?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
In line with the well-established ethnobotanical use of Arum maculatum for the treatment of hemorrhoidal disease, we sought to determine the activities of 30% or 70% ethanol extracts of the plant tubers in an array of pharmacological and biochemical models of some crucial events implicated in the pathogenesis of this disorder, namely angiogenesis, collagenase activity remodeling, cyclooxygenase activity, IL-2 secretion and oxidative stress. The tested hydro-alcoholic extracts from A. maculatum tubers inhibited the proliferation of EA.hy926 vascular endothelial cells, but even at the highest administered concentrations no decrease in viability was observed. The extracts induced a concentration-dependent decrease in collagenase activity, whereby the effects were more pronounced at the lower concentration of ethanol in the extragent. Moreover the tested extracts induced concentration-dependent suppression of cyclooxygenase activity (COX-1 and 2), albeit at very high and presumably supraphysiological concentrations. The extracts augmented the PHA/PMA-induced secretion of IL-2 from Jurkat E.6 (human T-cells), which was more pronounced following exposure to the 30% ethanol-derived product. The 30% EtOH extract demonstrated anti-radical properties against both stable free radicals (ABTS and DPPH) and biologically relevant reactive oxygen species (ROS), which could be considered as important mediators of inflammation and signaling molecules. Our findings give us reason to conclude that the hydroalcoholic extracts of A. maculatum tubers possess anti-inflammatory, antiangiogenic and antioxidant effects which in concert could contribute to its efficacy for the management of hemorrhoidal disease.
Introduction
Studies that explore the chemical diversity of the secondary metabolites in plants have been well established as a credible strategy for detection of promising lead compounds and new drugs [Citation1]. Despite the immense success of combinatorial chemistry and the availability of miniaturized high-throughput systems for biological screening, natural phytochemicals still pose significant advantages over synthetic combinatorial libraries, as the enzymatic systems of plants enable them to synthesize complex structures with complicated stereochemistry, chiral centers, fused heterocyclic scaffolds etc., which are virtually not possible to generate in a chemical lab [Citation2–5]. Moreover, since the number of thoroughly evaluated medicinal plants is very small compared to the vast number of available unexplored species, the pharmaceutical and medicinal exploitation of plant secondary metabolites remains a valuable approach for development of new therapeutic entities [Citation6]. An additional beneficial feature of natural products is their intrinsic propensity to act on various cellular targets (e.g. receptors, ion channels, enzymes) and to display in general low toxicological potential, which gives reason for these agents to be regarded as privileged structures in medicinal chemistry and drug design [Citation6–8].
By virtue of the complex crosstalk of pathological mechanisms implicated in the inflammatory processes with involvement of vascular events, pro-inflammatory and immune cells and an array of mediators, none of the existent antiphlogistic agents is capable of addressing the inflammation thoroughly [Citation9]. An exemplary condition where the conservative therapeutic modalities remain generally palliative is the hemorrhoidal disease. It is characterized by complex inflammatory, angiogenesis and tissue remodeling mechanisms, which current therapies fail to thoroughly address [Citation10–12]. The interest towards development of plant-based therapies for hemorrhoidal disease is further fueled by the fact that at present the vast majority of available medications are confined to plant derived extracts or purified phytochemicals [Citation13–17].
Among the diverse medicinal plants with documented ethnopharmacological use as remedies for hemorrhoidal disease, special attention is focused on Arum maculatum, and related species from this genus, by virtue of their well-known application in the traditional medicine of the Balkans, Asia Minor and the Middle East [Citation18–20]. The rationale for the medicinal use of this herb has been recently validated in a randomized clinical trial documenting significant improvement in health-related quality of life outcomes in patients with hemorrhoidal disease [Citation21].
In continuation of our preceding botanical, autecological and ethnopharmacological exploration of Arum maculatum [Citation18, Citation20, Citation22], we herein report a pharmacological evaluation of two hydro-alcoholic extracts of the plant using several pharmacological endpoints, namely modulation of collagenase and cyclooxigenase activities, effects on human vascular endothelial proliferation and viability and antioxidant and antiradical activities, to characterize its mode of action as a remedy for hemorrhoidal disease.
Materials and methods
Plant material, extract preparation and phytochemical analysis
Arum maculatum tubers were collected locally in Bulgaria. The extracts for the pharmacological studies were prepared with either 30% or 70% ethanol (EtOH) by dynamic maceration. Prior to the pharmacological evaluation, serial dilutions of the air-dried extracts were freshly prepared using dimethyl sulfoxide (DMSO).
The total amount of protein in the extracts was determined using the method of Lowry as a rough estimate for the lectin content. For more thorough phytochemical characterization, the extracts were further assayed by Gas chromatography–mass spectrometry (GC-MS). GC-MS analysis was carried out using a Trace 1310 GC gas chromatograph, tandem with Exactive GC mass spectrometer, using a TriPlus RSH autosampler for liquid probes. For the separation of the tested compounds, a capillary chromatographic column Termo TraceGold TG-5SilMS 30 m × 0.25 mm × 0.25 μm was used. The following temperature gradient was applied: the initial column temperature was 60.0 °C; thereafter it was increased from 60.0 °C to 270 °C with a temperature change rate of 15 K/min and maintenance of the final temperature for 15 min. Aliquots of 1 µL were applied using the split mode of the injector. Helium was used as eluent gas with a flow velocity of 1 mL/min.
Angiogenesis protocol
The human EA.hy926 cell line (ATCC: CRL-2922TM) was purchased from the American Type Culture Collection (Manassas, Virginia, USA). Cells were grown in Dulbecco’s Modified Eagle’s Medium containing 5% fetal bovine serum and 2 mmol/L L-glutamine. Exponentially growing cells were inoculated into 96-well microtiter plates in 100 μL at a density of 5000 cells/well. After cell seeding, the microtiter plates were incubated at 37° C, 5% CO2, 95% air and 100% relative humidity for 24 h prior to addition of the tested extract and the reference agent. After 24 h, two plates of each cell line were subjected to MTT-dye reduction assay protocol (see below), to serve as a measurement of the initial cell population at the time of drug treatment (Tz). The tested extracts and the stock solution of the reference compound (in DMSO) were freshly diluted to obtain the desired final concentrations with complete growth medium. Aliquots of 1 μL of these serial dilutions were added to the appropriate 96-well microplates already containing 100 μL of medium, to yield the required final drug concentrations. Following drug treatment, the plates were incubated for additional 72 h in a controlled environment (in an incubator with 37 °C, 5% CO2, 95% air and 100% relative humidity). Using seven MTT-absorbance measurements [time zero (Tz), control growth (C) and test growth in the presence of extracts or the reference compounds at the different concentration levels (Ti)], the percentage growth was calculated at each of the drug concentration levels. Percentage growth inhibition was calculated as:
From these bioassay data three dose response end-points were obtained for each tested agent/extract. Growth inhibition of 50% (GI50) is calculated from [(Ti-Tz)/(C-Tz)] × 100 = 50, which is the drug concentration resulting in a 50% reduction in the net formazan production increase (as measured by MTT-dye reduction assay) in control cells during the experimental exposure period. The test concentration resulting in total cell growth inhibition (TGI) is calculated from Ti = Tz. The LC50 (concentration of drug resulting in a 50% reduction in the measured formazan absorption at the end of the treatment as compared to that at the beginning) indicating a lethal cytotoxicity following treatment was calculated from [(Ti-Tz)/Tz] × 100 = −50. Values were calculated for each of these three growth inhibition end-points if the level of activity was reached; however, if the effect was not reached or was exceeded, the value for that parameter was expressed as greater or less than the maximum or minimum concentration tested.
Inhibition of collagenase and cyclooxygenase activity
The effects on collagenase activity were assessed using a commercially available colorimetric assay kit (BioVision Inc, CA, USA) using 1,10-phenanthroline as a positive control. The effects on cyclooxygenases COX-1 (from sheep erythrocytes) and COX-2 (human recombinant enzyme) were assessed using a commercially available inhibitor screening assay kit (Cayman Chemical, MI, USA). The selective COX-2 inhibitor nimesulide as well as the non-selective inhibitor indomethacin, were used as reference compounds in these investigations. The aforementioned enzymatic assays were conducted according to the instructions of the manufacturer. The inhibition graph for each inhibitor was constructed from data collected in three independent experiments. Data were analyzed by non-linear regression and the results were expressed as half maximal inhibitory concentration (IC50).
Immune modulating activity
The immune modulating activity was assessed by the ability of the tested extracts to influence the production of IL-2 upon mitogen stimulation in Jurkat (E6-1 clone) cells. Jurkat E6-1 cells were obtained from the American Type Culture Collection (Manassas, VA, USA). Exponentially growing cells seeded in 24-well microplates (106 cells/well), stimulated with 20 ng/mL 12-O-tetradecanoylphorbol 13-acetate (PMA) and 10 μg/mL phytohemaglutinin for IL-2 release and incubated in the presence of test samples over 24 h. The cultured supernatants were collected and the concentration of the released IL-2 was directly determined by a commercially available Interleukin-2 Diaclone™ ELISA (enzyme-linked immunosorbent assay) kit, which was purchased from Gen-Probe Inc. The procedure was carried out in compliance with the instructions of the manufacturer, and IL-2 was quantified (ng/mL) using a freshly prepared standard curve. The tests were run in triplicate.
Antioxidant and antiradical effects
ABTS method
The assay was performed according to Re et al. [Citation23]. It is based on the decrease of the absorbance value of the persistent radical cation of ABTS (2,2-azinobis (3-ethylbenzothiazoline-6-sulfonic acid). The radical has been pre-formed in buffered water by adding 14 mmol/L ABTS stock solution to potassium persulfate (2.45 mmol/L - final concentration). The working solution was prepared by diluting a volume of the prepared stock solution until its absorbance at 734 nm reaches 0.70 ± 0.02. Two milliliters for the working solution were mixed with different concentrations of the tested extract. The absorbance values were taken 60 min after the mixing at 734 nm.
DPPH method
The experiments were performed according to Goupy et al. [Citation24]. The method is based on the capability of the tested substance to reduce the purple colored DPPH radical into a non-radical form with yellow color. A working solution of the stable DPPH radical in ethanol with absorbance of 0.9 at 517 nm has been prepared. Again 2 mL of this solution were mixed with the extract in ethanol at different concentrations. The obtained mixtures were shaken vigorously and allowed to stand at room temperature for 1 h. Then the sample absorbance values were measured at 517 nm using a UV-VIS spectrophotometer.
Two types of samples were prepared for both assays. The first type were the controls containing the used stable free radical (where the extract was replaced with the used solvent). The second type were those containing the studied extract at different concentrations. Fresh radical solution was prepared for each experiment. The obtained data were presented as radical scavenging activity % calculated as follows:
where
is the absorbance of the control samples,
is the absorbance of the extract containing samples. All experiments were performed in triplicate and the results are presented as mean values with standard deviation (± SD).
Chemiluminescence assays
The chemiluminescence (CL) experiments were done using LKB 1251 luminometer connected with an AT-type computer via serial interface and automatic injector. Data collection and processing were done using MultiUse program ver. 1.08. All measurements were repeated three times and the luminometer was set up at 37 °C. The ratio between the chemiluminescent response in the presence and the absence of the extract was named chemiluminescent scavenging index (CL-SI).
Luminol-dependent chemiluminescence in a system of NaOCl generated hypochlorite
The assay was carried out using 1 mL of K2HPO4/KH2PO4, pH 7.4, containing 0.1 mmol/L luminol, 0.06 mmol/L NaOCl and the tested extract at different concentrations. In the control the extract was omitted. The chemiluminescence was measured every 50 milliseconds after the addition of NaOCl using the ‘flash assay’ option of the MultiUse program.
Luminol-dependent CL in a system of KO2 generated superoxide anion radical
The experiments were performed using 1 mL samples in 50 mmol/L buffer, pH 7.4, buffer containing 0.1 mmol/L luminol, and the tested extract (or buffer for the control sample). Due to the fast release of superoxide, the CL response was registered immediately after the addition of 20 µL KO2. It was measured for 1 min every 50 ms.
Data analysis
The data processing and statistical analysis of the experimental data were performed using GraphPad Prism software for PC. The statistical significance was analyzed using Student’s t-test with p ≤ 0.05 set as significance level.
Results and discussion
Phytochemical analysis
The total protein content, as a surrogate marker for the lectin content, was 9-fold higher in the extract obtained using 30% ethanol (6 mg/mL) vs. the 70% ethanolic extract (0.67%). The GC-MS data for the extracts are summarized in . The analysis revealed the presence of hydrocarbons, alcohols, thiols, carbonyl derivatives, terpenoids, fatty acid esters, as well as the nitrogen-containing compound (N-(2-ethyl phenyl) benzamide. With the only exception of the latter compound, the % area of the detected species was higher in the 70% ethanolic extracts.
Table 1. GC-MS data of the volatiles of the extracts of Arum maculatum roots.
Antiangiogenic effects
The antiproliferative effects of the hydro-alcoholic extracts of the plant were evaluated in an immortalized human umbilical vein endothelial cell line EA.hy926, using a modified MTT-dye reduction assay protocol to allow discrimination between the cell growth inhibitory effects from the lethal cytotoxicity, after 72 h continuous exposure. The sesquiterpene lactone hydroxyarthemisinine (ART-OH) was used as a reference compound in this study in line with its well established angiostatic activity.
As evident from the MTT-dye reduction assay dose-response curves () and from the calculated GI50 and TGI values () the tested A. maculatum extracts caused strong inhibition of the proliferation of EA.hy926 cells in a concentration-dependent manner. Noteworthy, even at the highest exposure intensities tested there was practically no significant decrease in cell viability. The angiostatic activity observed with our experimental system was more pronounced following exposure to the 30% ethanol-derived extract of the plant.
Figure 1. Antiangiogenic effects of A. maculatum extracts against EA.hy926 cells after 72 h. exposure (МТТ-assay).
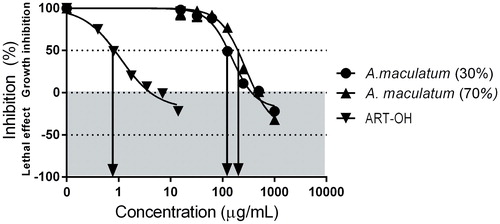
Table 2. Antiproliferative effects of the tested A. maculatum extracts against EA.hy926 vascular endothelial cells (MTT-dye reduction assay).
Angiogenesis is central for the pathogenesis of hemorrhoidal disease [Citation25–28]. Hence, the aforementioned findings indicate that the modulation of the endothelial proliferation, which is a pivotal event in neovascularization, at least partly mediates the in vivo effects of A. maculatum.
Effects on bacterial collagenase activity as a model of mammalian matrix metalloproteases
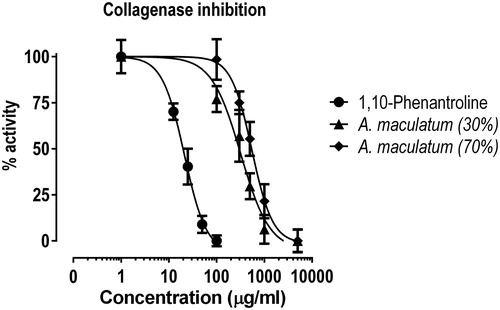
The modulatory effects of the tested extracts on matrix metalloproteases (MMP) were evaluated on bacterial collagenase as a model enzyme, using a commercially available colorimetric kit. The reference inhibitor 1,10-phenanthroline was used as a positive control in accordance to the manufacturer’s instructions. The data from this bioassay are summarized in and . The tested extracts caused concentration-dependent decrease of the collagenase activity, whereby the extract derived with the lower ethanol concentration proved to significantly outclass the other tested plant material, in line with the above presented MTT-bioassay data.
Table 3. Inhibitory activity of the tested extracts on bacterial collagenase.
The MMP activity is crucial for the angiogenesis processes and it has been well demonstrated that modulation of these enzymes is implicated in the mode of action of different drugs used to treat hemorrhoidal disease [Citation27, Citation29]. On these grounds, the established collagenase inhibitory activity of the tested extracts could be considered as an integral element of its multifaceted mode of action.
Effects on the activities of cyclooxygenases COX-1 and COX-2
Given the central role of prostaglandins and the related eicosanoids for the pathogenesis of the inflammatory process and tissue remodeling in hemorrhoid disease, we sought to determine the ability of the tested extracts to inhibit the activity of the constitutive and inducible forms of cyclooxygenase, namely COX-1 and COX-2. The evaluation was carried out using a commercially available colorimetric assay kit, where the clinically employed nonsteroidal anti-inflammatory drugs (NSAIDs) indomethacin and nimesulide served as reference inhibitors of the respective cyclooxygenase isoforms. The calculated IC50 values are presented in . Both extracts demonstrated inhibitory effects on cyclooxygenase, but only at extremely high concentrations, the activity of the 30% ethanolic extract being superior. Considering the IC50 values, however, these effects are not likely to contribute to the efficacy of the medicinal plant in the treatment of hemorrhoids.
Table 4. Inhibitory effects of the tested extracts on cyclooxygenase (COX-1 and COX-2) activities.
Immune modulating activity
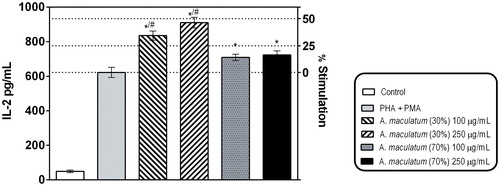
In line with the well-established immune modulating effects of Arum lectins, we tested the ability of the plant extracts to affect the mitogen-activated secretion of IL-2 in Jurkat E.6 cells as a model of human T-lymphocytes, using a commercially available ELISA kit (). As evident from the presented results, the tested extracts increased the production of IL-2. This effect was more pronounced at the extract derived with the lower ethanol concentration, which could be ascribed to the less pronounced denaturing of the plant lectins, as compared to that with the 70% ethanol extraction. These macromolecular compounds have been well established as central for some of the biological effects of the plant [Citation30, Citation31]. Moreover the Arum species also contains mucopolysaccharides, which are also known as immune-modulating bioactive substances to contribute to the established activity [Citation32].
Antioxidant and antiradical effects
As a next step, we evaluated the ability of the more active 30% EtOH derived extract to act as a free-radical scavenger and antioxidant, in line with the important contribution of oxidative stress to inflammation in general and to the pathogenesis of hemorrhoid disease in particular. To evaluate the anti-radical properties of the tested extract, a battery of in vitro chemical chemiluminescent and spectrophotometric model systems were employed. The spectrophotometric assays were used to deterime its capability to decrease the concentration of the ABTS•+ (2,2′-azino-bis(3-ethylbenzothiazoline-6-sulphonic acid) and DPPH (2,2-diphenyl-1-picrylhydrazyl) radicals. Both assays have been well described in the literature as indirect methods based on the reaction of the potential antioxidant with the unnatural, colored persistent radicals which are chemically different from the radicals normally responsible for the autoxidation in real systems. The methods are often used for preliminary screening procedures of pure compounds, biological samples, extract and many authors use them to compare the observed effectiveness of the tested compounds.
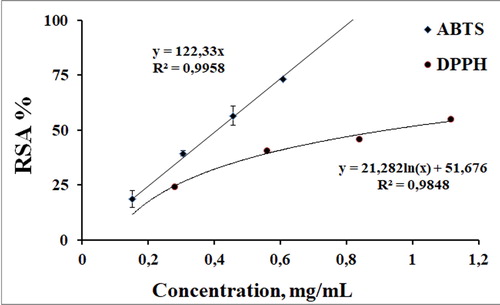
The tested extract demonstrated radical-scavenging activity against both the ABTS and the DPPH radicals (), suggesting the presence of components which could execute their antiradical properties by hydrogen atom transfer and single electron transfer mechanism. The scavenging abilities of the tested extract were concentration-dependent. As the sample concentration of the extract increased, the amount of the stable free radical in the sample decreased. A linear dose–RSA % dependence was observed only in the ABTS system. The studied concentration interval was from 0 to 0.61 mg/mL, and at the highest concentration, the RSA% reached around 75%. In the DPPH assay, the maximal tested concentration was 1.11 mg/mL and at this concentration of the extract, the observed RSA % was 55%. The concentration dependence of the radical-scavenging activity served as a basis to calculate the concentration at which this parameter decreased with 50%. Lower C50 values indicate better expressed radical scavenging properties. For the ABTS system, the C50 value was 0.409 ± 0.025 mg/mL and for the DPPH system, 0.924 ± 0.018 mg/mL.
Figure 4. Concentration dependence of the anti-radical activity of the 30% EtOH extract determined in stable free radical-based systems.
Further, we evaluated the capability of the extract to scavenge biologically relevant reactive oxygen species (ROS), superoxide anion radical and hypochlorite, using chemiluminescent model systems. For our experiments we chose luminol-enhanced chemiluminescence. Luminol was chosen as a luminophore due to its lack of selectivity and the fact that it can react with all ROS generated in the sample. This enabled us to compare the obtained data from assays evaluating anti-radical properties against different ROS.
The necessity to evaluate the capability of the extract to decrease ROS concentration is due to their role at the same time as signaling molecules and mediators of inflammation. The superoxide anion radical and hypochlorite are key targets when seeking substances influencing inflammation-induced oxidative stress. The former is a mediator of free radical toxicity: via participating in subsequent reactions it generates other ROS which contribute to the propagation of the oxidative stress injury (H2O2) or are the most potent species that oxidize the basic components of biological membranes – proteins and lipids (peroxynitrite, hypochlorite, hydroxyl radical). The superoxide anion radical is generated by NADPH oxidases which are present in a variety of cells, especially phagocytes and endothelial cells, playing a central role in the genesis of the inflammatory response. Hypochlorite is produced as a result of the myeloperoxidase induced oxidation of chloride ions accompanied with the reduction of hydrogen peroxide. The hypochlorite concentration in the samples corresponds to the levels detected during macrophage oxidative burst.
Typical chemiluminescent curves obtained in the chosen systems are summarized in . For each assay the program software requires control samples (containing PBS, luminol, the reaction-initiating agents – hypochlorite or potassium superoxide, but without addition of the extracts), blank samples (containing PBS, the tested extract, and the reaction-initiating agents) and the experimental samples with different concentration of the extract to calculate the chemiluminescent scavenging index (CL-SI, %). CL-SI was used as indicator of the scavenging properties in both systems. The obtained CL response is proportional to the area under the kinetics curves. In the presence of substances with scavenging properties, the concentration of the radicals and, respectively, the CL signal will decrease. Lower CL-SI corresponds to higher anti-radical potency. As evident from the data in , in both systems, the balnk sample (which contains all sample components except luminol) overlaps the X axes, which indicates lack of CL signal due to direct interaction of the tested extract and the other sample components. In both systems, the controls had a maximal peak of the CL curve and reaction curve area — integral suggesting capability of the tested extract to decrease the concentration of both the superoxide anion radical and hypochlorite in vitro. In other to quatify the observed scavenging effect in both systems, CL-SI was calculated and the obtained data are presented in .
Figure 5. Typical chemiluminescent curves obtained in the chemilumiescent model systems used for evaluation of the superoxide anion and hypochlorte scavenging activity.
Note: X-axis, time of analysis [s]; Y-axis, intensity of chemiluminescence signal - relative units [RU]. Blank samples contained PBS, the tested antioxidants, and the reaction-intitiating agents; control samples contained PBS, luminol and the reaction-initiating agents but without the tested antioxidants.
![Figure 5. Typical chemiluminescent curves obtained in the chemilumiescent model systems used for evaluation of the superoxide anion and hypochlorte scavenging activity.Note: X-axis, time of analysis [s]; Y-axis, intensity of chemiluminescence signal - relative units [RU]. Blank samples contained PBS, the tested antioxidants, and the reaction-intitiating agents; control samples contained PBS, luminol and the reaction-initiating agents but without the tested antioxidants.](/cms/asset/18816f31-5b16-4da5-966b-32ec8efb27ec/tbeq_a_1722239_f0005_b.jpg)
Figure 6. Effect of the 30% EtOH extract on the luminol dependent CL in model systems with different ROS: OCl¯ - system of NaOCl generated hypochlorite; O2•¯ - system of KO2 produced superoxide formation.
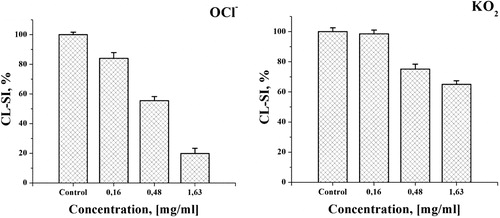
These findings imply that the extract possesses a capability to decrease the chemiluminescent signal in both experimental systems and hence is capable of scavenging the corresponding ROS. The observed effect was concentration dependent and the CL response tended to decrease with the increase in the concentration of the tested extract. This effect was more noticeable in the hypochlorite-containing system, where at the lowest tested concentration of 0.16 mg/mL, the observed decrease was more than 15% compared to the control. At the same concentration, there was no statistically significant decrease of the CL-SI value compared to the control in the superoxide system. This difference in the capability of the extract to influence the CL signal was observed at all the tested concentrations. At the maximal concentration of 1.63 mg/mL, the CL-SI ratio in the hypochlorite system was 5 times lower compared to the control and more than 3 times lower compared to the one determined in the superoxide system. Again C50 values were calculated. For OCl¯, the C50 value was 0.421 ± 0.023 mg/mL and for O2•−, 4.037 ± 0.121 mg/mL (determined using an extrapolation method, as it was not within the tested concentration range). These data indicate that the efficacy of A. maculatum as a traditional remedy for hemorrhoids is at least partly mediated by antioxidant and antiradical effects. This well corroborates the pharmacological profiles of most phytochemicals applied topically or systemically as antihemorrhoidal agents, the vast majority of which are potent antioxidants and free radical scavengers [Citation33–36].
Conclusions
Our study showed that A. maculatum is endowed with pleiotropic anti-phlogistic, antiangiogenic, antioxidant and antiradical activities. These activities, in concert, address some key elements of the inflammatory and tissue remodeling processes implicated in the pathogenesis of hemorrhoidal disease.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Cragg GM, Newman DJ. Natural products: a continuing source of novel drug leads. Biochim Biophys Acta. 2013;1830(6):3670–3695.
- Cragg GM, Newman DJ. Antineoplastic agents from natural sources: achievements and future directions. Expert Opin Investig Drugs. 2000;9(12):2783–2797.
- Cragg GM, Newman DJ. Discovery and development of antineoplastic agents from natural sources. Cancer Invest. 1999;17(2):153–163.
- Cragg GM, Boyd MR, Cardellina JH, 2nd, et al. Ethnobotany and drug discovery: the experience of the US National Cancer Institute. Ciba Found Symp. 1994;185:178–190, discussion 90–96.
- Heinrich M, Barnes J, Gibbons S, et al. Fundamentals of pharmacognosy and phytotherapy. 2nd ed. Edinburgh (UK): Elsevier; 2012. Chapter 1, Importance of plants in modern pharmacy and medicine; p. 3–9.
- Evans WC, Trease GE, editors. The plant and animal kingdoms as sources of drugs. In: Evans WC, Trease GE, editors. Trease and Evans’ pharmacognosy. 15th ed. New York (NY): WB Saunders; 2002. p. 13–54.
- Basmadjian C, Zhao Q, Bentouhami E, et al. Cancer wars: natural products strike back. Front Chem. 2014;2:20.
- Zhuang C, Zhang W, Sheng C, et al. Chalcone: a privileged structure in medicinal chemistry. Chem Rev. 2017;117(12):7762–7810.
- Lichota A, Gwozdzinski L, Gwozdzinski K. Therapeutic potential of natural compounds in inflammation and chronic venous insufficiency. Eur J Med Chem. 2019;176:68–91.
- Madoff RD, Clinical Practice Committee, American Gastroenterological Association, Fleshman JW. American gastroenterological association technical review on the diagnosis and treatment of hemorrhoids. Gastroenterology 2004;126(5):1463–1473.
- Dozois EJ, Pemberton JH. Chapter Sixty-Six – Hemorrhoids and other anorectal disorders. In: Wolfe MM, Davis GL, Farraye FA, Giannella RA, Malagelada J-R, Steer ML, editors. Therapy of digestive disorders. 2nd ed. Edinburgh (UK): W.B. Saunders; 2006. p. 945–958.
- Moult H-L, Aubert M, De Parades V. Classical treatment of hemorrhoids. J Visceral Surg. 2015;152(2):S3–S9.
- Erbay MŞ, Sarı A. Plants used in traditional treatment against hemorrhoids in Turkey. Marmara Pharm J. 2018;22(2):110–132.
- Dehdari S, Hajimehdipoor H, Esmaeili S, et al. Traditional and modern aspects of hemorrhoid treatment in Iran: a review. J Integr Med. 2018;16(2):90–98.
- Buckshee K, Takkar D, Aggarwal N. Micronized flavonoid therapy in internal hemorrhoids of pregnancy. Int J Gynaecol Obstet. 1997;57(2):145–151.
- Ho YH, Tan M, Seow-Choen F. Micronized purified flavonidic fraction compared favorably with rubber band ligation and fiber alone in the management of bleeding hemorrhoids: randomized controlled trial. Dis Colon Rectum. 2000;43(1):66–69.
- Mahboubi M. Effectiveness of Myrtus communis in the treatment of hemorrhoids. J Integr Med. 2017;15(5):351–358.
- Kochmarov V, Kozuharova E, Naychov Z, et al. Ethnobotany and ethnopharmacology of Arum maculatum L. (Araceae) in Bulgaria with an emphasis on its effect against haemorrhoids. Int J Pharm Chem Biol Sci. 2015;5(2):394–402.
- Leclerc H. [Wild arum (Arum maculatum L.); its use as food]. Presse Med. 1952;60(71):1514.
- Kozuharova E, Kochmarov V, Semerdjieva I, et al. Potential of wild populations resources of Arum maculatum L. (Araceae) in Bulgaria – a prospective medicinal plant. C R Acad Bulgare Sci. 2018;71(2):193–200.
- Zisis S, Giannakou K, Lavranos G, et al. Alternative herbal medicine for hemorrhoids, Effect of Arum maculatum on the quality of life of patients: a randomized controlled trial. J Appl Pharm Sci. 2019;9:40–45.
- Kozuharova E, Kochmarov V, Kachaunova E, et al. Distribution of Arum (Araceae) in Bulgaria. Flora Medit. 2014;24:51–62.
- Re R, Pellegrini N, Proteggente A, et al. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radical Biol Med. 1999;26(9-10):1231–1237.
- Goupy P, Dufour C, Loonis M, et al. Quantitative kinetic analysis of hydrogen transfer reactions from dietary polyphenols to the DPPH radical. J Agric Food Chem. 2003;51(3):615–622.
- Klink C, Binnebosel M, Kammer D, et al. Haemorrhoids are related to changes of cell function in mucosa and submucosa. Int J Colorectal Dis. 2009;24(12):1389–1394.
- Wang C, Lu H, Luo C, et al. miR-412-5p targets Xpo1 to regulate angiogenesis in hemorrhoid tissue. Gene 2019;705:167–176.
- Han W, Wang ZJ, Zhao B, et al. [Pathologic change of elastic fibers with difference of microvessel density and expression of angiogenesis-related proteins in internal hemorrhoid tissues]. Zhonghua Wei Chang Wai Ke Za Zhi. 2005;8(1):56–59.
- Diaz-Flores L, Gutierrez R, Gonzalez-Gomez M, et al. Segmentation of dilated hemorrhoidal veins in hemorrhoidal disease. Cells Tissues Organs 2018;205(2):120–128.
- Serra R, Gallelli L, Grande R, et al. Hemorrhoids and matrix metalloproteinases: a multicenter study on the predictive role of biomarkers. Surgery 2016;159(2):487–494.
- Mladenov I, Bulanov I, Stamenova M, et al. [The composition and structure of isolectins from Arum maculatum]. Eksp Med Morfol. 1990;29(1):36–39.
- Alencar VB, Alencar NM, Assreuy AM, et al. Pro-inflammatory effect of Arum maculatum lectin and role of resident cells. Int J Biochem Cell Biol. 2005;37(9):1805–1814.
- Koleva M, Achtardjieff C. [A glucomannan in the tubers of arum maculatum l]. Pharmazie 1975;30(2):111–113.
- Sapsrithong T, Kaewprem W, Tongumpai S, et al. Cissus quadrangularis ethanol extract upregulates superoxide dismutase, glutathione peroxidase and endothelial nitric oxide synthase expression in hydrogen peroxide-injured human ECV304 cells. J Ethnopharmacol. 2012;143(2):664–672.
- Kucukkurt I, Ince S, Keles H, et al. Beneficial effects of Aesculus hippocastanum L. seed extract on the body’s own antioxidant defense system on subacute administration. J Ethnopharmacol. 2010;129(1):18–22.
- Aggrawal K, Satija N, Dasgupta G, et al. Efficacy of a standardized herbal preparation (Roidosanal®) in the treatment of hemorrhoids: a randomized, controlled, open-label multicentre study. J Ayurveda Integr Med. 2014;5(2):117–124.
- Meshikhes AW. Efficacy of Daflon in the treatment of hemorrhoids. Saudi Med J. 2002;23(12):1496–1498.