Abstract
This study reports the high susceptibility of Propionibacterium acnes to four medicinal plants collected from western regions of Saudi Arabia. Plant extracts were obtained by using methanol and acetone. The susceptibility was tested by agar well diffusion method. Plants of interest, namely, Rhazya stricta, Azadirachta indica, Camellia sinensis and Ocimum basilicum, exhibited good antibacterial activity with minimum inhibitory concentration (MIC) values of 50 mg/mL, 25 mg/mL, 100 mg/mL and 100 mg/mL respectively. The maximum antibacterial effect was observed by A. indica followed by R. stricta. In addition, C. sinensis, and O. basilicum had moderate activities. The results show that these plants could be tested for use in skin care products to prevent or improve acne.
Introduction
The human skin acts as an external barrier which contains microorganisms that form commensal microbial community or microbiota and are linked with skin health. The most common microbiota found in the skin is Corynebacterium, Propionibacterium and Staphylococcus. The loss in the balance of natural microbial communities has been linked with skin diseases such as acne [Citation1–3]. Acne vulgaris is a chronic disorder, an inflammatory disease of the sebaceous glands and hair follicles of the skin marked by the eruption of pimples and found in areas of increased sebaceous production such as the face, upper arms, chest, and back [Citation4–6]. Disease studies have estimated acne to affect approximately (9.4%) of populations worldwide and consider it one of the most prevalent dermatologic conditions [Citation7]. Some psychological damages are severe and cause depression, low self-esteem, suicidal ideation and anxiety [Citation8, Citation9]. Propionibacterium acnes is a gram-positive facultative anaerobe, a pleomorphic rod-shaped bacterium, which thrives on the sebum and is believed to cause skin diseases such as acne [Citation10, Citation11].
Current therapies against acne primarily use 13-cis-retinoic acid and various antibiotics as the first line of defense [Citation12]. Standard oral and topical therapies can have significant side effects, including skin irritation, gastrointestinal upset and the development of drug-resistant bacteria [Citation13–17]. Current treatments are palliative, and there is an urgent need to find additional therapeutic options.
This research aimed to find an appropriate herbal treatment rather than available medicines with side effects to treat acne. Accordingly, the methanol and acetone extracts of four Saudi medicinal plants; Rhazya stricta, Azadirachta indica, Camellia sinensis and Ocimum basilicum were used to control the pathogenesis of P. acnes. Therefore, we proposed this research intending to discover new antimicrobial agents from natural sources as anti-acne agents and limit drug-resistant bacteria.
Materials and methods
Ethical approval
The study was approved by the unit of Biomedical Ethics Research Committee at King Abdul Aziz Hospital, Dermatology Department (Reference No. 230-17). The patients signed informed consent forms before the study.
Propionibacterium acnes clinical isolates
The isolates of P. acnes (22 isolates) were taken from patients with mild, moderate and severe acne conditions.
The acne severity was graded as severe, moderate and mild according to the classification of acne severity with some modification [Citation18]. Gender, age, and type of acne lesion were studied. The inflammatory acne lesions were determined based on counting and were (0–5), (6–20) and (21–50) for mild, moderate and severe, respectively. The main exclusion criteria were as follows: antibiotic intake, pregnant women, and patients receiving other therapy.
The bacterial strains were isolated from an inflammatory papule, pustule and cyst to screen for antibacterial activity. Each isolate was taken within a specified surface area of about 16 cm2 of the skin divided into three parts: the forehead, cheeks and jaw. An alcohol pad was used above the acne lesion to wipe microorganisms that are found on top of it. Acne lesion was then extracted with a scratch of the tiny thin lancet and the sebum was collected by slight pressure with the hand. In addition, the surface acne lesion was swabbed without an alcohol pad.
The swab was placed in a test-tube containing brain heart infusion (BHI), (Sigma-Aldrich Ltd) or Thioglycolate (THIO; HiMedia) as transport medium. The tubes were incubated for five days at 37 °C, followed by ten-fold serial dilution.
Identification of the isolates
Fermentation test
The fermentation test was made according to McDowell et al. method with some modification [Citation19]. Reinforced Clostridial Agar (RCA) media (Oxoid Ltd.) supplemented with a bromocresol purple (HiMedia) as an indicator (40 mg/L). About 50 μL of the culture was dispensed on Petri plates and a glass spreader was used to spread the culture. All plates were incubated at 37 °C for five days in an anaerobic chamber (Baker Co, UK) in an atmosphere of a mixed gas of (5.5%) hydrogen, (5%) carbon dioxide, and (89.5%) nitrogen. Fermentation was considered to occur when the colonies of P. acnes appeared yellow due to propionic acid production.
Molecular identification of strains
DNA extraction
DNA was extracted from pure cultures of P. acnes grown in BHI or THIO media after bacteria reached ideal growth. DNA extraction was made based on the binding of magnetic beads to nucleic acids and by combining 200 μL, 20 μL, 200 μL of lysis buffer, proteinase K buffer, and the sample, respectively with occasional shaking following the protocol of DNA/RNA Easy Extraction Kit (Genesig primer design, UK). The tube was incubated at room temperature for 15 min and 500 μL of magnetic beads were added to the solution. The next step was separating of magnetic beads and solution by attracting the beads to the magnetic rack. The beads were washed with 500 μL of wash buffer 1 and 2 separately for seconds then attracting the beads to the magnetic rack and the solution was discarded. Ethanol (80%) was added for seconds then the solution was removed and beads were left to air dry for 10 min. Finally, the elution buffer was added to elute nucleic acids, and the supernatant that contains DNA was stored at −20 °C.
The purity of DNA was measured by NanoDrop™ (Thermo Scientific). About 1 μL of elution buffer was used as a blank then reading was obtained by applying 1 μL of DNA sample at 260/280 (the ratio of absorbance).
Polymerase chain reaction (PCR) and amplification of 16S rRNA gene, Universal bacterial primers correspond to regions of the 16S rRNA gene that are highly conserved among divergent groups of prokaryotes and therefore would be expected to amplify partial DNA from the bacteria. The primer locations for P. acnes were Forward primer, 5′-AGAGTTTGATCCTGGCTCA-3′ (27), and Reverse Primer, 5′-AAGGAGGTGATCCAGCCGCA-3′ (1525).
The PCR reaction was conducted in a total volume of 25 μL, which contained 1 μL of 10 pmol of each primer, 12.5 μL of the commercial Master mix (GoTaq® Green Master Mix, 2X, Promega). Approximately 1 μL of 200 ng of DNA template was added to the PCRs tube. Nuclease-free water was added to adjust the final volume to 25 μL. PCR was performed with a thermal cycler (Applied Biosystems™ Veriti™ 96-Well Thermal Cycler) and programmed to perform 35 cycles that consist of initial step at 94 °C for 5 min, denaturation at 94 °C for 30 s, annealing of primers at 60 °C for 30 s, extension at 70 °C for 1.30 min and a final extra extension of 10 min at 70 °C.
Gel electrophoresis
After amplification, PCR products were analyzed by 2% agarose gel electrophoresis in 1X TAE (Tris Acetate EDTA). After the DNA migrated approximately (75%) of the way down the gel, DNA products were compared with molecular size markers (DNA ladder 1 kb, Bio lab Inc, US) and visualized under UV light using a gel documentation system (Biospectrum 410, UVP).
PCR products were sent to Macrogen Co. laboratories (Korea) to determine the sequence and identify the isolated strains. Data were analyzed and optimized by using MEGA X (Molecular Evolutionary Genetics Analysis) program and compared with sequences in the NCBI database.
Plant collection and preparation of powder
To prepare extracts, R. stricta, A. indica, C. sinensis and O. basilicum were collected from western regions (Makkah province) of Saudi Arabia in April 2017. Plant leaves were air-dried, homogenized by chopper blade blender into a fine powder, and stored in air-tight bottles at room temperature until use.
Methanol and acetone extract preparation
About 50 g of powdered plant materials were dissolved in 500 mL of 80% methanol and acetone separately then left in a laboratory shaker at room temperature for 48 h. The extract was filtered using filter paper (Whatman) and concentrated on a rotary evaporator (Eyela 0N-1110) at 45 °C. The residue was dissolved in dimethyl sulfoxide (DMSO; Thermo Fisher Scientific, US) to obtain a starting concentration of 500 mg/mL. A sterile syringe filter disk (Whatman) with 0.22 μm pore was used to filter the extract and finally stored in dark container glassware at −20 °C until use.
Preparation of different concentrations from a stock solution
Five different concentrations (400 mg, 200 mg, 100 mg, 50 mg) were prepared by diluting the extracts (stock) to the required concentration using sterile distilled water.
Agar well diffusion assay
The agar well diffusion assay was used according to Balouiri et al. [Citation20] with some modification. A loop inoculated with a single colony of P. acnes was dipped inside an Eppendorf tube containing 600 μL of sterile sodium chloride (0.9%) and set visually comparing it to the turbidity of the standard 0.5 McFarland tube. Sterilized cotton swab was used to inoculate freshly made Muller Hinton agar media (Micro-master). Cork borer with 6 mm in diameter sterilized and used to make the pores inside. About 100 μL of each plant extract was dispensed inside the wells and DMSO was used as a negative control. For positive control, Teicoplanin (TEI) 30 μg was used.
The plates were incubated anaerobically upwards at 37 °C for 36 h, and finally, the zones of inhibition were measured and recorded for each extract.
Minimum inhibitory concentration (MIC)
The minimum inhibitory concentration was determined according to Lima and de Aguiar [Citation21] with some modification. About 100 μL of Muller Hinton broth (Micro-master) was dispensed in a 96-well reaction plate. The first row was used as a negative control, the second row was used as a positive control and the third row contained an initial concentration of 100 mg of extract that was serially diluted horizontally up to well-labeled 12.
Approximately 30 μL of bacterial cultures (equal to 0.5 McFarland units) was dispensed in wells except for negative control. All plates were incubated in a sealed tight bag with a gas generating pack (BD, USA) for 36 h at 37 °C. Finally, 20 μL of resazurin (Sigma-Aldrich) was added to each well, then was incubated for two hours. Results read by changes in the color of media and the MIC was the lowermost concentration of plant extract that prevented this change.
Results and discussion
There was a correlation between acne and age. The data showed that high incidence of acne was found in the patients from the age group of 25–34 years, followed by those from the age group of 15–24 years, then the patients from the age group of 35–44 years with 50%, 36% and 9%, respectively. This study also estimated the distribution of acne vulgaris in both genders and the data showed that acne was more prevalent in females than in males: 91% vs. 9%, respectively. Similar results were reported in other studies [Citation22, Citation23]. In addition, another study reported that females were more likely to develop acne earlier than males [Citation24]. In contrast, other studies showed that acne occurred mostly in the age group of 13–20-year-olds and appeared in males more than in females, and severity was observed in males [Citation25, Citation26].
In the present study, the severity of acne in patients was classified as a severe, moderate and mild condition. The results showed that 46% had severe acne, followed by 27% who had a moderate condition, and the last group (27%) had a mild acne form. The severity of acne was associated with pustules (59%), cysts (32%) and nodules (9%). The position of acne lesions appeared mostly on the cheeks (50%) followed by the jaw (32%), then the forehead (14%) and (4%) near the nose.
The severity of acne in adolescence was associated with pustules and might be due to the production of more sebum, and hormonal changes such as testosterone, progesterone, glucocorticoids, insulin and insulin-like growth factors [Citation27]. On the other hand, Schäfer et al. [Citation28] pointed to the severity of acne associated with cigarette smoking. Other factors include physiological differences between men and women, the sampling site, drug administration, anxiety, study pressure and type of food [Citation29, Citation30].
Differentiation of P. acnes among different microbes (fermentation test)
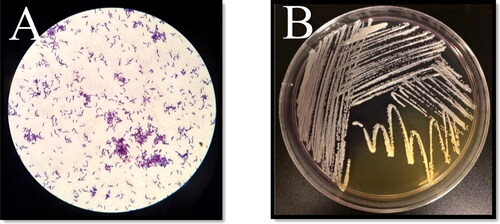
The fermentation test was considered positive when the color of the media changes from purple to yellow. Different microbes emerged in the early days and P. acnes began to appear as visible colonies at five days. P. acnes produces propionic acid (pH range 4–5) and colonies appeared as small circular yellowish dots or in an area of a complete change in the color of the medium. Other microbes appeared white or translucent in the purple region of the medium indicating that these microbes did not produce acids. In Gram stain, P. acnes cells appeared as rod-shaped bacilli (). After the incubation of pure culture on RCA, P. acnes appeared after 48 h, which was described as a small circular, yellowish, opaque, and semi-opaque convex colony. While P. acnes grows in a plate, the bacteria produce propionic acid that changes the color of the medium, indicating bacterial growth rate ().
Figure 1. Morphology of P. acnes. (A) P. acnes under the light microscope magnified 100×, cells occur singly or in pairs and arranged in Chinese letters. (B) Pure culture of P. acnes showing partial changes in the color of the medium due to the production of propionic acid.
P. acnes was isolated anaerobically from the pilosebaceous units; these sites were further occupied by it. These results are in agreement with other studies [Citation31, Citation32]. P. acnes reduces the growth of other microbes such as Staphylococcus aureus, Streptococcus pyogenes and further maintains an acidic pH in the sebaceous site due to hydrolysis of sebum contents and production of propionic acid [Citation33].
In contrast, the surface acne lesion was dominated by Staphylococcus epidermidis and it was described as small round whitish or translucent and flat colonies. This bacterium can gain access to P. acne sites. However, this interaction between S. epidermidis and P. acnes is important due to the antagonistic properties of S. epidermidis against P. acnes. These data are also in agreement with previous studies [Citation34, Citation35].
Sequencing of 16S rRNA gene
The 16S rRNA gene is used as a tool for the identification of bacteria because the similarity in this region is found in most microbes with slight differences in the sequence of nucleotides [Citation36]. This has made it possible to identify prokaryotic isolates because the 16S rRNA gene is flanked by a conserved sequence which enables the design of universal primers that can amplify the 16S rRNA from different bacteria [Citation37, Citation38].
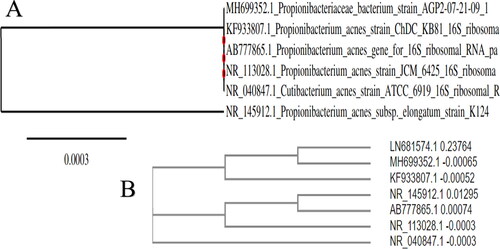
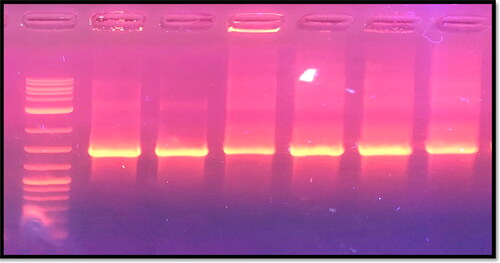
P. acnes isolates were identified by molecular test, and the 16S rRNA gene was amplified by PCR using specific primers. All strains (6 out of 22 isolates) of P. acnes in the present study were positive for the 16S gene. The PCR products were separated by electrophoresis in a 2% agarose gel ().
Figure 2. Gel electrophoresis of PCR products after amplification of 16S rRNA gene of P. acnes visualized under UV light. Lane1: 1 kb DNA marker; Lanes 2–6 are positive samples.
All samples were subjected to a partial sequencing of the 16S rRNA gene. Sequences were optimized using MEGA X software. Nucleotide sequence alignment was done against the corresponding GenBank sequences of known isolates of P. acnes.
The phylogenetic data and BLAST results of 16S rRNA genes showed that P. acnes displayed 99% nucleotide identities with sequences available in the GenBank database with accession numbers: MH699352.1, KF933807.1, AB777865.1, NR_113028.1, NR_040847.1, and NR_145912.1. Accession number LN681574.1 was used as the outgroup ().
Agar well diffusion assay
In the current study, the mean diameter (±SD) of inhibition zones of each plant extract was calculated after 36 h when the bacteria reached ideal growth. P. acnes was inhibited by all plant extracts in all tested isolates with varying degrees between each concentration.
The maximum antimicrobial activity was observed by acetone and methanol extracts of A. indica at 500 mg/mL, followed by R. stricta, C. sinensis, and the lowermost inhibition was achieved by the extracts from O. basilicum. In addition, the concentrations (400 mg/mL to 50 mg/mL) of each acetone and methanol extract showed varying degrees of inhibition zones with a correlation between the diameter of the inhibition zones and the concentration of extracts where the inhibition zones decreased as the concentrations decreased.
Each of A. indica and R. stricta inhibited P. acnes at all concentrations tested and showed strong activity against the bacteria, whereas C. sinensis and O. basilicum had moderate activity. The positive control used was Teicoplanin (TEI, 30 μg) with a mean inhibition zone of 30 ± 1.0 mm.
For A. indica, the acetone extract showed strong inhibition against the bacteria and the mean diameter of the inhibition zone at a concentration of 500 mg/mL was 40 ± 1 mm. In addition, even when the concentration was 50 mg/mL, the mean diameter of the inhibition zone was 25.4 ± 1.517 mm. The methanol extract had a similar effect, where the mean diameter at 500 mg/mL was 38.6 ± 1.67 mm, while at 50 mg/mL the mean diameter was also high 28 ± 1.414 mm.
The results showed strong inhibition by extracts of A. indica and these were higher than reported before by Charde et al. [Citation39], where A. indica at 20 mg/mL had a 20 mm zone of inhibition. In contrast, Nand et al. [Citation40] used methanol extract of leaves and bark of A. indica by agar disk diffusion method that showed insignificant effect against P. acnes. Another study reported that A. indica exhibits moderate inhibitory activity against P. acnes [Citation41]. However, Daud et al. [Citation42] showed that A. indica has a strong antibacterial activity against P. acnes.
Compared to other studies, the effect of A. indica against P. acnes in the present study may be attributed to differences in the extraction process, where a combination between water and methanol (80%) was used. Some studies agree that solvent combinations have effects mostly on phenolic compounds, antioxidants, and further to release most secondary metabolites [Citation43].
The R. stricta methanol extract was more effective with a zone of inhibition of 30.4 ± 2.96 mm (500 mg/mL) when compared to the acetone extract, whose inhibition zone was 25.6 ± 1.94 mm. At 50 mg/mL of methanol and acetone extracts, the mean diameter of the inhibition zone was 11.4 ± 2.074 and 9.2 ± 1.789 mm, respectively. Studies have shown that R. stricta has antimicrobial and antifungal activities due to the presence of active compounds found in the leaves such as alkaloids, tannins, triterpenes, glycosides and volatile bases [Citation44–47].
Methanol is the most used solvent in the screening of antimicrobial activities in plant extracts preparation [Citation48]. R. stricta showed strong activity against P. acnes and that was higher than recorded by the commercial antibiotic itself [Citation49, Citation50]. Our results are in agreement with Ahmad et al. [Citation51], who showed that the crude extract of R. stricta had antibacterial activities against a wide range of gram-positive and gram-negative bacteria.
The C. sinensis methanol extract at 500 mg/mL was more effective than the acetone extract and had moderate antimicrobial activity, where the inhibition zone was 18.6 ± 1.67 mm and 16 ± 2.34 mm, respectively. This was higher than previously reported by Nand et al. [Citation52], where the inhibition zone of C. sinensis was 13 mm.
On the other hand, O. basilicum had moderate to weak antimicrobial activities as compared to C. sinensis. The acetone and methanol extracts had similar antimicrobial activities with an inhibition zone of 13 mm and 12 mm, respectively. The methanol extract of O. basilicum showed resistance at 100 mg/mL. The inhibitory effect of O. basilicum may be due to the presence of a phenolic group, alkaloids, glycoside, flavonoids, anti-inflammatory and anti-oxidant agents, as suggested in other studies [Citation53–55]. The diameters of the inhibition zones obtained using all the plant extracts are shown in and and summarized in .
Figure 4. Antimicrobial activity of acetone plant extracts against P. acnes evaluated based on diameter of inhibition zones. Note: Extract concentrations were 500 mg, 200 mg, 50 mg
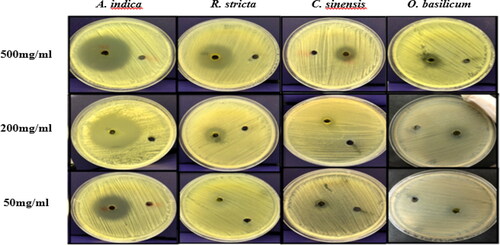
Figure 5. The diameter of the inhibition zone by methanol extract of plants against P. acnes at 500 mg, 200 mg, 50 mg.
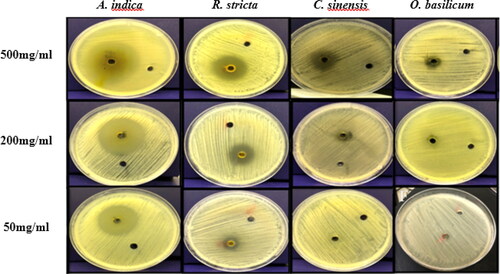
Table 1. Mean diameters of P. acnes inhibition zones obtained using different plant extracts.
The minimum inhibitory concentration (MIC)
The change in the color from blue to pink indicated the presence of the bacteria. In the negative control, Resazurin gave blue color indicating the absence of the bacteria. In contrast, positive control turned into pink color. The reaction between Resazurin and extracts in the presence of the bacteria determined the MIC and was noted as the lowermost concentration of plant extract that prevented this color change.
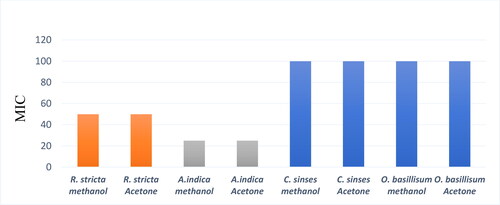
The MIC values of R. stricta, A. indica, C. sinensis and O. basilicum were determined. The MIC for acetone and methanol extracts of R. stricta was 50 mg/mL and this result is reported for the first time. The acetone and methanol extracts of A. indica had MIC of 25 mg/mL and it was higher than what has been reported before by Charde et al. [Citation39], where the MIC was 6.0 mg/mL. The MIC values were 100 mg/mL for both C. sinensis and O. basilicum. By comparison, Nand et al. [Citation52] showed that methanolic and petroleum ether extracts of C. sinensis had an antimicrobial effect against P. acnes with a MIC value of 1.25 mg/mL. In addition, phytochemical screening revealed the presence of alkaloids, flavonoids, glycosides and terpenoids, which indicates that these phytoconstituents may be responsible for the anti-acne activity. Furthermore, a study by Adigüzel et al. [Citation56] showed that O. basilicum methanol extract was active against a wide range of bacteria in the disk diffusion assay. The result showed that the extract had antibacterial effects and the MIC value was between 62.50 and 500 µl/ml. The results are shown in and summarised in .
Figure 6. Minimum inhibitory concentration (MIC) of plant extracts against P. acnes. Representative image. Note: Resazurin in the medium changes its color from blue to pink as an indication of the presence of bacteria. The MIC was the lowermost concentration of plant extract that prevented this change in color. NC, Negative Control, PC, Positive Control, MIC (RA), (RM)= where R for Rhazya, A for acetone, M for methanol, MIC (AA), (AM)= where A for Azadirachta, A for acetone, M for methanol, MIC (CA), (CM)= where C for Camelia, A for acetone, M for methanol, MIC (OA), (OM)= where O for Ocimum, A for acetone and M for methanol.

The medicinal plants have potent bioactive compounds, which are proven to have antimicrobial activity against a wide range of bacteria. Therefore, this leads to investigations into those bioactive compounds to decrease the emergence of antibiotic-resistant bacteria [Citation57]. The present study recommends using Saudi medicinal plants against acne-causing microbes. The plant extrcts in the present study exhibited good antimicrobial activity against P. acnes in vitro. Further studies are needed to isolate the active compounds from A. indica and R. stricta and to perform toxicity tests against animal and human cell lines.
The emergence of antibiotic-resistant bacteria has been high since the 1980s, and is still a serious problem nowadays with antibiotics that have side effects [Citation58, Citation59]. Hence, there is a great demand to use alternative medicine and safer treatment. Medicinal plants are generally safer and have low side effects [Citation15].
Until now, the relationship between acne and the causal bacterium is still in debate. It is not clear how P. acnes are involved in acne while being a major commensal of the normal skin microbiota [Citation2].
Conclusions
In this study, extracts from R. stricta, A. indica, C. sinensis and O. basilicum exhibited good antibacterial activities against P. acnes. The maximum antibacterial effect was observed with A. indica, followed by R. stricta. In addition, C. sinensis and O. basilicum had moderate activities.
Acknowledgment
The authors acknowledge the Dept. of Biological Sciences, Faculty of Science, King Abdulaziz University (KAU), Jeddah, and King Fahd Center For Medical Research (KFMRC).
Conflict of interest
No potential conflict of interest was reported by the authors.
Data availability
All data that support the findings from this study are available from the corresponding author upon reasonable request.
References
- Dréno B. What is new in the pathophysiology of acne, an overview. J Eur Acad Dermatol Venereol. 2017;31:8–12.
- Byrd AL, Belkaid Y, Segre JA. The human skin microbiome. Nat Rev Microbiol. 2018;16(3):143–155.
- McLaughlin J, Watterson S, Layton AM, et al. Propionibacterium acnes and acne vulgaris: new insights from the integration of population genetic, multi-omic, biochemical and host-microbe studies. Microorganisms. 2019;7(5):128.
- Gollnick HP, Finlay AY, Shear N, Global Alliance to Improve Outcomes in Acne. Can we define acne as a chronic disease? If so, how and when? Am J Clin Dermatol. 2008;9(5):279–284.
- Williams HC, Dellavalle RP, Garner S. Acne vulgaris. Lancet. 2012;379(9813):361–372.
- Tuchayi SM, Makrantonaki E, Ganceviciene R, et al. Acne vulgaris. Nat Rev Dis Primers. 2015;1(1):1–20.
- Tan JKL, Bhate K. A global perspective on the epidemiology of acne. Br J Dermatol. 2015;172:3–12.
- Yazici K, Baz K, Yazici AE, et al. Disease-specific quality of life is associated with anxiety and depression in patients with acne. J Eur Acad Dermatol Venereol. 2004;18(4):435–439.
- Haroon MZ, Alam A, Ullah I, et al. Quality of life and depression among young patients suffering from acne. J Ayub Med Coll Abbottabad. 2019;31(3):436–440.
- Perry AL, Lambert PA. Propionibacterium acnes. Lett Appl Microbiol. 2006;42(3):185–188.
- Capoor MN, Ruzicka F, Machackova T, et al. Prevalence of Propionibacterium acnes in intervertebral discs of patients undergoing lumbar microdiscectomy: a prospective cross-sectional study. PLoS One. 2016;11(8):e0161676.
- Kapadia NF, Khalid G, Burhany T, et al. Comparative efficacy and safety and efficacy of systemic 13-cis retinoic acid 20mg/day vs. 40mg/day in acne vulgaris. JPAD. 2016;15(3):238–241.
- Ross JI, Snelling AM, Eady EA, et al. Phenotypic and genotypic characterization of antibiotic-resistant Propionibacterium acnes isolated from acne patients attending dermatology clinics in Europe, the U.S.A., Japan and Australia. Br J Dermatol. 2001;144(2):339–346.
- Achermann Y, Goldstein EJ, Coenye T, et al. Propionibacterium acnes: from commensal to opportunistic biofilm-associated implant pathogen. Clin Microbiol Rev. 2014;27(3):419–440.
- Nasri H, Bahmani M, Shahinfard N, et al. Medicinal plants for the treatment of acne vulgaris: a review of recent evidences. Jundishapur J Microbiol. 2015; 8(11):e25580.
- Brown ED, Wright GD. Antibacterial drug discovery in the resistance era. Nature. 2016;529(7586):336–343.
- Zhu T, Zhu W, Wang Q, et al. Antibiotic susceptibility of Propionibacterium acnes isolated from patients with acne in a public hospital in Southwest China: prospective cross-sectional study. BMJ Open. 2019;9(2):e022938.
- Hayashi N, Akamatsu H, Kawashima M, et al. Establishment of grading criteria for acne severity. JDA. 2008;35(5):255–260.
- McDowell A, Valanne S, Ramage G, et al. Propionibacterium acnes types I and II represent phylogenetically distinct groups. J Clin Microbiol. 2005;43(1):326–334.
- Balouiri M, Sadiki M, Ibnsouda SK. Methods for in vitro evaluating antimicrobial activity: a review. J Pharm Anal. 2016;6(2):71–79.
- Lima-Filho JV, de Aguiar Cordeiro R. In vitro and in vivo antibacterial and antifungal screening of natural plant products: prospective standardization of basic methods. In: Lima-Fiho JV, Cordeiro RA (eds.), Methods and techniques in ethnobiology and ethnoecology. New York (NY): Humana Press; 2014. p. 275–291.
- Al-Khafaf DM, Sabry M, Al-Qaraghuli MA. Isolation and characterization of Probionibacterium acnes from acne vulgaris patients. Med J Babylon. 2006;3(3-4):265–272.
- Al‐Ameer AM, Al‐Akloby OM. Demographic features and seasonal variations in patients with acne vulgaris in Saudi Arabia: a hospital‐based study. Int. J. Dermatol. 2002;41(12):870–871.
- Adityan B, Thappa DM. Profile of acne vulgaris-A hospital-based study from South India. Indian J Dermatol Venereol Leprol. 2009;75(3):272–278.
- Miura Y, Ishige I, Soejima N, et al. Quantitative PCR of Propionibacterium acnes DNA in samples aspirated from sebaceous follicles on the normal skin of subjects with or without acne. J Med Dent Sci. 2010; 57(1):65–74.
- Abo El-Fetoh NM, Alenezi NG, Alshamari NG, et al. Epidemiology of acne vulgaris in adolescent male students in Arar, Kingdom of Saudi Arabia. J Egypt Public Health Assoc. 2016; 91(3):144–149.
- Arora MK, Yadav A, Saini V. Role of hormones in acne vulgaris. Clin Biochem. 2011; 44(13):1035–1040.
- Schäfer T, Nienhaus A, Vieluf D, et al. Epidemiology of acne in the general population: the risk of smoking. Br J Dermatol. 2001; 145(1):100–104.
- Wei B, Pang Y, Zhu H, et al. The epidemiology of adolescent acne in North East China. JEADV. 2010;24(8):953–957.
- Naghdi N, Ghane M. A comparison of culture and PCR methods for identifying Propionibacterium acnes in lesions isolated from patients with acne. Turk J Med Sci. 2017; 47(3):967–972.
- Fitz-Gibbon S, Tomida S, Chiu BH, et al. Propionibacterium acnes strain populations in the human skin microbiome associated with acne. J. Investing. Dermatol. 2013;133(9):2152–2160.
- Beylot C, Auffret N, Poli F, et al. Propionibacterium acnes: an update on its role in the pathogenesis of acne. J Eur Acad Dermatol Venereol. 2014;28(3):271–278.
- Dreno B, Martin R, Moyal D, et al. Skin microbiome and acne vulgaris: staphylococcus, a new actor in acne. Exp Dermatol. 2017;26(9):798–803.
- Wang Y, Kuo S, Shu M, et al. Staphylococcus epidermidis in the human skin microbiome mediates fermentation to inhibit the growth of Propionibacterium acnes: implications of probiotics in acne vulgaris. Appl Microbiol Biotechnol. 2014;98(1):411–424.
- Christensen GJ, Scholz CF, Enghild J, et al. Antagonism between Staphylococcus epidermidis and Propionibacterium acnes and its genomic basis. BMC Genomics. 2016;17(1):152..
- Greisen K, Loeffelholz M, Purohit A, et al. PCR primers and probes for the 16S rRNA gene of most species of pathogenic bacteria, including bacteria found in cerebrospinal fluid. J Clin Microbiol. 1994;32(2):335–351.
- Baker GC, Smith JJ, Cowan DA. Review and re-analysis of domain-specific 16S primers. J Microbiol Methods. 2003;55(3):541–555.
- Chakravorty S, Helb D, Burday M, et al. A detailed analysis of 16S ribosomal RNA gene segments for the diagnosis of pathogenic bacteria. J Microbiol Methods. 2007;69(2):330–339.
- Charde YM, Sharma PH, Choudhary NG, et al. Development and evaluation of herbal formulation for the treatment of acne. IJPSR. 2014;5(6):2250–2260.
- Nand P, Drabu S, Gupta RK. Insignificant anti-acne activity of Azadirachta indica leaves and bark. J Pharm Negative Results. 2012;3(1):29.
- Balakrishnan KP, Narayanaswamy N, Subba P, et al. Antibacterial activity of certain medicinal plants against acne-inducing bacteria. Int J Pharma Bio Sci. 2011;2:476–481.
- Daud FS, Pande G, Joshi M, et al. A study of antibacterial effect of some selected essential oils and medicinal herbs against acne causing bacteria. J Pharm Sci Invent. 2013;2:27–34.
- Namvar K, Mohammadi A, Ataei Salehi E, et al. Evaluation of solvent effect (methanol: water mixture) on the phenolic content and antioxidant activities of Stachys turcomanica Trautv. Pharm Sci. 2017;23(3):244–244.
- Emad AM, Gamal EGE. Screening for antimicrobial activity of some plants from Saudi folk medicine. Global J Res Med Plants Indigen Med. 2013;2(4):210–218.
- Marwat SK, Usman K, Shah SS, et al. A review of phytochemistry, bioactivities and ethno medicinal uses of Rhazya stricta Decsne (Apocynaceae). Afr J Microbiol Res. 2012;6(8):1629–1641.
- Baeshen MN, Khan R, Bora RS, et al. Therapeutic potential of the folkloric medicinal plant Rhazya stricta. Biol Syst Open Access. 2015;05(01):2.
- Obaid AY, Voleti S, Bora RS, et al. Cheminformatics studies to analyze the therapeutic potential of phytochemicals from Rhazya stricta. Chem Cent J. 2017;11(1):11.
- Das K, Tiwari RKS, Shrivastava DK. Techniques for evaluation of medicinal plant products as antimicrobial agents: current methods and future trends. J Med Plant Res. 2010;4(2):104–111.
- Guin JD, Huber DS, Gielerak PL. Antibiotic sensitivity of comedonal Propionibacterium acnes. Acta Derm Venereol. 1979;59(6):552–554.
- Oprica C, Emtestam L, Lapins J, et al. Antibiotic-resistant Propionibacterium acnes on the skin of patients with moderate to severe acne in Stockholm. Anaerobe. 2004;10(3):155–164.
- Ahmad S, Fatima K, Atiq R. Antibacterial activity of Pakistani Rhazya stricta. PJSIR. 2004;47(1):29–33.
- Nand P, Drabu S, Gupta RK. Phytochemical and antimicrobial screening of medicinal plants for the treatment of acne. Indian J Nat Prod Resour. 2012;3(1):28–32.
- Sirvi K, Goyal PK, Vyas B. Novel drug delivery system and its uses in the treatment of acne. IJPE. 2016;6(3):12–28.
- Aichalabi R, Fadhil A, Aibayati S. The antibacterial acivity of alcoholic extract of Ocimum basilicm on main causative agents acne in Iraqi teenagers. Int J Health Nutr. 2014;5(1):13–16.
- Güez CM, Souza ROD, Fischer P, et al. Evaluation of basil extract (Ocimum basilicum L.) on oxidative, anti-genotoxic and anti-inflammatory effects in human leukocytes cell cultures exposed to challenging agents. Braz J Pharm Sci. 2017;53(1):e15098. http://dx.doi.org/10.1590/s2175-97902017000115098.
- Adigüzel A, Güllüce M, Şengül M, et al. Antimicrobial effects of Ocimum basilicum (Labiatae) extract. Turk J Biol. 2005;29(3):155–160.
- Omojate Godstime C, Enwa Felix O, Jewo Augustina O, et al. Mechanisms of antimicrobial actions of phytochemicals against enteric pathogens–a review. J Pharm Chem Biol Sci. 2014;2(2):77–85.
- Sardana K, Gupta T, Kumar B, et al. Cross-sectional pilot study of antibiotic resistance in Propionibacterium acnes strains in Indian acne patients using 16S-RNA polymerase chain reaction: a comparison among treatment modalities including antibiotics, benzoyl peroxide, and isotretinoin. Indian J Dermatol. 2016;61(1):45–52.
- Doğan B, Bektöre B, Karabacak E, et al. Resistance status of antibiotics in Gram-positive bacteria isolated from acne lesions in İstanbul. Turkderm-Turk Arch Dermatol Venereol. 2017; 51(2):32–36.