ABSTRACT
Green synthesis of nanoparticles has an increasing benefit because of the rising need for developing environmentally friendly, cost-effective and safe strategies for nanomaterials synthesis. In this study, we investigated the fungus Trichoderma viride, which is used for the synthesis of biogenic silver nanoparticles. The bioreduction of silver nanoparticles (Ag NPs) was observed spectrophotometrically, and the studied Ag NPs were characterized by ultraviolet–visible spectroscopy (UV–Vis), transmission electron microscopy and scanning electron microscopy. The Ag NPs synthesized by T. viride were observed as stabilized and polydispersed globular particles, in sizes ranging from 1 to 50 nm. The antibacterial potential of Ag NPs was evaluated against human pathogenic bacteria. The biogenic Ag NPs significantly inhibited the growth of all tested pathogenic bacteria.
Introduction
Nanoparticles have an important role in the pharmaceutical and biotechnology industries.[Citation1] The development of silver nanoparticles (Ag NPs) biosynthesis is preferred because this is an eco-friendly and safe synthetic procedure. The synthesis of nanoparticles is one of the fastest growing fields because of their chemical, physical and biological features. Biogenic methods for nanoparticles forming by fungi,[Citation2,Citation3] bacteria [Citation4,Citation5] and plants [Citation6–8] have formerly been described. The biological synthesis of nanoparticles from fungi is considered to be a significant branch, due to the fungi's tolerance and metal bioaccumulation capability. The fungi are capable of biogenic Ag NPs formation and their biomass is easier to handle, hence, they are favoured over bacteria and other micro-organisms.[Citation5] Aspergillus terreus [Citation9] and biocontrol agents, such as Trichoderma harzianum, Trichoderma virens, Trichoderma asperellum, Trichoderma pseudokoningii and Trichoderma longibrachiatum [Citation10,Citation11] have been used for Ag NPs synthesis. Trichoderma viride is a mycoparasitic fungus that produces a great number of hydrolytic enzymes and is used against plant pathogenic fungi. Due to the high ability of biomass transformation, T. viride attracts great interest from research fields and biological control industries. This fungus is one of the best biocontrol agents, because of its ability to produce many enzymes, such as chitinases, glucanases and proteases.[Citation12,Citation13] Also, T. viride produces glycosyl hydrolases, such as xylanases, cellulases and mannanases [Citation14,Citation15] under suitable conditions. This study aimed to quickly synthetize biogenic Ag NPs by using T. viride, which is a nonpathogenic, fast growing and environmentally friendly fungus. Also, we studied the properties of the biogenic Ag NPs by several techniques and evaluated them against methicillin-resistant Staphylococcus aureus (MRSA) and four Gram-negative bacteria (Shigella boydii, Acinetobacter baumannii, Shigella sonnei and Salmonella typhimurium).
Materials and methods
Studied micro-organisms
T. viride was isolated from greenhouses soil in Riyadh, Saudi Arabia, and kept on potato dextrose agar medium (Sigma-Aldrich, Germany) at 5 °C. One Gram-positive bacterium (MRSA ATCC 43300) and four Gram-negative bacteria (A. baumannii ATCC BAA747, S. boydii ATCC 9207, S. sonnei ATTC 25931 and S. typhimurium ATCC 14028) were evaluated for their susceptibility to Ag NPs. All bacteria strains were obtained from Military Hospital, Riyadh, Saudi Arabia.
Preparation of biomass
T. viride was grown in liquid medium (Sigma-Aldrich, Germany) containing malt extract (0.3%), yeast extract (0.3%), glucose (1.5%) and peptone (0.5%). Erlenmeyer flasks were inoculated with spore suspension of T. viride and incubated at 25 °C on a rotary shaker (150 rpm) for 7 d. Mycelial mat was collected by using filter paper (Whatman No. 1). The biomass was washed with sterilized water. Mycelial mats (20 g) were added to flasks containing 200 mL sterilized distilled water and were incubated at 25 °C for 24 h. The biomass was refined, and the free crude filtrate was collected for the following tests.
Biosynthesis of Ag NPs
Ag NPs were biogenically synthesized by mixing 100 mL cell filtrate with 20 mL silver nitrate suspension (10 mmol/L) in a 250 mL Erlenmeyer flask incubated at 25 °C for 24 h in darkness. A flask without Ag+ was used as control. Ag NPs were intensified by centrifugation (Universal 320R, Hettich, Germany) at 11,000 rpm twice for 15 min and collected for additional tests.
Characterization of silver nanoparticles
UV–visible spectral analysis
The bio reduction of Ag+ in suspension was observed by ultraviolet–visible spectroscopy (UV–Vis) of the solution between 420 and 640 nm by using PerkinElmer LAMBDA 35 UV–Vis spectrophotometer (USA). The produced slimy substance was treated with acetone, leading to the deposition of Ag NPs. The Ag NPs were collected by centrifugation (14,000 rpm twice for 10 min) and were air-dried to obtain a powder.
Transmission electron microscopy (TEM)
TEM was performed by using JEOL microscope (JEM-1011), with an accelerating voltage of 80 keV. After drying of an aqueous Ag NPs' drop on the carbon-coated copper TEM grids, the specimens were dried under vacuum (Model 1:53 litres vacuum oven, Vinci Technologies, France) before loading them onto a sample hold. This assay was done to determine the Ag NPs shapes and sizes.
Scanning electron microscopy (SEM)
The SEM images were made by using JEOL microscope (JSM-6380 LA). The specimens were air-dried before measurements.
Energy dispersive spectroscopy (EDS)
For the EDS (Altima IV Make: Regaku, Japan) assay, the specimens were prepared on a copper substrate by drop-coating Ag NPs. The metal analysis of the single particles was done by JEOL JSM-6380 LA SEM.
Antibacterial activity of silver nanoparticles
The antibacterial activity of Ag NPs, biosynthesized by T. viride, was examined against pathogenic bacteria i.e. MRSA, S. boydii, A. baumannii, S. sonnei and S. typhimurium by using the agar well-diffusion method. The tested bacteria were grown on nutrient broth medium at 30 °C ± 2 °C for 48 h (3.0 g beef extract, 5.0 g/L peptone and a final pH of 6.8 ± 0.2; Sigma-Aldrich, Germany). After this, the bacteria were homogeneously swapped onto individual plates by using sterile cotton swabs. Wells of 3 mm diameter were made on Mueller–Hinton agar (3.0 g beef infusion, 1.5 g starch, 17.5 g peptone, 17.0 g/L agar; pH 7.4 ± 0.2; Sigma-Aldrich, Germany) by using cork borer. Ag NPs (75 μL) were poured in each well, and four antibiotic disks (gentamicin, ampicillin, tetracycline and neomycin) were used as controls. After a 24 h incubation period, the inhibition zones (mm) were measured. Four replicates of the trials were done.
Statistical analysis
The statistical results are presented as the mean ± standard deviation (SD) at P < 0.05.
Results and discussion
UV–Vis spectrum of Ag NPs
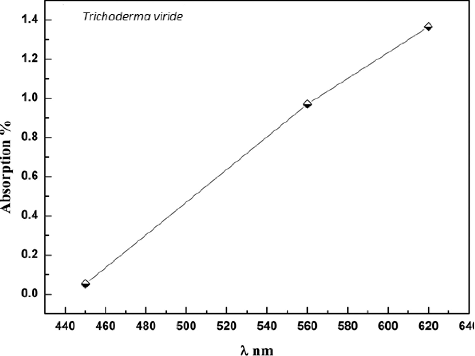
T. viride free cell extract with AgNO3 showed dark brown colour in water ().[Citation6,Citation16] The emergence of brown colour was due to the formation of Ag NPs.[Citation9] This colour is mainly referred to the surface plasmon resonance of the Ag NPs.[Citation9] No change in colour was detected in the control (without AgNO3) (A)).[Citation17] Therefore, it was clear from the obtained results that the ability of T. viride to synthesize Ag NPs was detected. The specimen was examined in the wavelength range from 420 to 640 nm; an extreme absorption was detected at 620 nm after 24 h of incubation (). The existence of Ag was proven through the obtained results.
Transmission electron microscopy (TEM)
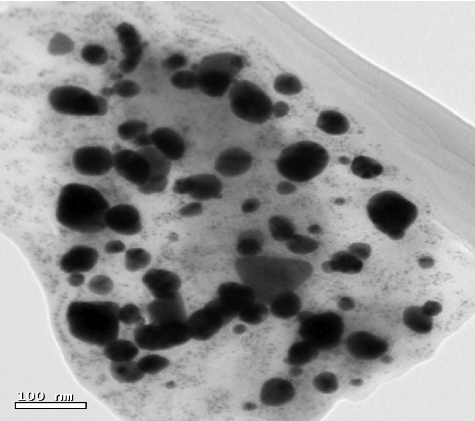
TEM was used to determine the shape of the synthesized Ag NPs. Individual Ag NPs as well as some assembled spherical Ag NPs can be seen as shown in . Ag NPs are seen to be linked to the surface of extremely huge biomolecules. Also, TEM metrics were conducted in order to assess the sizes and distribution of sizes for the prepared samples. The sizes of the nanoparticles depend on the nature of the used medium. Normally, by using a chemical method, the corresponding chemical may act as a stabilizing agent. But in the case of using a biosynthesis method, instead of chemicals, the fungal biomass filtrate is used as medium for the synthesis of the Ag NPs. In this case, the sizes of the nanoparticles cannot be controlled and the distribution of the sizes is wide – from 1 to 50 nm. Ag NPs were not in direct contact even in the aggregations and were enclosed by thin layer of organic material. This material may represent a capping agent, providing stability for the NPs.[Citation11]
Scanning electron microscopy (SEM)
The SEM images displayed spherical and irregularly formed Ag NPs with various sizes (). A presence of some Ag NPs groups can be seen, which was probably related to gathering of nanoparticles formed during the specimen production. These most probably contributed to the difference in the particles' sizes ranging from 1 to 50 nm.[Citation11,Citation18]
Energy dispersive spectroscopy (EDS)
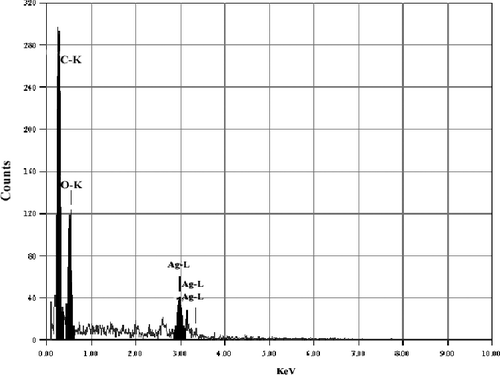
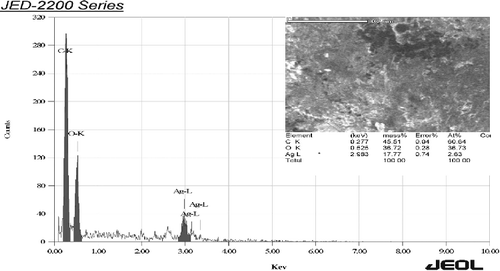
EDS spectrum was recorded in the spot-profile mode from one of the regions on the film surface which was densely populated with Ag NPs.[Citation19,Citation20] Powerful signals from the Ag atoms in the nanoparticle were noticed. Also, signals from carbon and oxygen were recorded ( and ). The carbon and oxygen signals were most probably due to X-ray emissions from the existing proteins in the fungus. It was noticed that silver gave weak signals compared to other elements, such as carbon and oxygen, whereas T. harzianum gave strong signals from silver, when compared to carbon and oxygen.[Citation11,Citation21] The optical absorption band peak at 3 KeV was an example for the absorption of mineral Ag nanocystallites.[Citation7,Citation22] Identification bands for the main emission energies for Ag are shown and are compatible with peaks in the spectrum, which gives confidence that the Ag was identified correctly.
Antibacterial activity of silver nanoparticles
The susceptibility of pathogenic bacteria to Ag NPs synthesized by T. viride is displayed in . The results showed that the Ag NPs had antibacterial activity against Gram-positive (MRSA) and Gram-negative bacteria (S. boydii, A. baumannii, S. sonnei and S. typhimurium). It was noticed that the Gram-negative bacteria exhibited bigger inhibition zones, whereas the Gram-positive bacterium showed smaller inhibition zone, compared to the antibiotics control. Ag NPs significantly inhibited the MRSA growth. In recent years, the nanoparticles have been considered as an interesting alternative technique to antibiotics and appear to have a high potential in solving bacterial multi-drug resistance in human pathogenic bacteria.[Citation23] Ag NPs have attracted great interest especially in antimicrobial activity.[Citation24–26] Ag has been permanently utilized to control a variety of diseases caused by both Gram-negative and Gram-positive bacteria.[Citation27–29]
Table 1. Antibacterial effect of Ag NPs synthesized by Trichoderma viride.
There are different results from other studies, which showed that the peptidoglycan layer of the Gram-negative bacteria could be the reason for the lower antibacterial activity of Ag NPs towards these bacteria, as this layer can restrain nanoparticles from entering the cell wall.[Citation30,Citation31] The high antibacterial activity of the Ag NPs was undoubtedly due to the Ag+, as the Gram-negative cell wall is attracted to them, resulting in cell wall tearing.[Citation9,Citation32] Hanna et al. [Citation33] found that the bacteria cell membrane and plasma were damaged by Ag NPs,[Citation34] causing exhaustion of the intracellular adenosine triphosphate. Silver compounds and Ag+ displayed a strong antibacterial activity, as these have a considerable surface region with strong reactive places. Previous studies revealed that the antimicrobial efficiency of Ag NPs was shape and size dependent.[Citation35,Citation36] Ag NPs (1–10 nm) attach to the cell membrane surface and significantly damage its permeability and respiratory function.[Citation37,Citation38]
Conclusions
In this study, Ag NPs were synthesized biologically by T. viride at room temperature. The synthesis of Ag NPs by using T. viride cell extract is a simple and appropriate method for a large-scale production. The capability of T. viride to synthesize Ag NPs as agents is highly promising for the green, sustainable manufacturing of nanomaterials. This also increases the widespread application of T. viride as an important strategy for the production of Ag NP.
Acknowledgements
The authors would like to extend their sincere appreciation to the deanship of scientific research at King Saud University for its funding this research group no. RGP-VPP-347.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Vijayakumar M, Priya K, Nancy FT, et al. Biosynthesis, characterization and anti-bacterial effect of plant-mediated silver nanoparticles using Artemisia nilagirica. Ind Crops Products. 2013;41:235–240.
- Gade A, Ingle A, Bawaskar M, et al. Fusarium solani: a novel biological agent for the extracellular synthesis of silver nanoparticles. J Nanoparticle Res. 2009;11:2079–2085.
- Neveen-Mohamed K. Biogenic silver nanoparticles by Aspergillus terreus as a powerful nanoweapon against Aspergillus fumigates. Afr J Microbiol Res. 2014;7:5645–5651.
- Husseiny MI, Aziz MAE, Badr Y, et al. Biosynthesis of gold nanoparticles using Pseudomonas aeruginosa. Spectrochimica Acta Part A. 2006;67:1003–1006.
- El-Shanshoury AER, ElSilk SE, Ebeid ME. Extracellular biosynthesis of silver nanoparticles using Escherichia coli ATCC 8739, Bacillus subtilis ATCC 6633, and Streptococcus thermophilus ESh1 and their antimicrobial activities. ISRN Nanotechnol. 2011;2011:385480.
- Peter L, Silambarasan S, Abraham J. Ecofriendly synthesis of silver nanoparticles from commercially available plant powders and their antibacterial properties. Scientia Iranica. 2013;20:1049–1054.
- Deenadayalan AK, Palanichamy V, Selvaraj MR. Green synthesis of silver nanoparticles using Alternanthera dentata leaf extract at room temperature and their antimicrobial activity. Spectrochimica Acta Part A. 2014;127:168–171.
- Peter L, Sivagnanam S, Abraham J. Synthesis of silver nanoparticles using plants extract and analysis of their antimicrobial property. J Saudi Chem Soc. 2015;19:311–317.
- Li G, Dan HE, Yongqing Q, et al. Fungus-mediated green synthesis of silver nanoparticles using Aspergillus terreus. Int J Mol Sci. 2012;13:466–476.
- Devi TP, Kulanthaivel S, Kamil D, et al. Biosynthesis of silver nanoparticles from Trichoderma species. Indian J Exp Biol. 2013;51:543–547.
- Vivek A, Jitendra K, Ritu S, et al. Green synthesis of silver nanoparticles by Trichoderma harzianum and their bio-efficacy evaluation against Staphylococcus aureus and Klebsiella pneumonia. Ind Crops Products. 2014;55:202–206.
- Grondona I, Hermosa MR, Tejada M, et al. Physiological and biochemical characterization of Trichoderma harzianum, a biocontrol agent against soil borne fungal plant pathogens. Appl Environ Microbiol. 1997;63:3189–3198.
- Lunge AG, Anita SP. Characterization of efficient chitinolytic enzyme producing Trichoderma species: a tool for better antagonistic approach. Int J Sci Environ Technol. 2012;1:37–385.
- Pragya T, Misra BN, Neelam SS. β-glucosidases from the fungus Trichoderma: an efficient cellulase machinery in biotechnological applications. BioMed Res Int. 2013;2013:203735.
- Jeffrey GL, Larry ET, John OB, et al. A constitutive expression system for glycosyl hydrolase family 7 cellobiohydrolases in Hypocrea jecorina. Biotechnol Biofuels. 2015;8:8–20.
- Silambarasan S, Abraham J. Biosynthesis of silver nanoparticles using Pseudomonas fluorescens. Res J Biotechnol. 2013;8:71–75.
- Smitha SL, Nissamudeen KM, Philip D, et al. Studies on surface plasmon resonance and photoluminescence of silver nanoparticles. Spectrochimica Acta Part A. 2009;71:186–190.
- Vahabi K, Mansoori GA, Karimi S. Biosynthesis of silver nanoparticles by fungus Trichoderma reesei. Insciences J. 2011;1:65–79.
- Mandal D, Bolander ME, Mukhopadhyay D, et al. The use of microorganisms for the formation of metal nanoparticles and their application. Appl Microbiol Biotechnol. 2006;69:485–492.
- Liao J, Anchun M, Zhu ZQY. Antibacterial titanium plate deposited by silver nanoparticles exhibits cell compatibility. Int J Nanomedicine. 2010;13:337–342.
- de Castro A, Pedro K, da Cruz J, et al. Trichoderma harzianum IOC-4038: a promising strain for the production of a cellulolytic complex with significant glucosidase activity from sugarcane bagasse cellulignin. Appl Biochem Biotechnol. 2010;162:2111–2122.
- Liao J, Zhu Z, Anchun MO, et al. Deposition of silver nanoparticles on titanium surface for antibacterial effect. Int J Nanomedicine. 2010;14:261–267.
- Rai MK, Deshmukh SD, Ingle AP, et al. Silver nanoparticles: the powerful nanoweapon against multidrug-resistant bacteria. J Appl Microbiol. 2012;112:841–852.
- Jana S, Pal T. Synthesis, characterization and catalytic application of silver nanoshell coated functionalized polystyrene beads. J Nanosci Nanotechnol. 2007;7:2151–2156.
- Stiufiuc R, Iacovita C, Lucaciu CM, et al. SERS active silver colloids prepared by reduction of silver nitrate with short-chain polyethylene glycol. Nanoscale Res Lett. 2013;8:47.
- Szmacinski H, Lakowicz JR, Catchmark JM, et al. Correlation between scattering properties of silver particle arrays and fluorescence enhancement. Appl Spectrosc. 2008;62;733–738.
- Lazar V. Quorum sensing in biofilms-How to destroy the bacterial citadels or their cohesion/power? Anaerobe. 2011;17:280–285.
- Donlan RM, Costerton JW. Biofilms: Survival mechanisms of clinically relevant microorganisms. Clinical Microbiol Rev. 2002;15:167–193.
- Taraszkiewicz A, Fila G, Grinholc M, et al. Innovative strategies to overcome biofilm resistance. Biomed Res Int. 2013;2013:150653.
- Colin P, Zheng D, Muhi MZ, et al. Silver nanoparticle synthesis using monosaccharides and their growth inhibitory activity against Gram-negative and positive bacteria. ISRN Nanotechnol. 2014;2014:480284.
- Shrivastava S, Bera T, Roy A. Characterization of enhanced antibacterial effects of novel silver nanoparticles. Nanotechnology. 2007;18:225103–225112
- Soo-Hwan K, Hyeong-Seon L, Deok-Seon R, et al. Antibacterial activity of silver-nanoparticles against Staphylococcus aureus and Escherichia coli. Korean J Microbiol Biotechnol. 2001;39:77–85.
- Hanna LK, Pontus C, Yolanda H, et al. Cell membrane damage and protein interaction induced by copper containing nanoparticles-Importance of the metal release process. Toxicology. 2013;13:59–69.
- Lok CN, Ho CM, Chen R, et al. Proteomic analysis of the mode of antibacterial action of silver nanoparticles. J Proteome Res. 2006;5:916–924.
- Elgorban AM, El-Samawaty AM, Yassin MA, et al. Antifungal silver nanoparticles: synthesis, characterization and biological evaluation. Biotechnol Biotechnol Equip. Forthcoming 2015. doi:10.1080/13102818.2015.1106339
- Shaban RS, Bahkali AH, Marwa MB, et al. Antibacterial activity of biogenic silver nanoparticles produced by Aspergillus terreus. Int J Pharmacol. 2015;11:858–863.
- Morones JR, Elechiguerra JL, Camacho A, et al. The bactericidal effect of silver nanoparticles. Nanotechnology. 2005;16:2346–2353.
- Kvitek L, Panacek A, Soukupova J, et al. Effect of surfactants and polymers on stability and antibacterial activity of silver nanoparticles (NPs). J Phys Chem. 2008;C112:5825–5834.