Abstract
The present study reports the isolation and analysis of a beta-glucosidase gene (accession number MN816859) from the fungus Auricularia heimuer. The Aa-bgl gene comprises 2583 bp and encodes a putative polypeptide of 860 amino acids. BlastP analysis revealed conserved glycoside hydrolase family 3 domains. Recombinant plasmid pET-32a-bgl was constructed to overexpress the target protein in Escherichia coli BL21, and sodium dodecyl sulphate polyacrylamide gel electrophoresis analysis revealed target bands between 100 kDa and 135 kDa, consistent with the expected molecular weight. Additionally, the transcription levels of Aa-bgl at different developmental stages were investigated, and the results showed that the expression levels increased with the maturation of the fruiting bodies. In mature fruiting bodies, the levels were 6.69-fold higher than in controls (mycelium at 6 days). We therefore speculate that Aa-bgl may be correlated with development in A. heimuer. The findings provide a theoretical basis for studying the function of this beta-glucosidase in future work.
Introduction
The fungus Auricularia heimuer is a traditional mushroom with both edible and medicinal value that has been cultivated in China for thousands of years [Citation1]. Notable for its nutritious and delicious characteristics, A. heimuer is consumed by many people around the world, and accounts for ∼6% of the global edible mushroom market [Citation2]. The polysaccharides present in A. heimuer possess anti-oxidative, anti-aging, anti-tumour and hypoglycaemic properties [Citation3–7]. Furthermore, due to its ability to accumulate elements, this species is believed to be a good source of trace elements, making it a valuable dietary supplement [Citation8].
Cellulose, the main component of lignocellulose biomass accounting for 30 − 50% [Citation9], is an abundant and renewable resource [Citation10], an important raw material for biofuel production [Citation11], and a common substrate used for the cultivation of edible mushrooms. Cellulase plays a critical role in the degradation of cellulose by microorganisms by complex enzyme systems composed of endoglucanases (EC: 3.2.1.4), cellobiohydrolases (EC: 3.2.1.91) and beta-glucosidases (EC: 3.2.1.21) [Citation12]. Among them, beta-glucosidase is the rate-limiting enzyme [Citation9], and this enzyme catalyses the degradation of cellobiose and other cellodextrins produced by endoglucanase and cellobiohydrolase [Citation13], thereby reducing feedback inhibition on cellobiohydrolase [Citation14], and accelerating the degradation of cellulose. Interestingly, beta-glucosidase is both inhibited by glucose and induced by glucose. Under high glucose concentrations, beta-glucosidase produces oligosaccharides such as cellobiose and sophorose via transglycosylation, considered to be an effective inducer of cellulase production [Citation15].
Beta-glucosidases are a heterogeneous group of hydrolytic enzymes present in various sources including plants, animals, fungi and bacteria [Citation16]. Members belong to different glycoside hydrolase (GH) families, and enzymes involved in the hydrolysis of cellulose mostly belong to GH1 and GH3 families [Citation17–19]. Due to their ability to hydrolyse and synthesise glycosidic bonds and wide substrate specificity, beta-glycosidases have great application value in the fields of medicine and energy [Citation20–21]. However, the function of beta-glucosidase gene in A. heimuer has not yet been studied. In the present work, we cloned and characterised a beta-glucosidase gene from A. heimuer, and measured transcription levels at different developmental stages; this aims to facilitate a better understanding of cellulase gene expression in A. heimuer and is ultimately directed at increasing the efficiency of cellulose utilization and raising mushroom yields. The results also lay a foundation for research on beta-glucosidase functions in A. heimuer and other fungi.
Materials and methods
Strains and plasmids
The A. heimuer strain was provided by the Edible Fungi R&D Center of Northeast Forestry University (Harbin, China). It was inoculated in preservation medium (48% sawdust, 50% wheat bran, 1% bean flour, 1% cornflour) and placed in the dark at 4 °C. Escherichia coli Top 10 and BL21 strains (Tsingke, Beijing, China) were cultured on Luria-Bertani (LB) medium. The pClone007 (Tsingke, Beijing, China) and pET-32a (Youbio, Changsha, China) vectors were used for cloning and expression, respectively.
RNA extraction and cDNA synthesis
Mycelia were inoculated into potato dextrose (PD) agar comprehensive medium and incubated at 25 °C for 1 week, and the most marginal mycelium was then inoculated into liquid PD medium using a punch and cultured in a constant temperature shaker incubator (25 °C, 160 rpm). Mycelia were collected 5 days later and stored at −80 °C. Total RNA was extracted using an RNAprep pure plant kit (Tiangen, Beijing, China) according to the manufacturer’s instructions, and cDNA was synthesised using a PrimeScript 1st strand cDNA synthesis kit (Takara, Dalian, China).
Amplification and sequence analysis of the Aa-bgl gene
Based on the A. heimuer transcriptome data and the NCBI database, the beta-glucosidase gene was determined and specific primers A-bglF and A-bglR () were designed. PCR Mix (DSBO, Guangzhou, China) was used to amplify the gene segment by polymerase chain reaction (PCR) in a mixture (20 μL) containing 10 μL 2× PCR Mix, 7.4 μL ultrapure H2O, 0.8 μL A-bglF, 0.8 μL A-bglR and 1 μL cDNA. Thermal cycling (TC-96/G/H, Bioer Technology, Hangzhou, China) involved denaturation at 94 °C for 3 min, followed by 35 cycles of denaturation at 94 °C for 30 s, annealing at 58 °C for 30 s, and extension at 72 °C for 50 s, and a final extension at 72 °C for 10 min. PCR products were cloned into the pClone007 vector for sequencing (Tsingke, Beijing, China).
Table 1. Primers used in this study.
Sequence analysis was performed using various software tools. Conserved region and protein family prediction were carried out using an NCBI Conserved Domains search (https://www.ncbi.nlm.nih.gov/). ExPASy ProtParam (https://www.expasy.org/) was utilised to analyse the molecular weight and isoelectric point of the deduced protein. TMHMM Server v. 2.0 was used for transmembrane region analysis. The SignalP 5.0 server was used to predict signal peptides (http://www.cbs.dtu.dk/services/SignalP/). The secondary structure of the protein was assessed by the Predictprotein tool (https://www.predictprotein.org/), and the three-dimensional structure was predicted using SWISS-MODEL (https://swissmodel.expasy.org/). A phylogenetic tree was generated using the neighbour-joining method in MEGA 6.0 [Citation22].
Construction of the prokaryotic expression vector
Using the characteristics of vaccinia topoisomerase I (Tsingke), the Aa-bgl gene was cloned into the pClone007 vector to generate p007-bgl. The restriction enzymes BamHI and HindIII were used to digest pET-32a (Youbio, Changsha, China), and primers A-bglhF and A-bglhR () containing these cleavage sites were designed for amplifying Aa-bgl. Using p007-bgl as template, the Aa-bgl product was amplified by PCR in a 20 μL mixture containing 10 μL 2× PrimeSTAR PreMix (Takara), 8 μL ultrapure H2O, 0.8 μL A-bglhF, 0.8 μL A-bglhR and 0.4 μL p007-bgl. Thermal cycling (TC-96/G/H, Bioer Technology, Hangzhou, China) involved an initial denaturation at 94 °C for 5 min, followed by 35 cycles at 94 °C for 30 s, annealing at 65 °C for 30 s, extension at 72 °C for 50 s, and a final extension at 72 °C for 10 min. PCR products were purified, digested with BamHI and HindIII, and ligated to pET-32a (digested with the same restriction enzymes) using a Trelief SoSoo Cloning Kit (Tsingke) according to the manufacturer’s instructions. Ligation products were transferred into E. coli Top10 competent cells; ampicillin at a final concentration of 100 μg/mL was added to LB medium for screening, and primers pET-F and pET-R were used for further testing of positive clones. The resulting pET-32a-bgl plasmid was extracted and subjected to DNA sequencing.
Heterologous expression of Aa-bgl in E. coli BL21
The pET-32a plasmid was transformed into E. coli BL21 as a blank control, and E. coli BL21 cells harbouring pET-32a-bgl were used to express the recombinant protein under the induction of isopropyl β-D-thiogalactoside (IPTG) at a final concentration of 1 mmol/L during culturing at 28 °C with shaking at 180 rpm. A 1 mL sample of the induced bacterial solution was collected at 2, 4, 6, 8 and 10 h, centrifuged at 13,770 × g for 10 min, and the cell pellet was stored at −20 °C. Cells were fully resuspended in SDS-PAGE Sample Loading Buffer (Biosharp, Shanghai, China) diluted to 1×,boiled in a water bath for 10 min, and 10 μL of the solution was used for sodium dodecyl sulphate–polyacrylamide gel electrophoresis (SDS-PAGE) analysis.
SDS-PAGE analysis of the Aa-bgl protein
SDS-PAGE with an 8% separation gel was performed using a dedicated kit (Solabio, Beijing, China) following the manufacturer’s instructions. Briefly, a 10 μL sample was added to each lane and electrophoresis was conducted with a voltage of 120 V for 2 h. The gel was stained with Coomassie Brilliant Blue R-250 (Solarbio, Beijing, China) for 2 h, then destained until there was no blue background.
Analysis of Aa-bgl transcription levels during different stages
A. heimuer mycelia activated on PDA were inoculated into 200 mL liquid medium containing 100 g PDA, 40 g wheat bran, 15 g brown sugar, 10 g dextrose, 2 g KH2PO4, 2 g peptone, 1 g MgSO4 and 10 mg VB1. After cultivation in a rotary shaker incubator (160 rpm) at 25 °C, mycelia were collected at 6, 8, 10 and 12 days and stored at −80 °C. Each test was repeated three times. Primordia, young fruiting bodies and matured fruiting bodies of A. heimuer () were obtained by artificial cultivation indoors, total RNA was extracted as described above, and cDNA was synthesised using a PrimeScript RT reagent kit (Takara). Subsequently, primers Aa-bglqF and Aa-bglqR () were designed, along with primers for amplifying the β-actin gene as an internal reference (), and quantitative real-time PCR (qRT-PCR) was performed using a CFX96 Real-Time PCR Detection System (Bio-Rad, Shanghai, China). The reaction mixture contained 12.5 μL 2× TB Green Premix ExTaq II (Takara), 2 μL cDNA template, 0.5 μL Aa-bglqF, 0.5 μL Aa-bglqR and 9.5 μL deionised water. Relative expression levels were calculated using the 2−ΔΔCt method [Citation23].
Results and discussion
Cloning and bioinformatics analysis of Aa-bgl
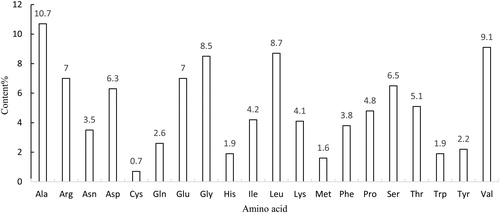
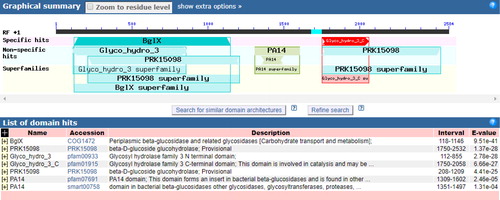
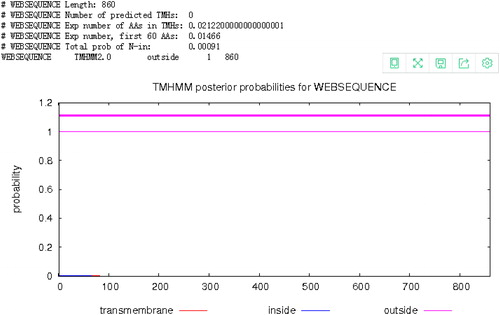
The A. heimuer cDNA was used as the template for gene cloning by RT-PCR, and the sequencing results showed that the full-length Aa-bgl gene is 2583 bp, encoding a putative polypeptide of 860 amino acids with a molecular weight of 93.969 kDa and a theoretical isoelectric point of 5.49 (). Amino acid sequence analysis () revealed that the most abundant amino acid is Ala (10.7%) and the least abundant is Cys (0.7%). The instability index of the predicted protein was computed to be 32.92, and the grand average of hydropathicity (GRAVY) was −0.202, indicating a stable hydrophilic protein. BlastP analysis indicated that the amino acid sequence possesses a conserved glycoside hydrolase family 3 domain (). Transmembrane analysis showed that amino acids 1 to 860 are not within the cell membrane (). Many secreted proteins include a signal peptide at the N-terminus, but a signal peptide was not predicted (), indicating that Aa-bgl is an intracellular protein. The absence of a cellulose-binding domain as reported for the extracellular β-glucosidase from P. chrysosporium further illustrates this point [Citation24]. Fungal cellulase is usually secreted into the matrix. However, this is not the case with beta-glucosidase. In Volvariella volvacea, most of the beta-glucosidase is intracellular and located in the apical area of the hypha, and a few present in the culture fluids are also distributed on the outer surface of the mycelium [Citation25]. The intracellular hydrolysis will not cause the glucose to be lost into the matrix, ensuring better growth and development of the microorganism.
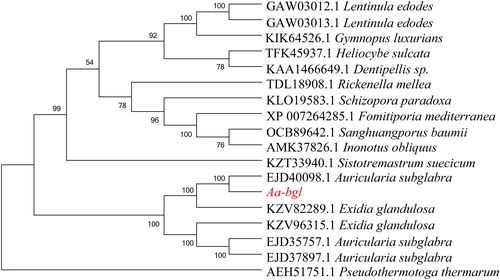
The protein secondary structure comprises 23.37% alpha helix, 21.05% beta sheet and 55.58% random coil. Tertiary structure prediction was performed using SWISS-MODEL with a beta-glucosidase from Kluyveromyces marxianus (SMTL id: 3abz.1) serving as the template (). Phylogenetic analysis using MEGA 6.0 was conducted with the amino acid sequence of Aa-bgl as a query, 16 homologous sequences from other fungi in the NCBI database (), and 1 external reference sequence from bacteria (). Aa-bgl was found to be most similar to bgl in Auricularia subglabra, while bgl in Pseudothermotoga thermarum was located in another branch, indicating greater genetic distance.
Figure 7. Structural and expression analysis of the Aa-bgl protein. (a) Tertiary structure prediction. (b) SDS-PAGE analysis following induction of expression by IPTG (1 mmol/L). M, protein molecular weight markers (Solarbio); Lane 1, E. coli transformed with pET-32a; Lanes 2–7, E. coli transformed with pET-32a-bgl induced with IPTG for 0, 2, 4, 6, 8 and 10 h, respectively.
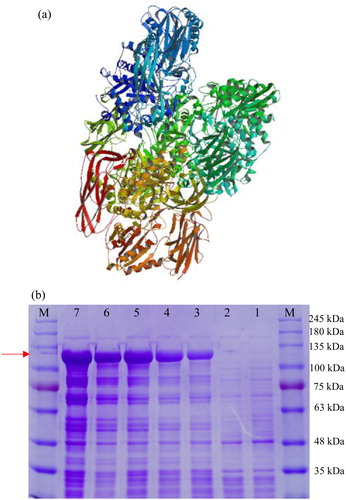
Table 2. Sequences of Aa-bgl homologs in various species.
SDS-PAGE analysis of the Aa-bgl protein
SDS-PAGE analysis of the recombinant Aa-bgl protein revealed bands with a molecular weight of ∼113.969 kDa between the 100 kDa and 135 kDa markers (), consistent with the theoretical molecular weight of the recombinant fusion protein [Citation26]. Additionally, SDS-PAGE showed that protein expression was highest when induced for 10 h.
Due to the limitations of the prokaryotic expression system, the activity of the target protease will be affected. As an important cellulase, its enzymatic properties are worthy of further study.
Analysis of Aa-bgl transcription levels
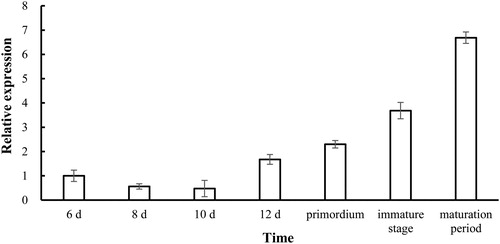
Expression levels of Aa-bgl during different developmental stages of A. heimuer were investigated, and the results showed that gene expression levels gradually increased over time, with highest levels in mature fruiting bodies that were 6.69-fold higher than those in controls (mycelia at 6 days; ). Interestingly, our findings are similar to the expression pattern of the beta glucosidase gene in V. volvacea [Citation27]. This may indicate an involvement in the synthesis of polysaccharides and other substances during the development of fruiting bodies. Of course, there may be differences in gene expression patterns under the induction of other different carbon sources.
Figure 9. Aa-bgl transcription levels at different stages of A. heimuer development.
Note: Values are means ± standard deviation from three experiments.
In recent years, as our awareness of environmental protection has grown, deforestation has been restricted, leading to a rise in sawdust prices. Thus, it would be of great economic benefit to increase the production of A. heimuer in the face of rising production costs. The relationship between extracellular endocellulase activity and fruiting body yield has been investigated in Agaricus bisporus, and the results showed a positive correlation [Citation28]. In Trichoderma asperellum, four strains expressing cbh genes were obtained through genetic transformation, and their cellulase activities were increased by 30–40% compared with the wild-type strain [Citation29]. Experiments have clearly shown that additional copies of the bgl1 gene in Trichoderma reesei can increase the hydrolysis of cellulose [Citation30]. Similar findings were found in tobacco plants, and the biomass yield and life cycle of the transformants overexpressing BglB were 52% higher and 36% shorter than those of the wild-type, respectively [Citation31]. Beta-glucosidases also play important roles in the regulation of cellulase genes in T. reesei [Citation19], but the effect of cellulose-related genes in A. heimuer requires further study.
Conclusions
In the present study, the beta-glucosidase gene Aa-bgl from A. heimuer was cloned and successfully expressed in E. coli. The protein contains a conserved glycoside hydrolase 3 family domain, and it is an intracellular enzyme lacking a signal peptide. The expression levels of Aa-bgl during different developmental stages revealed highest expression in mature fruiting bodies. The results provide valuable information for further functional studies of Aa-bgl in A. heimuer. To study gene function, further studies are needed in addition to prokaryotic expression. At present, we have cloned the glyceraldehyde 3-phosphate dehydrogenase (gpd) promoter of A. heimuer and constructed a binary expression vector based on pCAMBIA1301. In future work, we will establish a set of genetic transformation procedures for A. heimuer, using genetic engineering methods to enhance or suppress the expression of Aa-bgl to further explore its functions.
Disclosure of interest
The authors report no conflict of interest.
Acknowledgments
The authors are grateful to Professor Li Zou (Department of Forest Protection, College of Forestry, Northeast Forestry University) for her guidance and help.
Data availability statement
The data that support the findings of this study are available on request from the corresponding author, [Z]. The data are not publicly available due to their containing information that could compromise the privacy of research participants.
References
- Li L, Zhong CH, Bian YB. The molecular diversity analysis of Auricularia auricula-judae in China by nuclear ribosomal DNA intergenic spacer. Electron J Biotechn. 2014;17(1):27–33.
- Royse DJ, Singh M. A global perspective on the high five: Agaricus, Pleurotus, Lentinula, Auricularia & Flammulina. International Conference on Mushroom Biology & Mushroom Products. 2014. New Delhi, India.
- Zeng WC, Zhang Z, Gao H, et al. Characterization of antioxidant polysaccharides from Auricularia auricular using microwave-assisted extraction. Carbohydr Polym. 2012;89(2):694–700.
- Cai M, Lin Y, Luo YL, et al. Extraction, antimicrobial, and antioxidant activities of crude polysaccharides from the wood ear medicinal mushroom Auricularia auricula-judae (Higher Basidiomycetes). Int J Med Mushrooms. 2015;17(6):591–600.
- Lu A, Yu M, Shen M, et al. Antioxidant and anti-diabetic effects of Auricularia auricular polysaccharides and their degradation by artificial gastrointestinal digestion-Bioactivity of Auricularia auricular polysaccharides and their hydrolysates. Acta Sci Pol Technol Aliment. 2018;17(3):277–288.
- Qiu J, Zhang H, Wang Z, et al. The antitumor effect of folic acid conjugated-Auricularia auricular polysaccharide-cisplatin complex on cervical carcinoma cells in nude mice. Int J Biol Macromol. 2018;107(Pt B):2180–2189.
- Lu A, Yu M, Shen M, et al. Preparation of the Auricularia auricular polysaccharides simulated hydrolysates and their hypoglycaemic effect. Int J BIol Macromol. 2018; 106:1139–1145.
- Hu T, Li L, Hui GF, et al. Selenium biofortification and its effect on multi-element change in Auricularia auricular. Food Chem. 2019; 295:206–213.
- Rajasree KP, Mathew GM, Pandey A, et al. Highly glucose tolerant β-glucosidase from Aspergillus unguis: NII 08123 for enhanced hydrolysis of biomass. J Ind Microbiol Biotechnol. 2013;40(9):967–975.
- Tilman D, Hill J, Lehman C. Carbon-negative biofuels from low-input high-diversity grassland biomass. Science. 2006;314(5805):1598–1600.
- Parisutham V, Kim TH, Lee SK. Feasibilities of consolidated bioprocessing microbes: from pretreatment to biofuel production. Bioresour Technol. 2014; 161:431–440.
- Lindenmuth BE, Mcdonald KA. Production and characterization of Acidothermus cellulolyticus endoglucanase in Pichia pastoris. Protein Expr Purif. 2011;77(2):153–158.
- Bohlin C, Praestgaard E, Baumann MJ, et al. A comparative study of hydrolysis and transglycosylation activities of fungal β-glucosidases. Appl Microbiol Biotechnol. 2013;97(1):159–169.
- Bhatia Y, Mishra S, Bisaria VS. Microbial beta-glucosidases: cloning, properties, and applications. Crit Rev Biotechnol. 2002;22(4):375–407.
- Singhania RR, Patel A, Pandey A, et al. Genetic modification: a tool for enhancing beta-glucosidase production for biofuel application. Bioresour Technol. 2017;245(Pt B):1352–1361.
- Jiang W, Wang W, Pan B, et al. Facile and green fabrication of biocatalytic chitosan beads by one-step genipin-mediated β-glucosidase immobilization for production of bioactive genistein. ACS Appl Mater Interfaces. 2014;6(5):3421–3426.
- Tian L, Liu S, Wang S, et al. Ligand-binding specificity and promiscuity of the main lignocellulolytic enzyme families as revealed by active-site architecture analysis. Sci Rep. 2016; 6(1):23605.
- Crespim E, Zanphorlin LM, de Souza FH, et al. A novel cold-adapted and glucose-tolerant GH1 β-glucosidase from Exiguobacterium antarcticum B7 . Int J Biol Macromol. 2016; 82:375–380.
- Singhania RR, Patel AK, Sukumaran RK, et al. Role and significance of beta-glucosidases in the hydrolysis of cellulose for bioethanol production. Bioresour Technol. 2013; 127:500–507.
- Salgado JCS, Meleiro LP, Carli S, et al. Glucose tolerant and glucose stimulated β-glucosidases - A review. Bioresour Technol. 2018; 267:704–713.
- Bayer EA, Lamed R, Himmel ME. The potential of cellulases and cellulosomes for cellulosic waste management. Curr Opin Biotechnol. 2007;18(3):237–245.
- Zhang SC, Tong YX, Li YJ, et al. Genome-wide identification of the HKT genes in five Rosaceae species and expression analysis of HKT genes in response to salt-stress in Fragaria vesca. Genes Genomics. 2019;41(3):325–336.
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25(4):402–408.
- Li B, Renganathan V. Gene cloning and characterization of a novel cellulose-binding beta-glucosidase from Phanerochaete chrysosporium. Appl Environ Microbiol. 1998;64(7):2748–2782.
- Cai YJ. Purification, characterization and localization of cellulolytic enzymes produced by the straw mushroom. (Ph.D Dissertation). Volvariella volvacea. 1996.
- Sun J, Wang SX, Wang XT, et al. Cloning and expression analyses of a cellobiohydrolase gene from Auricularia heimuer. Biotechnol Biotec Eq. 2019;33(1):1327–1334.
- Ding SJ, Ge W, Buswell JA. Molecular cloning and transcriptional expression analysis of an intracellular beta-glucosidase, a family 3 glycosyl hydrolase, from the edible straw mushroom, Volvariella volvacea. FEMS Microbiol Lett. 2007;267(2):221–229.
- Claydon N, Allan M, Wood DA. Fruit body biomass regulated production of extracellular endocellulase during periodic fruiting by Agaricus bisporus. Transact Br Mycol Soc. 1988;90(1):85–90.
- Wang Q, Chen L, Fang CY, et al. The overexpression of one single cbh gene making Trichoderma asperellum T-1 a better cellulase producer. Ann Microbiol. 2019;69(7):673–683.
- Barnett CC, Berka RM, Fowler T. Cloning and amplification of the gene encoding an extracellular beta-glucosidase from Trichoderma reesei: evidence for improved rates of saccharification of cellulosic substrates . Biotechnology (NY).). 1991;9(6):562–567.
- Cho EJ, Nguyen QA, Lee YG, et al. Enhanced biomass yield of and saccharification in transgenic tobacco over-expressing β-glucosidase. Biomolecules. 2020;10(5):806. [cited 2020 Jun 03]