Abstract
Marigold (Tagetes erecta L.) flowers can be used to produce lutein, but the production process generates fermentation wastewater, which is rich in lactic acid. Currently, most fermentation wastewater treatment methods are relatively expensive and time-consuming. To develop a practical marigold flower fermentation wastewater treatment method, in this study, we treated the fermentation wastewater with calcium oxide to prepare a neutralization solution, which was then used to irrigate maize that in turn exhibited a significant increase in growth. Then, we investigated soil pH, conductivity and enzyme activity, as well as microbial community and diversity of the maize rhizosphere and non-rhizosphere soil. Compared with the control group, the neutralization solution had no significant effect on soil pH and four kinds of enzyme (soil urease, catalase, sucrase and acid phosphatase) activity, but the electrical conductivity of the treatment group increased by 24.3%. The 16s rDNA and ITS rDNA sequencing results showed that the neutralization solution treatment influenced the diversity and abundance of bacteria and fungi in the non-rhizosphere samples, as well as the number of bacterial species in rhizosphere soil. The effect of neutralization solution treatment on bacteria was greater than that on fungi. Proteobacteria and Ascomycota were the predominant bacterial and fungal phyla in maize rhizosphere soil. The relative abundance of Paenibacillus, Bacillus and Penicillium genera significantly increased in non-rhizosphere soils. We hereby present a potential method for treating marigold flower fermentation wastewater for fertilizer utilization and show that the neutralization solution promotes maize growth and influences the soil microbial structure.
Introduction
Marigold (Tagetes erecta L.) is an annual herbaceous plant of genus Compositae which is rich in carotenoids such as lutein and other carotenoids [Citation1]. Marigold flower is the main raw material for extracting lutein, which plays an important role in preventing and controlling diseases in the human body [Citation2]. Lutein is widely used in food [Citation3], medicine, health care products, feed and other industries [Citation4]. Xanthin plays an important role in the prevention and treatment of diseases in humans and has potential applications for functional food and medicine production [Citation5]. Currently, the production of pigment particles from marigold requires fermentation by lactic acid bacteria [Citation6]. After fermentation, water is squeezed out of the flowers, resulting in a large amount of wastewater. Generally, about 0.4 tons of wastewater is produced per ton of marigold flowers during fermentation, and the dehydrated solution contains lactic acid and other acidic substances [Citation7]. A large number of acid dehydrated liquid is produced in the production and processing of marigold [Citation8], which does not meet the requirements of environmental protection. Currently, air floatation and biological contact oxidation are widely used in marigold processing. However, these two methods are complex and require sophisticated environmental protection equipment, the operation cost of equipment is high, and the effect of biochemical treatment is not ideal. Therefore, it is necessary to develop practical wastewater treatment technology with low investment and operating cost.
Marigold flower fermentation wastewater is rich in lactic acid [Citation6], and inexpensive calcium lactate solution can be obtained by adding a calcium source. Calcium lactate is a good exogenous calcium supplement due to very high bioavailability, high solubility and high stability in solution [Citation9]. Calcium lactate can facilitate plant growth and phytic acid degradation [Citation10]. Calcium lactate and gibberellic acid treatment promotes growth, hormone metabolism and phytic acid degradation in germinating soybean [Citation11]. Calcium lactate solution prepared by marigold flower fermentation wastewater may thus promote plant growth.
In this study, calcium oxide was used to treat marigold flower fermentation wastewater, resulting in a neutralization solution (calcium lactate solution) which was then applied to maize, and its growth was monitored for one month. We thus assessed the effect of the neutralization solution on the growth of the maize, soil physiochemical properties and the rhizosphere and non-rhizosphere microorganisms of maize. This study provides a method in resolving the problem of marigold flower fermentation wastewater and evaluated the feasibility of this method by studying the effect of neutralization solution on soil and maize.
Materials and methods
Neutralizing of marigold flower fermentation wastewater and field experiment
The marigold flower fermentation wastewater at various volumes (i.e. 100 mL, 200 mL, 500 mL, 800 mL and 1000 mL) was neutralized with calcium oxide solid to a pH of 7.0 and repeated thrice. The linear equation of neutralization reaction is Y = 5.4507 g/L × X – 0.0444, where Y is the amount of calcium oxide (g) and X is the volume of the marigold flower fermentation wastewater (L). The amount of calcium oxide used for neutralizing was calculated to be 5.41 g/L.
Subsequently, we neutralized 10 m3 of marigold flower fermentation wastewater, and the amount of calcium oxide was calculated to be 54.1 kg. Approximately 49.1 kg of calcium oxide was added to 10 m3 marigold flower fermentation wastewater and thoroughly mixed, pH change was monitored using a pH metre, and the remaining 5 kg of calcium oxide was slowly added to the mixture and then thoroughly mixed until the pH was 6.8–7.2. Next, the mixture was left to stand for one day, then the mixture was used as the neutralization solution.
The test maize variety was Xingnong 998 sown in 1 ha in Zhangjiawan, Weichang County, Chengde City, Hebei Province, China, on 23 June 2018. One month later, in the normally managed maize field, four areas of 5 m × 5 m were randomly selected for the experiment, and the other four planting areas of the same size were selected as control. Neutralization solution was used to irrigate the test area, and the rest of the control area was watered with the same amount of water.
Soil sampling
Rhizosphere soil sampling
In each experimental area, five maize roots were dug out using the five-point sampling method, loosely bound soil was shaken off and tightly bound soil was collected and pooled into one sample. The rhizosphere soil of maize in the treatment groups was named R-T, while that in the control group was named R-CK. After sampling, these were stored at −20 °C until DNA extraction.
Non-rhizosphere soil sampling
In each experimental area, the same five-point sampling method was adopted, and five areas more than 20 cm away from maize root were selected. The surface soil was removed using a sampling shovel, and the soil samples of the plowing layer of 10–20 cm were taken as one sample. The non-rhizosphere soil of maize in the treatment group was named NR-T, while the non-rhizosphere soil of the control group was named NR-CK. One part of the soil sample was air-dried and ground, and a 60-mesh screen was used for physiochemical experiments, and the other part was stored at −20 °C for DNA extraction.
Determination of maize growth index and soil physicochemical properties
One month later, the plant height, stem thickness and chlorophyll content of 10 maize plants in different treatments were measured [Citation12]. The soil pH was measured by a pH metre, and the soil conductivity was measured by a conductivity metre.
The activities of soil urease (S-UE), catalase (S-CAT), sucrase (S-SC) and acid phosphatase (S-ACP) were determined using a soil urease activity detection kit, soil catalase activity detection kit, soil sucrase activity detection kit and soil acid phosphatase activity detection kit (Solarbio, China), respectively.
Soil DNA extraction
Total DNA was extracted from 0.25 g of soil using a PowerSoil DNA Isolation Kit (Mobio Laboratories, Carlsbad, CA, USA) according to the manufacturer’s recommended protocol. The purity of DNA was detected by electrophoresis, and the quality was evaluated using a NanoDrop 1000 spectrophotometer (Thermo Fisher Scientific, Waltham, MA, USA).
High-throughput sequencing of soil microbes
The construction of 16S MetaVx™ library, construction of Illumina MiSeq sequencing high-throughput sequencing library, and sequencing based on Illumina MiSeq platform were performed by GENEWIZ Company (Suzhou, China). The concentration of DNA samples was detected by a Qubit 2.0 Fluorometer (Invitrogen, Carlsbad, CA, USA), and the sequencing library was constructed using a MetaVx™ library construction kit (GENEWIZ, Inc., South Plainfield, NJ, USA).
Using 30–50 ng of DNA as a template, a series of PCR primers designed by GENEWIZ were used to amplify two highly variable regions of V3 and V4 on prokaryote 16s rDNA. The V3 and V4 regions were amplified by upstream primers containing the ‘3′ The V3 and V4 regions were 5′’ sequence and downstream primers containing the ‘3′ sequence and downstream 5′’ sequence. In addition, an index linker is added to the end of the PCR product of the 16s rDNA by PCR for sequencing. The V3 and V4 regions were amplified by upstream primers containing the “3’CCTACGGRRBGCASCAGKVRVGAAT 5’” sequence and downstream primers containing the “3’GGACTACNVGGGTWTCTAATCC 5’” sequence.
Using 5–50 ng DNA as a template, the variable region of ITS2 on fungi ITS rDNA was amplified by PCR, and the ITS2 region was amplified by upstream primers containing the “3’GTGAATCATCGARTC 5’” sequence and downstream primers containing the “3’TCCTCCGCTTATTGAT 5’” sequence. An Index linker was added to the end of PCR product of ITS rDNA by PCR
The library quality was assessed using an Agilent 2100 Biological Analyzer (Agilent Technologies, Palo Alto, CA, USA), and library concentration was determined using a Qubit2.0 Fluorometer (Invitrogen, Carlsbad, CA, USA). After the DNA library was mixed, PE250/300 double-terminal sequencing is conducted according to the instructions of the Illumina MiSeq (Illumina, San Diego, CA, USA) instrument (the specific platform is based on the contract), and the sequence information was read by the MiSeq Control Software (MCS) implemented in the MiSeq.
Statistical analysis
After filtering, the chimera sequence was removed, and the final sequence was used for OTU (Operational taxonomic unit) analysis. VSEARCH (1.9.6) [Citation13] and QIIME (1.9.1) [Citation14] were used for sequence clustering (sequence similarity was set to 97%). The 16s rRNA reference database is Silva 132 [Citation15], and the ITS rRNA reference database is UNITE ITS database (https://unite.ut.ee/) [Citation16]. Then the representative sequences of OTU were analysed by RDP classifier version 2.2 (Ribosomal Database Program) [Citation17] with Bayesian algorithm, and the community composition of each sample was counted at different species classification levels. Based on the analysis results of OTU, the α diversity indices such as Shannon and Chao1 were calculated by random sampling of sample sequences with QIIME. Principal co-ordinate analysis (PCoA) visualization map was used to display β diversity based on the Bray-Curtis inter-sample distance matrix. Excel software version 2016, GraphPad Prism version 8 and R software version 3.3.1 were used for statistical analysis and mapping.
Results and discussion
Growth index of maize and physiochemical properties of soil
After one month of sowing, the experiment was initiated, the test area was irrigated with the neutralization solution, and the rest of the area was irrigated with water. After one month of treatment, plant height, stem diameter and chlorophyll content of the two groups were measured. The results showed that compared with the control group, stem diameter, plant height and chlorophyll content of maize significantly increased by 15.12%, 7.84% and 10.45%, respectively (p < 0.05; ). Thus, the neutralization solution could potentially promote the growth of maize. The main component of the neutralization solution is calcium lactate, and it has been reported that calcium lactate can promote plant growth [Citation9,Citation10], so calcium lactate in neutralization solution may be an important pro-growth factor in this study.
Table 1. Effects of neutralization solution on maize.
Soil pH, electrical conductivity (EC) and the activities of soil enzymes (S-UE, S-CAT, S-SC and S-ACP) were measured to clarify the effect of the neutralization solution on soil of the two groups. Compared to the control group, the neutralization solution had little effect on the soil pH, but the EC of the treatment group increased by 24.3% (p < 0.05; ). Many studies have shown that soil pH and EC have a significant effect on soil microbial activity [Citation18,Citation19], while soil pH did not change significantly and soil EC increased significantly in this study, which indicated that the neutralization solution may affect soil microorganisms by affecting soil EC. Soil enzyme activity is an important indicator of soil fertility [Citation20]. Assessment of the activity of four enzymes in maize field soil between the control and treatment groups did not reveal statistically significant differences (), which indicated that the neutralization solution does not affect soil enzymatic activity and may not have much effect on soil fertility. These results indicate that the use of the neutralization solution has minimal effects on some of soil properties and may be safe to be used for maize field irrigation for fertilizer utilization.
Table 2. Soil pH, EC and enzyme activity in the non-rhizosphere soil of maize.
Analysis of sequencing data
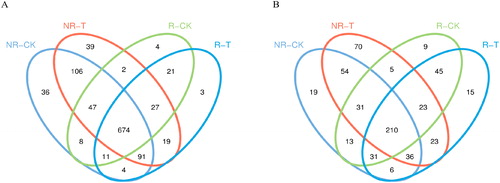
To explain the effect of neutralization solution on maize rhizosphere and non-rhizosphere microorganisms, 16s rDNA and ITS rDNA sequencing were conducted. A total of 11,428,02 effective reads were obtained by 16s rDNA high-throughput sequencing of bacteria, and the effective reads of each sample were between 44,909 and 92,886. The sequencing coverage for all samples was 99.6%–99.8%, indicating that the sequencing data well reflect the bacterial community structure of all samples. Among these, a total of 1092 OTUs were obtained by QIIME with 97% sequence identity. The number of OTUs in R-T, R-CK, NR-T and NR-CK samples was 850, 794, 1005 and 977, respectively. The abundance of OTUs of bacteria in rhizosphere soil was significantly lower than that in non-rhizosphere soil. The abundance of OTUs of non-rhizosphere and rhizosphere soil in the neutralization solution treatment group was higher than the control group. The results of Venn analysis showed that the number of core OTUs of the four groups was 674. The number of core OTUs in the neutralization solution treatment group was 813, whereas that in the control group was 740 ().
A total of 983,914 effective sequences were obtained by ITS rDNA high-throughput sequencing of fungi, and the effective reads of each sample were between 33,916 and 78,496. The sequencing coverage for all samples was 99.8%–99.9%, indicating that the sequencing data well reflect the fungal community structure of all samples. Among these, a total of 596 OTUs were obtained by QIIME with 97% sequence identity. The number of OTUs in R-T, R-CK, NR-T and NR-CK samples was 389, 367, 452 and 454, respectively. OTU abundance of fungi in rhizosphere soil was significantly lower than in non-rhizosphere soil. The results of Venn analysis showed that the number of core OTUs of the four groups was 210. The number of core OTUs in the neutralization solution treatment group was 292, whereas that in the control group was 285 ().
Compared with the control group, we found that neutralization solution treatment increased the abundance of bacterial OTUs in the soil samples, but not the abundance of fungal OTUs in the soil samples.
Alpha diversity of soil samples
The α diversity of different samples was analysed by QIIME, and the calculated results of each diversity index are shown in . The diversity of bacteria in non-rhizosphere soil was significantly higher than that in the rhizosphere soil, and neutralization solution treatment had no significant effect on bacterial diversity in non-rhizosphere soil. According to the ace and Chao1 indices, the number of bacterial OTUs in neutralization solution treatment rhizosphere soil was significantly higher than that in the control soil, but there was no significant difference between the Shannon and Simpson indices, which may be due to low species evenness in the treatment group. There was no significant difference in fungal α diversity index among samples.
Table 3. Analysis of microbial diversity indices in the rhizosphere and non-rhizosphere soil of maize.
The α diversity analysis of different samples indicated that the number of bacterial OTUs in the neutralization solution treatment rhizosphere samples was significantly higher than that in control samples. Neutralization solution treatment had no significant effect on the number of bacterial OTUs in non-rhizosphere soil. There was no significant difference in the number of fungal OTUs between non-rhizosphere and rhizosphere soil.
Beta diversity among different groups
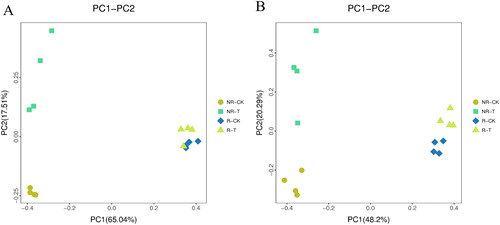
The differences among the four samples were analysed by PCoA. The first and second PCoA axes explained 17.51% and 65.04% of the observed variation in the bacterial community, respectively (). PCoA revealed that the bacteria in the rhizosphere soil samples were minimally affected by the neutralization solution treatment and could be grouped roughly together. In the non-rhizosphere soil samples, NR-CK and NR-T were separated from each other. The results showed that there was no significant difference in the structure of rhizosphere soil between the treatment and control groups, but there was a significant difference between the rhizosphere and non-rhizosphere soil samples.
PCoA of fungi () indicated that all soil samples could be distinguished from each other, but the distance between R-CK and R-T was smaller than that between NR-CK and NR-T. The results showed that there were significant differences in the fungal community structure of rhizosphere and non-rhizosphere soil between the treatment and control groups. The first and second PCoA axes explained 20.29% and 48.2% of the observed variations in the fungal community, respectively.
PCoA of bacteria and fungi showed that the neutralization solution treatment groups could be distinguished from the control groups in non-rhizosphere soils. These results showed that neutralization solution treatment has a highly significant effect on the diversity and abundance of bacteria and fungi in non-rhizosphere samples and the number of bacterial species in rhizosphere soil.
Dominant microbes observed in different groups
At the phylum level, the predominant bacteria phylum was Proteobacteria in all the soil samples (). However, the relative abundance of Proteobacteria in the rhizosphere samples was at least 77.13% higher than that in the non-rhizosphere samples. In the non-rhizosphere samples, there were significant differences (p < 0.05) in the relative abundance of 9 of the top 10 dominant phyla between NR-CK and NR-T (the abundance of Proteobacteria, Firmicutes, Patescibacteria and Verrucomicrobia increased in NR-T, whereas the Acidobacteria, Actinobacteria, Gemmatimonadetes, Chloroflexi and Nitrospirae decreased in NR-T), except for Bacteroidetes. Among these, the relative abundance of Patescibacteria in the treatment group increased by 5.32-fold. In the rhizosphere samples, there were significant differences (p < 0.05) in the relative abundance of 4 of the top 10 predominant phyla between R-CK and R-T (the abundance of Proteobacteria decreased in R-T, whereas that of Bacteroidetes, Patescibacteria and Chloroflexi increased in R-T). Among these, the relative abundance of Patescibacteria in the treatment group increased by 4.32-fold.
Figure 3. Relative abundance of bacterial (A) and fungal (B) species in different soil samples at the phylum level.
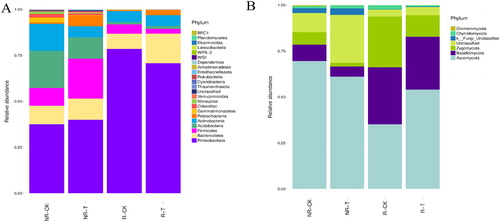
The ITS sequences were annotated to a total of seven clusters at the phylum level, and the predominant fungal phylum was Ascomycota in all the soil samples (). In the non-rhizosphere samples, there were no significant differences (p > 0.05) in the relative abundance of all phyla between NR-CK and NR-T. In the rhizosphere samples, there were significant differences (p < 0.05) in the relative abundance of Ascomycota (which increased by 53.51% in R-T) and Zygomycota (which decreased by 57.75% in R-T) phyla between R-CK and R-T.
By analysing the predominant microbes in different samples, we found that the effect of the neutralization solution treatment on bacteria was greater than that on fungi. In the non-rhizosphere samples, there were significant differences in the relative abundance of 9 of the top 10 predominant bacterial phyla between the treatment and control groups, whereas no significant differences were observed among the fungal phyla. The relative abundances of Acidobacteria and Chloroflexi were reported strongly positive correlated with soil pH and EC values in greenhouse soils [Citation20]. However, the neutralization solution treatment caused an increase in soil EC value but reduced the relative abundance of these bacteria phyla in this study, which indicated that the neutralization solution has a significant effect on maize non-rhizosphere bacteria community.
In the rhizosphere samples, there were significant differences in the relative abundance of 4 of the top 10 predominant bacterial phyla between the treatment and control groups, whereas there were 2 of the 7 predominant fungal phyla. Proteobacteria and Ascomycota were the predominant bacterial and fungal phyla in the rhizosphere soil of maize in different treatments. The similar results have shown in some reports after different treatments in the rhizosphere soil of maize [Citation21,Citation22], indicating that the most important influential factor of microbial community structure in the rhizosphere of the maize is the root system and not the other external environmental factors [Citation23]. The predominant bacterial phylum in wheat [Citation24] and rice [Citation25] rhizosphere soil were also reported as Proteobacteria. Most members of Proteobacteria play important roles in nitrogen fixation [Citation22], indicating that Proteobacteria plays an important role in the rhizosphere of many crops. The relative abundance of Proteobacteria in all the above reports was less than 55%, however, the relative abundance of Proteobacteria was more than 70% in all rhizosphere samples of maize in this study. We speculate that the maize variety and its growing environment may have led to this result together.
After neutralization solution treatment, the relative abundance of Patescibacteria most significantly increased in both rhizosphere and non-rhizosphere soils. Patescibacteria is a recently established bacterial phylum that encompasses mostly unculturable bacterial taxa [Citation26]. Recent studies have found that some species in this phylum have lost important genes associated with de novo biosynthesis of essential amino acids, nucleotides, fatty acids and cofactors [Citation27]. Neutralization solution treatment increased the relative abundance of Patescibacteria, indicating that some of the components in the neutralization solution may supplement nutrients for the growth of some species in Paescibachia.
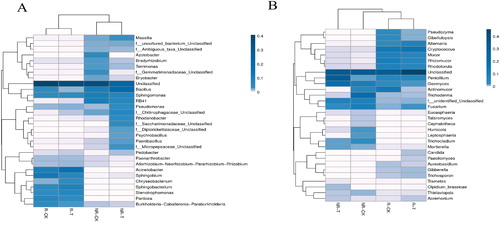
To further clarify the effect of neutralization solution treatment on microbial communities in soil at the genus level, the relative abundance of the top 30 genera of bacteria and fungi was analysed using heat maps, which showed significant differences in various soil samples ().
In the non-rhizosphere groups of bacteria, the relative abundance of Bryobacter, Rhizobium, Paenarthrobacter, Bradyrhizobium and Sphingomonas significantly decreased after neutralization solution treatment, including Bryobacter, which decreased by 65.7% (p < 0.01) (). Neutralization solution treatment significantly enriched the relative abundance of Paenibacillus, Rhodanobacter, Pseudomonas, Psychrobacillus and Bacillus, in which Paenibacillus increased by 19.19-fold (p < 0.01) and Bacillus increased 96.8% (p < 0.05). The predominant genus changed from Sphingomonas to Bacillus after treatment. In the rhizosphere groups of bacteria, the relative abundance of Sphingobium significantly decreased after neutralization solution treatment by 45.63% (p < 0.05) (). The treatment significantly enriched the relative abundance of Psychrobacillus and Chryseobacterium by 4.33- and 1.38-fold (p < 0.01), respectively. The predominant genus changed from Sphingobium to Acinetobacter after treatment.
Neutralization solution treatment had obvious influences on fungal genera. In the non-rhizosphere soil samples, Trichoderma, Geomyces, Fusarium, Actinomucor and Trichocladium were the top 5 predominant genera in NR-CK. However, Penicillium, Geomyces, Fusarium, Mortierella and Trichocladium were the top 5 most abundant genera in NR-T. The relative abundance of Paecilomyces, Actinomucor, Trichoderma, Leptosphaeria, Talaromyces, Cephalotheca and Humicola decreased after the treatment, in which Paecilomyces was not detected in the treatment group, whereas Humicola decreased by 87.13% (p < 0.05) (). Neutralization solution treatment significantly increased the relative abundance of Penicillium by 20.07-fold (p < 0.05). Among the rhizosphere groups, Cryptococcus, Actinomucor, Rhizomucor, Rhodotorula and Mucor were the top 5 predominant genera in R-CK. However, Cryptococcus, Alternaria, Mucor, Penicillium and Rhizomucor were the top 5 most abundant genera in R-T. The relative abundance of Actinomucor and Geomyces significantly decreased after neutralization solution treatment by 95.93% and 45.95% (p < 0.05) (). The treatment significantly increased the relative abundance of Alternaria and Fusarium by 1.27- and 0.74-fold (p < 0.05).
The relative abundance of the top 30 genera of bacteria and fungi showed significant differences in various soil samples. The relative abundance of Paenibacillus and Bacillus genera significantly increased in non-rhizosphere soils after neutralization solution treatment. Several members of genus Paenibacillus have been shown to produce diverse antimicrobial peptides (AMPs) [Citation28] and promote plant growth [Citation29]. Many members of genus Bacillus have been utilized as biocontrol strains [Citation30] because these can promote plant growth [Citation31] and inhibit pathogenic fungal [Citation32–34]. Neutralization solution treatment significantly enriched the relative abundance of genus Penicillium by 20.07-fold in non-rhizosphere soil. Penicillium can promote the plant growth and induce resistance by activating multiple defence signals [Citation35]. The increase in the relative abundance of these probiotics may be one of the reasons why the neutralization solution can promote maize growth.
Conclusion
For the reuse of the wastewater, we treated the fermentation wastewater with calcium oxide to prepare the neutralization solution and then used it to irrigate maize. We found that the growth index of maize significantly increased with neutralization solution treatment. However, no one has reported the effect of calcium lactate on soil properties and plant rhizosphere and non-rhizosphere microbial structure. Therefore, this study investigated the soil pH, conductivity, enzyme activity and the microbial community structure in maize rhizosphere and non-rhizosphere soil to reveal the effect of marigold flower fermentation wastewater calcium oxide neutralization solution on soil property and microbial community structure.
In conclusion, the use of the marigold flower fermentation wastewater neutralization solution can promote maize growth and has minimal effects on soil properties. Neutralization solution treatment has a very significant effect on the diversity and abundance of bacteria and fungi in non-rhizosphere samples, as well as the number of bacterial species in rhizosphere soil. The effect of neutralization solution treatment on bacteria was greater than that on fungi. Proteobacteria and Ascomycota were the predominant bacterial and fungal phyla in the rhizosphere soil of maize after treatment, indicating that the most important factors influencing microbial community structure in the rhizosphere of the maize are the root system and not the other external environmental factors. The relative abundance of Paenibacillus, Bacillus and Penicillium genera significantly increased in non-rhizosphere soil after neutralization solution treatment. The increase in the relative abundance of these probiotics may be one of the reasons why the neutralization solution can promote maize growth. We have proposed a potential method of treating marigold flowers fermentation wastewater and illustrate that the neutralization solution promotes the growth of maize and affects soil microbial structure.
Acknowledgements
We thank LetPub (www.letpub.com) for its linguistic assistance during the preparation of this manuscript.
Disclosure statement
No potential conflict of interest was reported by the authors.
Data availability statement
The sequence data generated in this study were deposited to the NCBI database (NCBI accession numbers: SRR10609229–SRR10609260).
Additional information
Funding
References
- Hadden WL, Watkins RH, Levy LW, et al. Carotenoids composition of marigold (Tagetes erecta) flower extract used as nutritional supplement. J Agric Food Chem. 1999;47(10):4189–4194.
- Samsudin H, Soto-Valdez H, Auras R. Poly(lactic acid) film incorporated with marigold flower extract (Tagetes erecta) intended for fatty-food application. Food Control. 2014;46:55–66.
- Álvarez MV, Hincapié S, Saavedra N, et al. Exploring feasible sources for lutein production: food by-products and supercritical fluid extraction, a reasonable combination. Phytochem Rev. 2015;14(6):891–897.
- Liu C, Chang D, Zhang X, et al. Oral fast-dissolving films containing lutein nanocrystals for improved bioavailability: formulation development, in vitro and in vivo evaluation. AAPS PharmSciTech. 2017;18(8):2957–2964.
- Richard AB. Lutein and zeaxanthin dietary supplements raise macular pigment density and serum concentrations of these carotenoids in humans. J Nutr. 2003;133(4):992–998.
- Wu X, Zhang H, Wang X, et al. Fermented marigold flowers suitable for the fine screening of lactic acid bacteria strains. Food Sci Tech. 2011;36(2):16–18 (in Chinese).
- Rong H. Comprehensive treatment to marigold flower fermentative waste. Chem Eng. 2007;140(5):36–38 (in Chinese).
- Luis NBJ, Hugo JI, Enrique BA, et al. An optimization study of solid-state fermentation: xanthophylls extraction from marigold flowers. Appl Microbiol Biotechnol. 2004;65(4):383–390.
- Pathomrungsiyounggul P, Grandison AS, Lewis MJ. Effect of calcium carbonate, calcium citrate, tricalcium phosphate, calcium gluconate and calcium lactate on some physicochemical properties of soymilk. Int J Food Sci Tech. 2010;45(11):2234–2240.
- Hui Q, Yang R, Shen C, et al. Mechanism of calcium lactate facilitating phytic acid degradation in soybean during germination. J Agric Food Chem. 2016;64(27):5564–5573.
- Hui Q, Wang M, Wang P, et al. Gibberellic acid promoting phytic acid degradation in germinating soybean under calcium lactate treatment. J Sci Food Agric. 2018;98(2):644–651.
- Yang F, Zhang F, Sun J, et al. Identification and preliminary application of antagonistic strains against potato fungal diseases. China Vegetables. 2019;369(11):56–62.
- Rognes T, Flouri T, Nichols B, et al. VSEARCH: a versatile open source tool for metagenomics. Peer J. 2016;4:e2584.
- Caporaso JG, Kuczynski J, Stombaugh J, et al. QIIME allows analysis of high-throughput community sequencing data. Nat Methods. 2010;7(5):335–336.
- Yilmaz P, Parfrey LW, Yarza P, et al. The SILVA and ‘All-species Living Tree Project (LTP)’ taxonomic frameworks. Nucleic Acids Res. 2014;42(Database issue):D643–648.
- Nilsson RH, Larsson KH, Taylor AFS, et al. The UNITE database for molecular identification of fungi: handling dark taxa and parallel taxonomic classifications. Nucleic Acids Res. 2019;47(D1):D259–D264.
- Wang Q, Garrity GM, Tiedje JM, et al. Naive Bayesian classifier for rapid assignment of rRNA sequences into the new bacterial taxonomy. Appl Environ Microbiol. 2007;73(16):5261–5267.
- Xiao K, Yu L, Xu J, et al. pH, nitrogen mineralization, and KCl-extractable aluminum as affected by initial soil pH and rate of vetch residue application: results from a laboratory study. J Soils Sediments. 2014;14(9):1513–1525.
- Kim JM, Roh AS, Choi SC, et al. Soil pH and electrical conductivity are key edaphic factors shaping bacterial communities of greenhouse soils in Korea. J Microbiol. 2016;54(12):838–845.
- Pascual JA, Moreno JL, Hernández T, et al. Persistence of immobilised and total urease and phosphatase activities in a soil amended with organic wastes. Bioresource Technol. 2002;82(1):73–78.
- Hollister EB, Engledow AS, Hammett AJM, et al. Shifts in microbial community structure along an ecological gradient of hypersaline soils and sediments. ISME J. 2010;4(6):829–838.
- Yin A, Jia Y, Qiu T, et al. Poly-γ-glutamic acid improves the drought resistance of maize seedlings by adjusting the soil moisture and microbial community structure. Appl Soil Ecol. 2018;129:128–135.
- Will C, Thurmer A, Wollherr A, et al. Horizon-specific bacterial community composition of german grassland soils, as revealed by pyrosequencing-based analysis of 16S rRNA genes. Appl Environ Microbiol. 2010;76(20):6751–6759.
- Maike R, Pérez-Jaramillo JE, Kavamura VN, et al. Multitrophic interactions in the rhizosphere microbiome of wheat: from bacteria and fungi to protists. FEMS Microbiol Ecol. 2020;96(4):4.
- Hernández M, Dumont MG, Yuan Q, et al. Different bacterial populations associated with the roots and rhizosphere of rice incorporate plant-derived carbon. Appl Environ Microbiol. 2015;81(6):2244–2253.
- Brown CT, Hug LA, Thomas BC, et al. Unusual biology across a group comprising more than 15% of domain bacteria. Nature. 2015;523(7559):208–211.
- Lemos LN, Medeiros JD, Dini-Andreote F, et al. Genomic signatures and co-occurrence patterns of the ultra-small Saccharimonadia (phylum CPR/Patescibacteria) suggest a symbiotic lifestyle. Mol Ecol. 2019;28(18):4259–4271.
- Baindara P, Nayudu N, Korpole S. Whole genome mining reveals a diverse repertoire of lanthionine synthetases and lanthipeptides among the genus Paenibacillus. J Appl Microbiol. 2020;128(2):473–490.
- Trinh CS, Jeong CY, Lee WJ, et al. Paenibacillus pabuli strain P7S promotes plant growth and induces anthocyanin accumulation in Arabidopsis thaliana. Plant Physiol Biochem. 2018;129:264–272.
- Gutierrez-Manero FJ, Ramos-Solano B, Probanza A, et al. The plant-growth-promoting rhizobacteria Bacillus pumilus and Bacillus licheniformis produce high amounts of physiologically active gibberellins. Physiol Plant. 2001;111(2):206–211.
- Chen XH, Koumoutsi A, Scholz R, et al. Comparative analysis of the complete genome sequence of the plant growth-promoting bacterium Bacillus amyloliquefaciens FZB42. Nat Biotechnol. 2007;25(9):1007–1014.
- Wu WJ, Park SM, Ahn BY. Isolation and characterization of an antimicrobial substance from Bacillus subtilis BY08 antagonistic to Bacillus cereus and Listeria monocytogenes. Food Sci Biotechnol. 2013;22(2):433–440.
- Liu H, Yin S, An L, et al. Complete genome sequence of Bacillus subtilis BSD-2, a microbial germicide isolated from cultivated cotton. J Biotechnol. 2016;230:26–27.
- Liu H, Wang Y, Yang Q, et al. Genomics and LC-MS reveal diverse active secondary metabolites in Bacillus amyloliquefaciens WS-8. J Microbiol Biotechnol. 2020;30(3):417–426.
- Hossain MM, Sultana F, Kubota M, et al. The plant growth-promoting fungus Penicillium simplicissimum GP17-2 induces resistance in Arabidopsis thaliana by activation of multiple defense signals. Plant Cell Physiol. 2007;48(12):1724–1736.