/L of each inhibitor was added to the suspension solution. As a result, the cell viability was maintained and the cell growth was accelerated. These findings could be essential for future developments in large scale culture of licorice cells devoted to browning prevention and cell viability maintaining.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Zhang QY, Ye M. Chemical analysis of the Chinese herbal medicine Gan-Cao (licorice). J Chromatogr A. 2009;1216:1954–1969.
- Li YL, Yang Y, Fu CH, et al. Production of Glycyrrhizin in cell suspension of Glycyrrhiza inflata batalin cultured in bioreactor. Biotechnol Biotechnol Equip. 2012;26(5):3231–3235.
- Zhang HC, Liu JM, Lu HY. Enhanced flavonoid production in hairy root cultures of Glycyrrhiza uralensis Fisch by combining the over-expression of chalcone isomerase gene with the elicitation treatment. Plant Cell Rep. 2009;28:1205–1213.
- He Y, Guo XL, Lu R. Changes in morphology and biochemical indices in browning callus derived from Jatropha curcas hypocotyls. Plant Cell Tiss Org. 2009;98:11–17.
- Ko WH, Su CC, Chen CL. Control of lethal browning of tissue culture plantlets of Cavendish banana cv. Formosana with ascorbic acid. Plant Cell Tiss Org. 2009;96:137–141.
- Altunkaya A, Gokmen V. Effect of various inhibitors on enzymatic browning, antioxidant activity and total phenol content of fresh lettuce (Lactuca sativa). Food Chem. 2008;107:1173–1179.
- Queiroz C, da Silva AJR, Lopes MLM. Polyphenol oxidase activity, phenolic acid composition and browning in cashew apple (Anacardium occidentale, L.) after processing. Food Chem. 2011;125:128–132.
- Yu YC, Tang T, Qian Q. Independent losses of function in a polyphenol oxidase in rice: differentiation in grain discoloration between subspecies and the role of positive selection under domestication. Plant Cell. 2008;20:2946–2959.
- Yoruk R, Marshall MR. Physicochemical properties and function of plant polyphenol oxidase: a review. J Food Biochem. 2003;27:361–422.
- Mayer AM. Polyphenol oxidases in plants and fungi: going places? A review. Phytochem. 2006;67:2318–2331.
- Dörnenburg H, Knorr D. Evaluation of elicitor-and high-pressure-induced enzymatic browning utilizing potato (Solanum tuberosum) suspension cultures as a model system for plant tissues. J Agr Food Chem. 1997;45:4173–4177.
- Zhang CY, Dong YS, Li YL, et al. Unstructured models for suspension cultures of Taxus media cells in a bioreactor under substrate-sufficient conditions. Biochem Eng J. 2013;71:62–71.
- Xu QM, Cheng JS, Ge ZQ. Effects of organic solvents on membrane of Taxus cuspidata cells in two-liquid-phase cultures. Plant Cell Tiss Org. 2004;79:63–69.
- Segovia-Bravo KA, Jaren-Galan M, Garcia-Garcia P. Treatments to inhibit the browning reactions in model solutions of olive fruit extracts. Food Chem. 2010;123:741–746.
- Segovia-Bravo KA, Jaren-Galan M, Garcia-Garcia P. Browning reactions in olives: mechanism and polyphenols involved. Food Chem. 2009;114:1380–1385.
- Loc NH, Tuan VC, Binh DHN, et al. Accumulation of sesquiterpenes and polysaccharides in cells of zedoary (Curcuma zedoaria Roscoe) cultured in a 10 L bioreactor. Biotechnol Bioprocess Eng. 2009;14(5):619–624.
- Kajani AA, Moghim S, Mofid MR. Optimization of the basal medium for improving production and secretion of taxanes from suspension cell culture of Taxus baccata L. DARU J Pharm Sci. 2012;20(1):816–822.
- Xu Q, Zhao Q, Zhao C, et al. Effects of Ce4+ on membrane integrity of rice in seedling hydroponic cultures. Agric Sci. 2014;5:785–792.
- Ding CK, Chachin K, Ueda Y. Inhibition of loquat enzymatic browning by sulfhydryl compounds. Food Chem. 2002;76:213–218.
- Lopez J, Uribe E, Vega-Galvez A. Effect of air temperature on drying kinetics, vitamin C, antioxidant activity, total phenolic content, non-enzymatic browning and firmness of blueberries variety O' Neil. Food Bioproc Tech. 2010;3:772–777.
- Shams-Ardakani M, Mohagheghzadeh A, Ghannadi A. Formation of glycyrrhizin by in vitro cultures of Glycyrrhiza glabra. Chem Nat Compd. 2007;43:353–354.