?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
To isolate nitrite-oxidizing bacteria to control the nitrite concentration in freshwater aquaculture systems, a Nitrobacter enrichment culture was obtained from a freshwater shrimp pond using a continuous enrichment and gradient dilution method. There was only one species of Nitrobacter in the enrichment culture, with an abundance of more than 99%. The Nitrobacter strain was identified as Nitrobacter vulgaris XY-12 on the basis of its 16S rRNA gene and NxrA gene sequences. The effects of pH, temperature, salinity and nitrate on the nitrification activity of the enrichment culture were examined, and its ability to remove nitrite in aquaculture water was also tested. The results showed that the optimum pH for the nitrite-oxidation activity of the enrichment culture was 7.5, the optimum temperature range was 27 °C–30 °C, the optimum salinity was 0.38%, the half maximal inhibitory concentration (IC50) of NO3−–N was 400 mg L−1, and the optimum concentration of NO2−–N was 200 mg L−1. In optimum conditions, the maximum NO2−–N removal rate afforded by the enrichment was 1.62 mg L−1 h−1, and the minimum doubling time was 12 ± 2 h. Moreover, XY-12 could multiply in field aquaculture water, and the maximum NO2−–N removal rate was 0.15 mg L−1 h−1. These results indicated that the enrichment had good application prospects in aquaculture waters.
Introduction
Nitrite is one of the main pollutants in aquaculture water, especially when the temperature is high because ammonia-oxidizing bacteria have a greater growth rate and can out-compete nitrite-oxidizing bacteria (NOB) at temperatures higher than 25 °C [Citation1,Citation2]. A concentration of NO2−–N in water >1 mg L−1 is harmful to aquatic organisms [Citation3].
NOB mainly include five groups: Nitrobacter, Nitrospira, Nitrospina, Nitrococcus and Candidatus Nitrotoga [Citation4]. Generally, Nitrospira, Nitrotoga and Nitrobacer were considered to be the main NOB in aquaculture water. Brown et al. [Citation5] found that Nitrospire was the main dominant genus in shrimp recirculating aquaculture water. Hüpeden et al. [Citation6] found that Nitrotoga was the main dominant genus in a low temperature recirculating aquaculture water system. Navada et al. [Citation7] reported that Nitrobacter was the main dominant genus in some brackish water biofilms. Nitrobacter and Nitrospira were the main NOB in a freshwater recirculating aquaculture system (RAS) [Citation8] and in a moving-bed biofilm reactor (MBBR) [Citation9].
Nitrobacter was the earliest NOB to be studied and was mainly divided into four species: N. winogradskyi, N. hamburgensis, N. vulgaris and N. alkalicus [Citation10]. In natural water environment and aquaculture waters, N. winogradskyi was found to be the dominant NOB in brackish water [Citation11], marine environment and coastal shrimp RAS [Citation12]. N. vulgaris was widely distributed in freshwater, brackish water, seawater [Citation13] and freshwater aquarium biofilter [Citation14], while N. alkalicus [Citation15] and N. hamburgensis [Citation16,Citation17] were the dominant species in sediments of soda lakes and freshwaters, respectively.
To date, most of the known purified Nitrobacter strains have been isolated from soil, sand filters of waterworks, sewage treatment plants and freshwater rivers [Citation15, Citation18–20]. However, only one strain of N. winogradskyi ZS-1 was reportedly isolated from brackish aquaculture water [Citation11], and there was no report of Nitrobacter isolates from freshwater aquaculture waters.
The application of NOB in aquaculture water is considered to have great potential in the control of nitrite concentration. However, the sensitivity to environmental factors, accompanied by the slow growth of NOB [Citation4], has greatly prevented the application of NOB in aquaculture systems, especially when the water temperature is high [Citation1,Citation2]. Thus, the isolation of a fast-growing and high-temperature tolerant NOB would be of benefit for the application of NOB in aquaculture water. Therefore, in this study, a high-temperature tolerant Nitrobacter enrichment culture was obtained from a freshwater aquaculture pond, and the effects of various environmental factors on its ability to remove nitrite were examined.
Materials and methods
Sampling
The water sample was collected from a shrimp pond (N30°41′49.6″E113°46′51.0″) located in Hanchuan, Hubei Province, China.
The collected water sample was subjected to 16S rRNA gene high-throughput sequencing. The sample DNA was extracted by a HiPure soil DNA kit B kit (Magen, Guangzhou, China), and the DNA concentration was determined by a qubit@ds DNA HS assay kit (Magen). The primers used for the template of polymerase chain reaction (PCR) amplification of the V3–V4 region of the 16S rRNA gene were F-CCTACGGRRBGCASCAGKVRVGAAT and R-GGACTACNVGGGTWTCTAATCC [Citation21]. The reaction system was contained in 25 μL: buffer, 2.5 μL; deoxyribonucleoside (dNTP) mixture, 2 μL; upstream and downstream primers, 1 μL; Taq DNA, 0.5 μL; template, 2 μL; and ultrapure water, 16 μL. The reaction conditions were 24 cycles of predenaturation at 94 °C for 3 min, denaturation at 94 °C for 5 s, annealing at 57 °C for 90 s, extension at 72 °C for 10 s, and final extension at 72 °C for 5 min. The PCR products were sequenced by Genewiz Co. Ltd. (Suzhou, China).
For the enrichment of NOB, 1 mL of sample was inoculated into 100 mL of enrichment medium (NaHCO3 0.4 g, KH2PO4 1 g, K2HPO4 1.31 g, EDTA 2 mg, ZnSO4·7H2O 1.1 mg, CoCl2·6H2O 0.8 mg, MnCl2·4H2O 2.55 mg, CuSO4·4H2O 0.8 mg, Na2MoO4·2H2O 0.1 mg, CaCl2·2H2O 2.75 mg, FeCl3·6H2O 2.5 mg, MgSO4·7H2O 88.8 mg and ddH2O 1000 mL) [Citation22]. In the mixture, the NO2−–N concentration in the form of NaNO2 was adjusted to 20 mg L−1 and the pH was set to 7.4–7.6. The mixture was cultured at 27 °C, 150 rpm for 35 days. During this period, the concentration of nitrite was measured every 7 days. After 35 days, 1 mL of the enriched culture was counted on a BD FACS Calibur flow cytometer to determine its total bacterial count.
To purify the enrichment culture, when the total bacterial count (determined by flow cytometry, see below) reached ∼3.0 × 106 cells mL−1, take 1 mL enriched sample and dilute it 107 times by step dilution method, so that there were only approximately three bacteria in 100 mL. Subsequently, 100 mL of the diluted sample was equally divided into 20 tubes (5 mL per tube), which were then incubated at 27 °C, 150 rpm for 30 days. The positive samples were identified by measuring the concentration of NO2−–N on the 30th day.
NXR gene sequencing
The amplification of the NxrA gene was conducted as described by Franck et al. [Citation23] using the primers F1370 F1 nxrA CAG ACC GAC GTG TGC GAA AG and F2843 R2 nxrA TCC ACA AGG AAC GGA AGG TC. The amplification of the NxrB gene was conducted as described by Pester et al. [Citation24] using the primers nxrB 169f TAC ATG TGG AAC A and nxrB 638r CGG TTC TGG TCR ATC A. All primers were synthesized by Invitrogen (Shanghai, China), and the PCR product was sequenced by Shanghai Sangon Biotech Co., Ltd.
Physiological characterization
To prepare the inoculum, 5 mL of the positive sample with the highest nitrification activity was inoculated into 100 mL of medium containing 20 mg L−1 NO2−–N and the mixture was cultured at 27 °C, 150 rpm until the NO2−–N removal rate reached ∼0.2 mg L−1 h−1.
To determine the optimum temperature of the nitrification activity, 9 mL of inoculum was inoculated into 900 mL of medium containing 20 mg L−1 NO2−–N. Subsequently, 50-mL samples of the mixture were collected into 100-mL flasks and incubated at 18 °C, 21 °C, 24 °C, 27 °C, 30 °C and 33 °C each for 4 days (pH 7.5, 150 rpm).
To determine the optimum pH of the nitrification activity, 9 mL of inoculum was inoculated into 900 mL of medium containing 20 mg L−1 NO2−–N. Subsequently, 50-mL samples of the mixture were collected into 100-mL flasks, and the pH was set to 6.0, 6.5, 7.0, 7.5, 8.0 and 8.5. The samples were then incubated at 27 °C, 150 rpm for 4 days.
To determine the salinity tolerance of the enrichment culture, a 10.5 mL inoculum was inoculated into 1050 mL of medium containing 20 mg L−1 NO2−–N. Subsequently, 50 mL samples of the mixture were collected into 100 mL flasks, and the salinity was set to 0.13%, 0.38%, 0.63%, 0.88%, 1.13%, 1.38% and 1.63%. The samples were then incubated at pH 7.5, 27 °C, 150 rpm for 4 days.
To determine the nitrate tolerance of the enrichment culture, a 10.5-mL inoculum was inoculated into 1050 mL of enrichment medium containing 20 mg L−1 NO2−–N. Subsequently, 50-mL samples of the mixture were collected into 100-mL flasks, and the NO3−–N concentration (in the form of NaNO3) was set to 0, 25, 50, 100, 200, 400 and 800 mg L−1, respectively. Moreover, NaCl was added to those mixtures so that the initial salinity was the same for all the treatments. The samples were then incubated at pH7.5, 27 °C, 150 rpm for 4 days.
For all of the tests of temperature, pH, salinity and nitrate concentration described above, subsamples were collected every 24 h to measure the concentration of NO2−–N; moreover, the concentration of NO3−–N was measured at the end of the experiment.
In the test to determine the optimum nitrite concentration (the maximum initial NO2−–N concentration was up to 600 mg L−1), because the high initial concentration of nitrite significantly influenced the sensitivity of the measurement of NO2−–N, dissolved oxygen (DO) uptake, rather than the elimination of NO2−–N used in the former tests, was applied to indicate the nitrification activity. To achieve a considerable decrease in DO within an hour, 2700 mL of inoculant was concentrated by centrifugation (27 °C, 5432 g, 15 min). Subsequently, 27 mL of the concentrated sample was added to 1350 mL of basic medium and divided equally into 27 flasks. The initial NO2−–N concentration was then set to 10, 25, 50, 100, 200, 300, 400, 500 and 600 mg L−1. Next, the flasks were completely sealed and incubated for 1 h at 27 °C with 10 rpm of magnetic stirring, during which the DO uptake was measured with a Hamilton Bonaduz AG dissolved oxygen probe every 15 min.
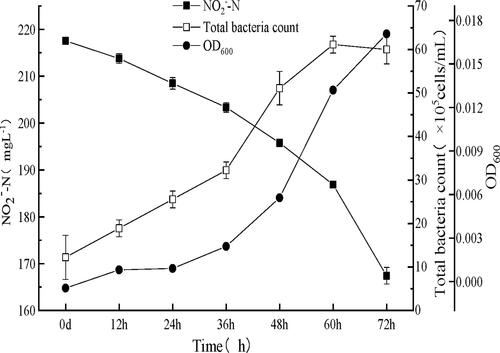
According to the results of the tests, a 1.5-mL inoculum was inoculated into 150 mL of modified basic medium (pH7.5; salinity, 0.38%; NO2−–N, 200 mg L−1). Subsequently, 50-mL samples of the mixture were collected into 100-mL flasks and incubated at 27 °C, 150 rpm for 7 days. The concentration of NO2−–N, OD600 and total bacterial count were determined every 12 h; moreover, the concentration of NO3−–N was measured at the end of the experiment.
Nitrification activity in aquaculture water
The test water samples were collected from a freshwater shrimp pond, NanHu Lake (a freshwater lake) and brackish water (salinity1%). NaNO2 was added to 250 mL of freshwater from shrimp pond, NanHu Lake and Brackish water, respectively, to yield an initial concentration of NO2−–N of ∼13 mg L−1. Subsequently, a 2.5-mL inoculum of the enrichment culture was added to the tested treatments, whereas the control treatments were inoculated with bacteria-free medium. The samples were then incubated at pH 7.5, 27 °C, 150 rpm for 8 days. Subsamples were collected every 24 h to measure the concentration of NO2−–N, and the concentration of NO3−–N was measured at the end of the experiment.
Measurement and data analysis
Triplicate independent tests were conducted for all experiments. NO2−–N and NO3−–N were detected via the N-(1-naphthalene)-diaminoethane spectrophotometric method and the phenol disulfonic acid method, respectively.
The total bacterial count was determined via flow cytometry, as follows: A 1 mL water sample was filtered through a 2000 mesh sieve to remove big particles in the water. Subsequently, SYBR Green I was added to obtain a final concentration of 1× in the sample, followed by its incubation at 25 °C for 10 min [Citation25] and measurement by a BD FACS Calibrated flow cytometer at 488 nm excitation.
The oxidation ratio of NO2−–N was calculated as follows:
(1)
(1)
where C0 is the concentration of NO3−–N (mg L−1) at the end of the experiment and Ct is the initial concentration of NO2−–N (mg L−1).
The generation time was calculated as follows:
(2)
(2)
where Bt is the total bacterial count of the enrichment culture and Δt is the time interval.
Data analysis
Origin 2017 was used to plot the data. Data are mean values with standard deviation (±SD). Analysis of variance (ANOVA) tests (with multiple comparisons of LSD) were performed using SPSS 20.0.
Results and discussion
The NOB in the enrichment was identified as N. vulgaris
Only one NOB strain was identified by high-throughput sequencing in the field sample, although its relative abundance was only 0.0687‰. Moreover, the partial 16S rRNA gene sequence of the NOB was 100% identical to that of N. vulgaris (NR 042449.1).
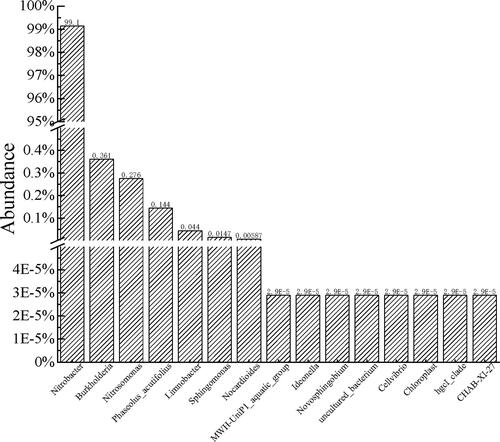
In the NOB enrichment culture, as shown in , only one NOB strain was identified via high-throughput sequencing, with a relative abundance of 99.1%. Its partial 16S rRNA gene was 100% identical to that of N. vulgaris (NR042449.1).
According to the phylogenetic tree of the NxrA gene sequence (), the NOB in the enrichment had the closest genetic distance to N. vulgaris, and its identity was 100% compared with Nitrobacter vulgaris (DQ 421375.1). Nitrite oxidoreductase (NXR) is a key enzyme in NOB [Citation26], and NxrA and NxrB were used in the PCR amplification of Nitrobacter [Citation13] and Nitrospira [Citation24], respectively. In the present study, the amplification of the NxrB gene yielded negative results, indicating that there was no Nitrospira in this enrichment. Hence, the only NOB strain detected in the enrichment was termed N. vulgaris XY-12.
Figure 2. Phylogenetic tree of XY-12 based on the partial 16S rRNA sequence (a) and NxrA gene sequence (b).

Till now, N. vulgaris has been found in different kinds of waters, but particularly in low nitrite environment [Citation19]. Cai et al. [Citation13] found that N. vulgaris was the main Nitrobacter in the biofilter of a freshwater fish tank through enrichment culture. Navada et al. [Citation7] found that N. vulgaris was the main NOB in the nitrifying filter of brackish water with salinity of 12 ‰. However, the abundance of N. vulgaris in a biofilm reactor of RAS was significantly higher when the salinity was 6 ‰∼12 ‰ than the salinity of 32 ‰ [Citation27].
Physiological characteristics of N. vulgaris XY-12
As shown in , an obvious nitrification activity of XY-12 was observed when the temperature was between 21 °C and 30 °C, and the highest nitrification activity occurred at 27 °C–30 °C (with a maximum nitrification rate of 0.46 mg L−1 h−1 and an oxidation ratio of NO2−–N of 97%–100%). Temperature can directly affect the growth and metabolism of microorganisms by affecting the activity of key enzymes and the optimum cell membrane fluidity for microbial growth [Citation28]; moreover, it can indirectly affect the activity of nitrifying bacteria by affecting pH and FNA [Citation29]. Huang et al. [Citation30] found that Nitrobacter exhibited a negative correlation with temperature and a higher abundance under lower temperature conditions in aerobic activated sludge bioreactors. However, Sorokin et al. [Citation15] reported that the optimum temperature of Nitrobacter AN1 was 25 °C–28 °C. In this paper, the optimum temperature of XY-12 was between 27 °C and 30 °C (), which was higher than that reported for AN1. From the aspect of application, the relatively higher optimum temperature of XY-12 might contribute to its application in high-temperature conditions, at which the accumulation of nitrite is more likely to occur [Citation1].
Figure 3. Effects of temperature (a), pH (b), salinity (c) and nitrate (d) on the removal efficiency of nitrite (mean ± SD).
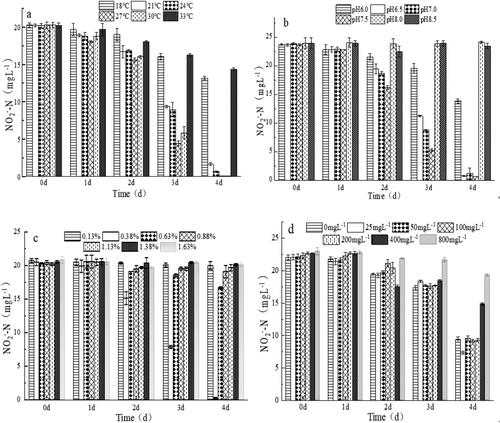
As shown in , XY-12 had obvious nitrification activity between pH 6.0 and 7.5. The nitrification activity reached 0.457 mg L−1 h−1 at pH 7.5 (with an oxidation ratio of NO2−–N of 92%) but was only 52% of the maximum nitrification activity at pH 6.0. Thus, the optimum pH for XY-12 was between 6.5 and 7.5. pH is an essential factor in the oxidation of nitrite, which can influence NOB directly by affecting their metabolism or indirectly by affecting the ionization equilibrium of NO2−/HNO2 [Citation31]. As shown in , the isolated Nitrobacter grew well under neutral or alkaline conditions, i.e. Zhang et al. [Citation33] found that the optimum pH of nitrifying bacteria was 7–9 in a sequencing batch reactor [Citation33], which is comparable with that observed for XY-12. From the perspective of application, XY-12 may be able to adapt to the pH range in freshwater aquaculture systems, which is usually between 6.5 and 8.5, and particularly favours relative low pH conditions when the toxic effect of HNO2 is more pronounced.
Table 1. Adaptability of different Nitrobacter strains to various environmental factors.
As shown in , the nitrification activity of XY-12 was obvious at a salinity of 0.38%–0.63% (with an oxidation ratio of NO2−–N of 87%–93%). In this salinity range, the nitrification activity reached up to 0.32 mg L−1 h−1 when the salinity was 0.38%, whereas it was only 25% of the maximum nitrification activity when the salinity was 0.63%, indicating that the optimum salinity for the nitrification of XY-12 was ∼0.38% and that the half maximal inhibitory concentration was between 0.38% and 0.63%. Salinity could affect cell osmotic pressure, which in turn modulates the transfer of substrates and oxygen, thereby affecting the rate of nitrification [Citation34–36]. The tolerance of salinity varies between different Nitrobacter strains. For instance, the nitrite oxidation rate of N. winogradskyi decreased to 42% when the salinity reached 1.17% [Citation37], whereas the nitrite oxidation rate of Nitrobacter sp. was not affected when the salinity was up to 1.5% [Citation38]. In this paper, the nitrification activity of XY-12 was highest at a salinity of 0.38% but was completely inhibited when the salinity reached 1.13%. As the salinity of freshwater aquaculture is usually lower than 0.5%, XY-12 could at least adapt to the salinity of freshwater aquaculture waters.
High nitrate concentrations inhibit the nitrification activity [Citation39]. As shown in , XY-12 had significant nitrification activity when the initial NO3−–N concentration was between 0 and 400 mg L−1, and the highest nitrification activity of 0.462 mg L−1 h−1 was reached at a NO2−–N concentration of 20 mg L−1 (with an oxidation ratio of NO2−–N of 86%). When the NO3−–N concentration was 400 mg L−1, the nitrification activity decreased to only 46% of the maximum nitrification activity, indicating that the optimum NO3−–N concentration range for XY-12 was 0–200 mg L−1 and the IC50 was 400 mg L−1. There are few studies of the nitrate inhibition of Nitrobacter; moreover, the IC50 of N. winogradskyi ZS-1 (isolated from brackish aquaculture water) was only 100 mg L−1 [Citation11], which was much lower than that of XY-12.
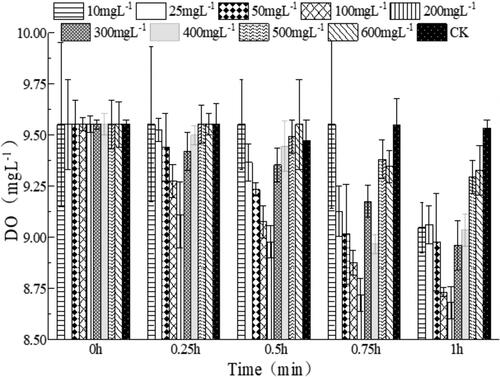
As shown in , XY-12 exhibited a strong DO consumption during the first 15 min, when the initial concentration of NO2−–N was 50–200 mg L−1. Moreover, the DO consumption rate was highest when the initial concentration of NO2−–N was 200 mg L−1 (p < 0.05), indicating that the optimum initial concentration of NO2−–N was approximately 200 mg L−1. Nitrobacter is considered to be an r-strategist with low substrate affinity [Citation40]. Generally, the optimum NO2−–N concentration of NOB ranges from 100 to 1500 mg L−1 [Citation41]. Thus, the affinity of XY-12 was higher than that of most other NOB. For instance, the optimum NO2−–N concentration of N. winogradskyi ZS-1 was as high as 500 mg L−1 [Citation11], which was much higher than that of XY-12 (200 mg L−1). From the perspective of application, its relatively high affinity to NO2−–N might benefit the application of XY-12 in freshwater aquaculture systems because the NO2−–N concentration is usually no more than 20 mg L−1 in these systems [Citation3].
Figure 4. Effect of different initial nitrite concentrations on the uptake of dissolved oxygen concentration (mean ± SD).
In optimum culture conditions, XY-12 removed ∼50 mL−1 NO2−–N in 3 days (), with a maximum NO2−–N removal rate of approximately 1.62 mg L−1 h−1 and an oxidation ratio of NO2−–N of 97%. Moreover, according to the total bacterial count, the shortest generation time of XY-12 was 12 ± 2 h. Thus, the growth of XY-12 was comparable to N. vulgaris Ab1 isolated by Bock [Citation19], but was much faster than that of N. vulgaris ‘Z’ [Citation19]. The previously reported generation time of Nitrobacter was 12–43 h (), suggesting that XY-12 is among the fastest-growing Nitrobacter strains. Because the growth of NOB is usually very slow [Citation16], a NOB strain with rapid proliferation might contribute to its population establishment and a subsequent efficient removal of nitrite.
As shown in , the removal of nitrite by XY-12 in various aquaculture waters was obvious: the concentrations of nitrite in all tested treatments were significantly lower than those observed for the control treatments (p < 0.05). In the freshwater shrimp pond water (), the maximum rate of removal of NO2−–N in the tested treatment was 0.15 mg L−1 h−1, and NO2−–N in the tested treatment was below the detection limit within 7 days, whereas the rate of removal of NO2−–N in the control treatment was only 0.02 mg L−1. To verify the growth of XY-12 in the field sample, the samples of the tested treatment were subjected to 16S rRNA gene high-throughput sequencing, with the results indicating that the abundance of XY-12 increased from 0.034‰ to 0.58% within 7 days. Hence, XY-12 multiplied and contributed greatly to the removal of nitrite in the freshwater shrimp water. Similarly, Lin and Zhu [Citation42] reported that inoculated Nitrobacter became one of the dominant species in a shrimp pond within 48 h after inoculation.
Figure 6. Removal efficiency of nitrite (mean ± SD) in waters from a freshwater shrimp pond (a), NanHu Lake (b) and brackish water (c).
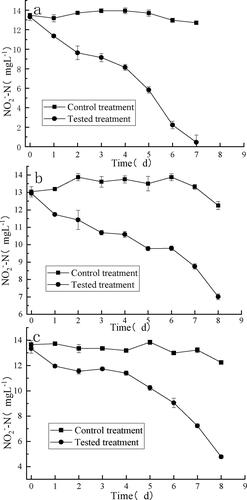
In NanHu Lake water (), the average removal rate of NO2−–N in the tested treatment was 0.03 mg L−1 h−1, which was also significantly higher than the control treatment.
In brackish water (), XY-12 was affected by salinity: there was no significant difference between the tested treatment and the control treatment in the first 4 days. However, from the fifth day, the removal rate of nitrite in the tested treatment increased significantly, and the average NO2−–N removal rate reached 0.07 mg L−1 h−1, which was comparable with the NO2−–N removal rate observed in freshwater shrimp pond water. In other words, after 4 days of inhibition by the high salinity in the brackish water, the nitrification activity of XY-12 finally recovered. Comparable to our result, Navada et al. [Citation27] also found that, after 14 days of gradual increasing of 10 ‰ salinity, the nitrification rate was 35% higher comparing to a one-step increasing of salinity.
Moreover, the oxidation ratios of NO2−–N were all higher than 85% for the three tested treatments (), suggesting that nitrification was the main route used for the removal of nitrite in those tested aquaculture waters. Kuhn et al. [Citation43] added a commercial bacterial agent containing N. winogradskyi to a circulating aquaculture system, and the concentration of NO2−–N was maintained at 1 mg L−1 within 28 days. Ren et al. [Citation44] added NOB to a shrimp-breeding pond; the concentration of NO2−–N in the experimental treatment was less than 0.02 mg L−1 within 7 days. Comparably, nitrite in various aquaculture waters was obviously removed within 7–8 days in the present study.
Table 2. Activity of XY-12 in different aquaculture waters.
By comparison of the nitrification activity of XY-12 in different media and in aquaculture water, we noted that the NO2−–N removal rate ranged greatly from 0.03 mg L−1 h−1 (in NanHu Lake water, with the NO2−–N concentration of 13 mg L−1) to 1.62 mg L−1 h−1 (in optimum culture condition, with the NO2−–N concentration of 200 mg L−1). The result was in accordance with the understanding that Nitrobacter is a typical r-strategist and grows faster at relatively high NO2−–N concentrations [Citation40].
According to its characteristics, the potential bioaugmentation of XY-12 in wastewater treatment of RAS at high temperature will include two approaches. One is that, if the hydraulic retention time (HRT) is significantly longer than the generation time of XY-12 (about 12 h), which is quite common for the wastewater treatment of RAS [Citation45,Citation46], the enrichment can be seeded to the system directly. Otherwise, in the conditions of shorter HRT, since nitrifying bacteria are suitable for adhesive growth [Citation47], XY-12 should be fixed in some carriers, such as filters [Citation48] or MBBR [Citation49], to avoid being washed out by the high flow rate. Thus, future research should pay special attention to the practical technology of how to use such a NOB enrichment in different aquaculture scenes, especially when the commercial cost is concerned.
Conclusions
In this study, XY-12, a N. vulgaris enrichment culture with high nitrification efficiency, was isolated and identified from freshwater aquaculture water. Efficient nitrification was achieved in a wide range of temperatures and pH values, and XY-12 had a good removal effect of nitrite in both freshwater and brackish aquaculture water. These characteristics will be of great value for application in aquaculture waters.
Acknowledgements
The authors thank Yuheng Cai from Hubei University of Technology for laboratory support in total bacterial counting.
Disclosure statement
Authors have no conflict of interest to declare.
Data availability
The data that support the findings of this study are available in the public domain: https://blog.csdn.net/weixin_57734968/article/details/116226160.
Additional information
Funding
References
- Hellinga C, Schellen AAJC, Mulder JW, et al. The sharon process: an innovative method for nitrogen removal from ammonium-rich waste water. Water Sci Technol. 1998;37(9):135–142.
- Shourjeh MS, Kowal P, Drewnowski J, et al. Mutual interaction between temperature and DO set point on AOB and NOB activity during shortcut nitrification in a sequencing batch reactor in terms of energy consumption optimization. Energies. 2020;13(21):5808.
- Kroupova HK, Valentova O, Svobodova Z, et al. Toxic effects of nitrite on freshwater organisms: a review. Rev Aquacult. 2018;10(3):525–542.
- Spieck E, Lipski A, Klotz M. Cultivation, growth physiology, and chemotaxonomy of nitrite-oxidizing bacteria. Method Enzymol. 2011;486:109–130.
- Brown MN, Aurelio B, James D, et al. Ammonia-oxidizing archaea and nitrite-oxidizing nitrospiras in the biofilter of a shrimp recirculating aquaculture system. FEMS Microbiol Ecol. 2013;83(1):17–25.
- Hüpeden J, Wegen S, Off S, et al. Relative abundance of nitrotoga spp. in a biofilter of a cold-freshwater aquaculture plant appears to be stimulated by slightly acidic pH. Appl Environ Microbiol. 2016;82(6):1838–1845.
- Navada S, Olav V, Frédéric G, et al. Biofilms remember: osmotic stress priming as a microbial management strategy for improving salinity acclimation in nitrifying biofilms. Water Res. 2020;176:115732.
- Bartelme RP, Mclellan SL, Newton RJ. Freshwater recirculating aquaculture system operations drive biofilter bacterial community shifts around a stable nitrifying consortium of ammonia-oxidizing archaea and comammox nitrospira. Front Microbiol. 2017;8:101.
- Gonzalez-Silva BM, Jonassen KR, Bakke I, et al. Nitrification at different salinities: biofilm community composition and physiological plasticity. Water Res. 2016;95:48–58.
- Liang F, Wen YK, Dong X, et al. Response of activity and community composition of nitrite-oxidizing bacteria to partial substitution of chemical fertilizer by organic fertilizer. Environ Sci Pollut Res. 2021;4:1–12.
- Ma S, Zhang D, Zhang W, et al. Ammonia stimulates growth and nitrite-oxidizing activity of Nitrobacter winogradskyi. Biotechnol Equip. 2014;28(1):27–32.
- Krishnani KK. Detection and diversity of nitrifying and denitrifying functional genes in coastal aquaculture. Aquaculture. 2010;302(1–2):57–70.
- Cai M, Siu-Kin N, Kent LC, et al. Physiological and metagenomic characterizations of the synergistic relationships between ammonia- and nitrite-oxidizing bacteria in freshwater nitrification. Front Microbiol. 2018;9:280.
- Elling FJ, Hemingway JD, Evans TW, et al. Vitamin B12-dependent biosynthesis ties amplified 2-methylhopanoid production during oceanic anoxic events to nitrification. Proc Natl Acad Sci U S A. 2020;117(52):32996–33004.
- Sorokin DY, Muyzer G, Brinkhoff T, et al. Isolation and characterization of a novel facultatively alkaliphilic nitrobacter species, N. alkalicus sp. nov . Arch Microbiol. 1998;170(5):345–352.
- Hagopian DS, Riley JG. A closer look at the bacteriology of nitrification. Aquacult Eng. 1998;18(4):223–244.
- Navarro E, Fernandez MP, Grimont F, et al. Genomic heterogeneity of the genus nitrobacter. Microbiology Society. 1992; 42(4):554–560.
- Bock E, Sundermeyer-Klinger H, Stackebrandt E. New facultative lithoautotrophic nitrite-oxidizing bacteria. Arch Microbiol. 1983; 136(4):281–284.
- Bock E, Koops H-P, Möller UC, et al. A new facultatively nitrite oxidizing bacterium, Nitrobacter vulgaris sp. nov. Arch Microbiol. 1990;153(2):105–110.
- Hunik JH, Meijer H, Tramper J. Kinetics of Nitrobacter agilis at extreme substrate, product and salt concentrations. Appl Microbiol Biotechnol. 1993;40(2–3):442–448.
- Fu S-F, Wang F, Shi X-S, et al. Impacts of microaeration on the anaerobic digestion of corn straw and the microbial community structure. Chem Eng J. 2016; 287:523–528.
- Vadivelu VM, Yuan Z, Fux C, et al. The inhibitory effects of free nitrous acid on the energy generation and growth processes of an enriched nitrobacter culture. Environ Sci Technol. 2006; 40(14):4442–4448.
- Franck P, Sophie W, Elisabeth B, et al. First exploration of nitrobacter diversity in soils by a PCR cloning-sequencing approach targeting functional gene nxrA. FEMS Microbiol Ecol. 2008;63(1):132–140.
- Pester M, Maixner F, Berry D, et al. NxrB encoding the beta subunit of nitrite oxidoreductase as functional and phylogenetic marker for nitrite-oxidizing nitrospira. Environ Microbiol. 2014;16(10):3055–3071.
- Li SN, Wang XJ, Zhou J, et al. Application of flow cytometry to enumerate small plankton. journal of lake sciences. Chinese. 2015;27(5):757–766.
- Vanparys B, Spieck E, Heylen K, et al. The phylogeny of the genus nitrobacter based on comparative rep-PCR, 16S rRNA and nitrite oxidoreductase gene sequence analysis. Syst Appl Microbiol. 2007;30(4):297–308.
- Navada S, Vadstein O, Tveten AK, et al. Influence of rate of salinity increase on nitrifying biofilms. J Cleaner Prod. 2019;238:117835.
- Zhang SF, Wang YY, He WT, et al. Impacts of temperature and nitrifying community on nitrification kinetics in a moving-bed biofilm reactor treating polluted raw water. Chem Eng J. 2014;236:242–250.
- Arthur H, Watson K. Thermal adaptation in yeast: growth temperatures, membrane lipid, and cytochrome composition of psychrophilic, mesophilic, and thermophilic yeasts. J Bacteriol. 1976;128(1):56–68.
- Huang ZH, Gedalanga PB, Asvapathanagul P, et al. Influence of physicochemical and operational parameters on nitrobacter and nitrospira communities in an aerobic activated sludge bioreactor. Water Res. 2010;44(15):4351–4358.
- Jimenez E, Gimenez B, Ruano MV, et al. Effect of pH and nitrite concentration on nitrite oxidation rate. Bioresour Technol. 2011;102(19):8741–8747.
- Karin M, Eberhard B. Isolation and partial characterization of inner and outer membrane fractions of Nitrobacter hamburgensis. FEMS Microbiol Lett. 2010;2:137–141.
- Zhang X, Wang CF, Yu X, et al. Effects of pH on the kinetics of NOB and functional genes. China Environ Sci. 2020;40(4):1537–1544. Chinese.
- Gao Y, Wang X, Li J, et al. Effect of aquaculture salinity on nitrification and microbial community in moving bed bioreactors with immobilized microbial granules. Bioresour Technol. 2020;297:122427.
- Paul S, Bag SK, Das S, et al. Molecular signature of hypersaline adaptation: insights from genome and proteome composition of halophilic prokaryotes. Genome Biol. 2008;9(4):R70.
- Soppa J. From genomes to function: haloarchaea as model organisms. Microbiology (Reading). 2006;152(Pt 3):585–590.
- Ilgrande C, Leroy B, Wattiez R, et al. Metabolic and proteomic responses to salinity in synthetic nitrifying communities of nitrosomonas spp. and nitrobacter spp. Front Microbiol. 2018;9:2914.
- Moussa MS, Sumanasekera DU, Ibrahim SH, et al. Long term effects of salt on activity, population structure and floc characteristics in enriched bacterial cultures of nitrifiers. Water Res. 2006;40(7):1377–1388.
- Boon B, Laudelout H. Kinetics of nitrite oxidation by Nitrobacter winogradskyi. Biochem J. 1962; 85(3):440–447.
- Andrews JH, Harris RF. R-selection and k-selection and microbial ecology. Adv Microbial Ecol. 1986;9:99–147.
- Grunditz C, Dalhammar G. Development of nitrification inhibition assays using pure cultures of nitrosomonas and nitrobacter. Water Res. 2001; 35(2):433–440.
- Lin WT, Zhu YN. Analysis of microbial diversity of nitrifying bacteria by terminal restriction fragment length polymorphism. Chin J Biotechnol. 2010;26(4):483–488.
- Kuhn DD, Drahos DD, Marsh L, et al. Evaluation of nitrifying bacteria product to improve nitrification efficacy in recirculating aquaculture systems. Aquacult Eng. 2010;43(2):78–82.
- Ren J, Lin WT, Shen YJ, et al. Optimization of fermentation media for nitrite oxidizing bacteria using sequential statistical design. Bioresour Technol. 2008;99(17):7923–7927.
- Chen Z, Chang ZQ, Qiao L, et al. Effect of hydraulic retention time on solid-phase denitrification reactor in recirculating aquaculture system. Aquaculture. 2021;543:736928.
- Yedong G, Liang G, Meng YS, et al. Heterotrophic denitrification strategy for marine recirculating aquaculture wastewater treatment using mariculture solid wastes fermentation liquid as carbon source: optimization of COD/NO3−–N ratio and hydraulic retention time. Bioresour Technol. 2020;304(122982). DOI: 10.1016/j.biortech.2020.122982.
- Li Q. The microbial structure and the selection of attachment growth mode of nitrifying bacteria in nitrification process. Liaoning Urban Rural Environ Sci Technol. 2000;20(6):34–38. Chinese.
- Sclenickova K, Kolousek D, Pecenka M, et al. Application of zeolite filters in fish breeding recirculation systems and their effect on nitrifying bacteria. Aquaculture. 2020; 516(734605)
- Forrest D, Delatolla R, Kennedy K. Carrier effects on tertiary nitrifying moving bed biofilm reactor: an examination of performance, biofilm and biologically produced solids. Environ Technol. 2016;37(6):662–671.