Abstract
Plants have evolved an adaptive mechanism named drought stress memory to improve resistance and increase survival chances, by means of coordination of physiological responses and changes of gene expression patterns. The ATP-binding cassette (ABC) superfamily G proteins are membrane proteins that are involved in abscisic acid (ABA) transport and the response to drought stress. The ABC protein AhATL1 could play an important role in drought resistance by affecting ABA importation and stomatal closure and could participate in drought stress memory in Arachis hypogaea L. Here, we screened the interacting proteins of AhATL1 by co-immunoprecipitation and mass spectrometry, in order to further explore the function of AhATL1 in response to repeated dehydration stress. Subsequently, we screened for proteins that may be related to abscisic acid transport and identified AhLBP, AhGCP, AhSHT and AhHSP. Furthermore, we predicted their signal peptides, transmembrane structures, functional domains, and analyzed their gene expression levels, demonstrating they may play a role in peanut drought stress memory. Yeast two-hybrid assay showed that AtSLAC1 but not AhLBP, AhGCP, AhSHT, AhHSP or AhSLAC1 interacted with AhATL1. However, confocal microscopic analysis demonstrated that AhATL1 was co-localized with AhHSP, AhSLAC1 and AtSALC1. And BiFC assays showed that AhATL1 interacted with AhSLAC1 and AtSALC1, indicating that a close relationship existed between them. The findings provide further insights into the role of AhATL1 in drought stress memory and a useful reference for exploring the role of the ABA signaling pathway in plant drought stress memory.
Supplemental data for this article is available online at https://dx.doi.org/10.1080/13102818.2021.2013734 .
Introduction
As sessile organisms, plants are constantly exposed to the vagaries of repeated biotic and abiotic stresses during their whole life. To cope with repeated drought stress and increase their survival chances, plants have evolved a class of adaptive strategies for stress memory, which is also known as information storage [Citation1,Citation2]. Recently, relevant agricultural practices and scientific researches have shown that plants have the ability to remember drought stress. Arabidopsis seedlings subjected to several cycles of drought and water recovery treatments maintained higher relative water content and induced higher expression levels of dehydration responsive genes than plants encountering the initial drought stress [Citation2–5]. Physiological and biochemical responses, transcriptome and proteome analysis of some short-term repeated drought stressed crops (including wheat, rice, peanut, soybean and maize) indicate that these crops have drought stress memory, scilicet, crops could adapt or remember the first dehydration stress through ‘training’, and show different effects on repeated dehydration stress [Citation6–10]. According to recent studies, the establishment of stress memory is closely associated with epigenetic regulation [Citation11,Citation12], such as histone modifications and DNA methylation [Citation13–15]. However, what mechanism provides for their appearance before the stress memory and how transcriptional memory of stress is transformed into a comprehensive and integrated physiological response to repeated dehydration stress are unclear.
Abscisic acid (ABA) is the most important phytohormone for the stress response and tolerance of plants. It is rapidly induced and transported under drought stress, triggering a series of physiological responses and signal transduction, such as the production of reactive oxygen species (ROS), stomatal closure, the increase in intracellular Ca2+ and the activation of downstream signal transduction pathways related to drought tolerance [Citation16,Citation17]. The fast adjustment of the stomatal aperture is necessary for effective drought tolerance in plants, which is achieved by the activity of the S-type anion channel (SLAC1) that triggers the release of anions and K+ from the guard cells, resulting in the reduction of osmotic content [Citation18–20]. SLAC1 is the direct target of the ABA signaling pathway. SLAC1 interacts with calcium-dependent protein kinases (CPKs) and calcium-independent protein kinases OST1 (open stomata 1) to activate guard cell anion channels and promote stomata closure quickly through ABA signal transduction [Citation21–24]. However, whether SLAC1 interacts with other components of the ABA signaling pathway to regulate stomatal movement is still unknown.
Physiological responses mediated by ABA are regulated by the concentration of ABA within and surrounding the cell, and the sensitivity of the cell to ABA. However, the concentration of ABA at the site of action is also influenced by the mobile nature of ABA in addition to the rate of ABA biosynthesis and catabolism. The ABA transporters coordinate with the transpiration force to redistribute and transport ABA to the site of action, thereby regulating ABA-dependent stress responses [Citation25–27]. For example, under drought stress, ABA is rapidly synthesized in root vascular parenchyma and transported to the leaves via the xylem, leading to a series of stress responses such as stomatal closure in peanut and Arabidopsis [Citation26,Citation28]. The ATP-binding cassette (ABC) superfamily G proteins belong to ABA transporters with two basic domains, TMD (transmembrane domain) and NBD (nucleotide-binding domain), which play essential roles in seed germination, stomatal movement, lateral root development and various environmental stress responses in plants [Citation29–31]. Mutation in the AtMRP5 ABC protein impairs abscisic acid and cytosolic Ca2+ activation of slow (S-type) anion channels in guard cells [Citation32]. The atabcg22 mutant increased water loss and decreased drought tolerance through affecting stomatal movement and increasing transpiration [Citation33]. RCN1/OsABCG5 is involved in the accumulation of ABA in guard cells, which is necessary for stomata closure in rice [Citation34]. The researches show that ABC transporters may regulate stomatal movement by affecting the ion channel protein activity of guard cells and ultimately affect the drought tolerance of plants. However, it is still unclear whether they play a role in plant drought stress memory or not.
Peanuts are an important economic and oil crop in China. The peanut yield reduction caused by drought accounted for more than 20% of the total peanut production in China. In the actual production of peanuts, drought and water shortage environments are repeated [Citation35,Citation36]. Recent studies have revealed that peanuts under repeated dehydration conditions show more rapid and stronger physiological defense and recovery, as well as growth and stress-related gene expression [Citation8,Citation36]. Therefore, it prompted us to explore whether and how ABA transporters are involved in repeated drought stress in peanuts. ABA transporter-like1 (AhATL1) gene from A. hypogaea that encodes a plasma membrane-localized protein containing ABC family domains has been cloned in our laboratory. The previous studies found that AhATL1 could reduce ABA sensitivity by specifically affecting ABA import and the overexpression of AhATL1-OX in Arabidopsis displayed larger stomatal aperture than Col, in which SLAC1 expression level was also lower [Citation37,Citation38]. AhATL1 expression was induced more significantly in recurring dehydration stress relative to the expression in the first exposure, indicating AhATL1 participated in the drought stress memory in peanuts [Citation8,Citation38]. Here, to further explore the function of AhATL1 and its role in peanut drought stress memory, we obtained AhATL1 data by using mass spectrometry assay and screened its interacting proteins during repeated dehydration, providing a reference for exploring the molecular mechanism of plant drought stress memory.
Materials and methods
Plant materials and experimental design
The peanut cultivar ‘Yueyou 7′ used in this study was provided from the Crop Research Institute, Guangdong Academy of Agricultural Sciences, China. Seeds were soaked in water for 12 h, and then placed on moist filter paper in a growth chamber with a cycle of 16 h light from fluorescent and incandescent lamps (200 µmol m−2 s−1) at 26 °C followed by 8 h darkness at 22 °C for 48 h until the cusp was exposed. Then germinated seeds were sown in saturated peat-containing soil in a growth chamber. Peanut plants were grown to the four-leaf stage under normal conditions as described previously [Citation37].
Drought ‘train’ treatment was conducted in a replicated experiment on peanut seedlings in the four-leaf stage for 8 h of dehydration followed by a 48-h cycle of full rehydration recovery [Citation8,Citation38]. The peanut seedlings were dehydrated for 8 h (D1), and then rehydrated with 1/8 Murashige and Skoog solution (pH 6.0) for 48 h (R1). In the same way, the peanut plants were subjected to the second drought (D2) and then rehydrated (R2). Aerial tissue was harvested at noon on the day specified. The excised samples were immediately frozen and stored at −80 °C until use. All treatments were performed in at least three independent experiments.
Gene expression analysis
Total RNA of 100 mg of peanut leaves were extracted using the Trizol Kit (TaKaRa) and 2 μg of high-quality RNA was reverse transcribed (RT) using a Prime Script TMRT Reagent Kit (Perfect Real Time, TaKaRa, Dalian, China) according to the company’s protocol. Quantitative real-time PCR was performed with the ChamQ SYBR qPCR Master Mix (Vazyme Biotech Co., Ltd) on the CFX96 Touch system (Bio-Rad, USA) to quantify gene transcript levels. The validated housekeeping gene AhACTIN was used as reference gene to normalize the total amounts of cDNA present in each reaction. The expression level of each gene was calculated using the relative 2-ΔΔCT method in three independent biological replicates [Citation37]. The specific primers used in real-time RT-PCR are shown in .
Table 1. Analysis of AhATL1 candidate interacting proteins in MS.
Affinity purification and mass spectrometry
Total protein of approximately 200 mg of peanut leaves was extracted using Plant Total Protein Extraction Kit (KeyGEN BioTECH, KGP7100) and protein assay was performed by using BCA Protein Assay Kit (KeyGEN BioTECH, KGP902) following the manufacturer’s instructions. Protein extract (500 µL) was added into 500 µL IP (immunoprecipitation) buffer (50 mmol L−1 Hepes (pH7.5), 150 mmol L−1 KCl, 5 mmol L−1 MgCl2, 10 mmol L−1 ZnSO4, 1% Triton X-100, 0.05% sodium dodecyl sulphate) mixed with 20 µl protein A/G Plus-agarose beads which had been balanced for 10 min, and gently rotated end-over-end at 4 °C for 1 h. Half of the supernatant was incubated with specific antibody for AhATL1 (Willget Biotech, Shanghai, China) and the other with horseradish peroxidase -IgG on a rotatory shaker at 4 °C for 3 h. Then protein A/G Plus-agarose beads were added to the solution and incubated for 1 h while rotated. Beads were washed three times with 500 µL IP buffer and re-suspended in 200 µL Elution buffer (50 mmol L−1 Tris − HCl (pH 8.0), 1% SDS, 10 mmol L−1 ethylenediaminetetraacetic acid), boiled at 65 °C for 15 min. The eluted protein samples were identified by mass spectrometry which was done by the company Beijing Protein Innovation (Beijing, China).
Bioinformatics analysis of proteins screened by MS
The physicochemical properties of the proteins were analyzed using ExPASy-ProtParam (http://web.expasy.org/protparam/). The N-terminal signal peptide and transmembrane structure of the proteins were predicted on the Signal P and TMHMM (http://www.cbs.dtu.dk/services/SignalP/; http://www.cbs.dtu.dk/services/TMHMM/) websites, and the conserved domains were analyzed using SMART (http://smart.embl-heidelberg.de/).
Subcellular co-localization assay
The full-length coding sequence of AhATL1, AhSLAC1, AtSLAC1, AhLBP and AhHSP were respectively introduced into pGreen-GFP and pGreen-mCherry to generate GFP-AhATL1, mCherry-AhSLAC1, mCherry-AtSLAC1, mCherry-AhLBP, mCherry-AhHSP fusion constructs. All constructs were then transformed into Arabidopsis protoplasts. The protoplast isolation and transient expression were carried out as described previously [Citation37]. The distribution of all fusion proteins was observed by a confocal microscope (LSM800, Carl Zeiss, Germany) after the protoplasts were incubated at 23 °C for 17 h in the dark. The specific primer sequences are provided in .
Table 2. Properties of AhATL1 candidate interacting proteins.
Yeast two-hybrid assay
The full coding sequences of AhATL1, AhSLAC1, AtSLAC1, AhLBP, AhGCP, AhSHT and AhHSP were cloned into pGADT7 and pGBKT7 vectors respectively to generate AD-AhATL1, BD-AhSLAC1, BD-AtSLAC1, BD-AhLBP, BD-AhGCP, BD-AhSHT, BD-AhHSP combinatory constructs. Shorter fragments of AhATL1 (AN and AC), AhSLAC1 (HN and HC) and AtSLAC1 (TN and TC) coding regions were separately cloned into pGADT7 and pGBKT7 vectors respectively to generate AD-AN, AD-AC, BD-HN, BD-HC, BD-TN and BD-TC constructs. All constructs were transformed into the AH109 yeast strain by the lithium acetate method, and grown on minimal medium lacking Trp and Leu according to manufacturer (Clontech) instructions. Transformed colonies were plated onto medium SD/-Trp-Leu-His but supplemented with various concentrations of 3-AT and SD/-Trp/-Leu/-His/-Ade to assess possible interactions [Citation39]. The primers used are summarized in Supplemental Table S3.
Bimolecular fluorescence complementation (BiFC) assay
Full-length fragments of AhATL1, AhSLAC1 and AtSLAC1 were recombined into the pGreen binary vector HY105 containing C- or N-terminal fusions of EYFP, and then co-transformed into Arabidopsis protoplasts as previously described [Citation39]. YFP fluorescence signals were detected under a confocal microscope (LSM800, Carl Zeiss, Germany) after 12–20 h incubation at 23 °C in the dark. The primers used are listed in Supplementary Table S4.
Results
Screening and bioinformatics of candidate proteins interacting with AhATL1
To further explore the function of the dehydration-responsive gene AhATL1, the affinity purification followed by mass spectrometry was performed with AhATL1 protein as the bait, and several proteins that may interact with AhATL1 were found according to the protein profiles, namely AhLBP, AhGCP, AhSHT and AhHSP (). AhLBP is a luminal-binding protein. There are 90 AhLBP in the proteome of AhATL1, including 4 unique peptides and 6 peptides, and its sequence coverage is 11.43. AhGCP is a glycine cleavage protein. There are 11 AhGCP in the proteome of AhATL1, including 2 unique peptides and 6 peptides, and its sequence coverage is 6.50. AhSHT is a serine hydroxymethyltransferase. There are 24 AhSHT in the proteome of AhATL1, including 1 unique peptide and 5 peptides, and its sequence coverage is 13.32. AhHSP is a heat shock protein. There are 138 AhHSP in the proteome of AhATL1, including 2 unique peptides and 4 peptides, and its sequence coverage is 7.09.
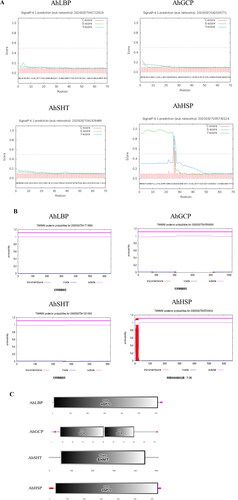
The analysis of the physicochemical properties of the candidate proteins by ExPASy-ProtParam found that all of them were hydrophobic proteins, among which AhLBP and AhHSP were stable, while AhGCP and AhSHT were unstable (). Most of them were 120–160 kDa in molecular weight and their theoretical isoelectric point was approximately 5.00. However, the molecular weight of AhGCP was 261.23 kDa and the theoretical pI was 4.82 ().
Furthermore, Signal P and TMHMM were used to predict the secondary structure of the proteins. The results showed that there was no signal peptide cleavage site and no transmembrane structure at the N-terminus of AhLBP, AhGCP and AhSHT, but AhHSP had a signal peptide cleavage site between 1–25 amino acids and a transmembrane structure between 7–26 amino acids at the N-terminal of the protein (). A prediction of their conserved domains was performed using SMART and revealed that a heat shock protein domain was discovered in AhLBP and AhHSP protein sequences. Besides, there was a signal peptide sequence in AhHSP that was consistent with the results of signal peptide prediction. The alignment of AhGCP contained two GDC-P domains, and AhSHT was classified as the SHMT family ().
Figure 1. Conserved functional domain and transmembrane domain analysis of AhATL1 candidate interacting proteins. (A) Signal peptide analysis of AhLBP, AhGCP, AhSHT and AhHSP. (B) Transmembrane structure analysis of AhLBP, AhGCP, AhSHT and AhHSP. (C) Conserved functional domain of AhLBP, AhGCP, AhSHT and AhHSP.
Transcriptional responses of AhLBP, AhGCP, AhSHT and AhHSP during multiple exposures to drought stress
In our previous research, the transcript levels of AhATL1 during the second dehydration stress was significantly different from that in the initial stress, indicating AhATL1 was involved in peanut drought stress memory [Citation8]. To determine whether genes encoding proteins identified by mass spectrometry (AhLBP, AhGCP, AhSHT and AhHSP) respond to repeated drought stress, we analyzed their transcriptional response during the four treatment phases in peanut. As displayed in , the overall transcript level of AhLBP increased during dehydration stress (D1 and D2), especially more strongly in the second drought stress, which was in accordance with the responses of AhATL1 to recurring drought stress. In addition, it was worth noting that the AhLBP expression level in the first rehydration period (R1) decreased sharply and was significantly lower than that in the second rehydration period (R2). In contrast, the transcript levels of AhGCP and AhHSP in the subsequent treatments were generally less than their expression levels in the period of the initial drought and re-watering. However, there was almost no change in the overall expression of AhSHT comparing the two stress treatments. Combined with mass spectrometry, the expression patterns of AhLBP, AhGCP and AhHSP under repeated drought stress suggested that AhLBP, AhGCP and AhHSP might play a role in peanut drought stress memory that was related to ABA transport.
Figure 2. Expression patterns of genes encoding AhLBP, AhGCP, AhSHT and AhHSP during repeated dehydration stress in Arachis hypogaea L. The x-axis illustrates different drought and re-watering time. Transcript levels measured in recovered plants before initiating stress treatments are designated as pre-stressed (D1-0) levels. Respectively, D1-2, D1-4 and D1-8 represent that plants are dry for 2, 4, 8 h during the first dehydration stress, while R1-2, R1-4, R1-8, R1-24, R1-48 are defined as the first rehydration of plants for 2, 4, 8, 24, 48 h. And so on to the second drought and re-watering treatments. The y-axis shows the gene expression levels after normalization to internal control gene AhACTIN. Results are the average of at least two independent experiments, each with three replicates, and the representative experiment shown indicates the mean ± s.e.m., n = 3 replicates.
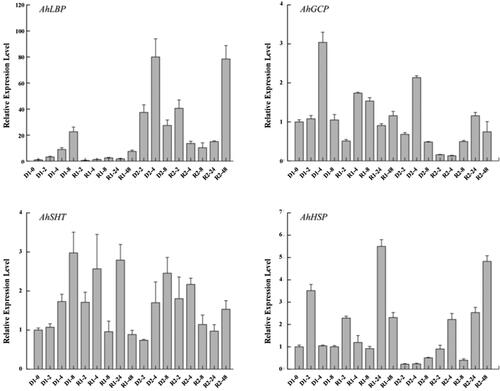
Furthermore, according to their gene expression patterns, AhLBP was gradually induced in D1, and vigorously up-regulated followed by down regulation after 4 h of drought in D2. AhGCP was gradually induced to 4 h after drought in D1, then the gene expression level began to decrease, and the D2 period change was similar to that in the D1 period. AhHSP was increased in advance and began to decrease after 2 h of drought in D1; the changes of AhHSP expression were eliminated in D2. These results indicated that the trends of gene expression may be the same or different between D1 and D2 periods in response to the discontinuous drought faced by plants.
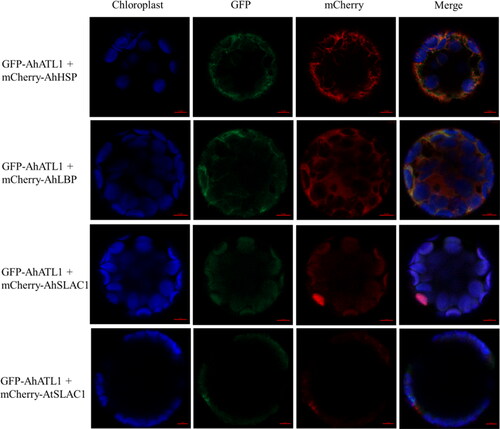
Co-localization of AhATL1 with AhLBP, AhHSP, AhSLAC1 and AtSALC1
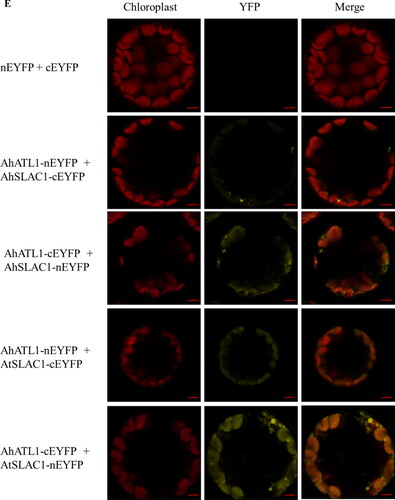
To verify whether there is a connection between AhATL1 and candidate interacting proteins as shown in MS, we selected AhLBP and AhHSP for the assay of co-localization with AhATL1 separately on the basis of their bioinformatics and gene expression characterization. Our preliminary research in the laboratory showed that the expression level of AtSLAC1 in the heterologous overexpression Arabidopsis line AhATL1-OX was lower than Col under normal, dehydrated and ABA treatments (). Furthermore, relative researches also revealed that ABC transporters could affect the ion transport function of SLAC1 [Citation29,Citation30]. Therefore, to explore the connection of ABC transporters and SLAC1 in peanut, we also performed subcellular co-localization analysis of AhSLAC1 and AtSALC1 with AhATL1. The GFP-tagged plasmid vector fused to AhATL1 and the mCherry-tagged plasmid vector fused to AhLBP, AhHSP, AhSLAC1 and AtSALC1 were transiently expressed in isolated protoplasts, whose fluorescence signal was detected by confocal microscopy. As shown in , eGFP-AhATL1 was co-localized with mCherry-AhHSP, mCherry-AhSLAC1 and mCherry-AtSALC1 on the plasma membrane of protoplasts. However, the fluorescence signals of mCherry-AhLBP fused protein were diffused throughout the protoplasts, indicating that AhLBP was located in the cytoplasm. These indirectly indicated that there was a certain functional relationship between AhATL1 and AhHSP or SLAC1 in ABA transport during repeated drought stress.
Figure 3. Analysis of subcellular co-localization of AhATL1 and AhLBP, AhHSP, AhSLAC1 or AtSALC1. Full coding sequences of AhLBP, AhHSP, AhSLAC1 and AtSALC1 were integrated to the mCherry-tagged plasmid and co-transformed respectively with the GFP-tagged fusion protein integrated with Full-length fragment of AhATL1 into Arabidopsis protoplasts. Fluorescence images were captured with a confocal microscope by using 63x oil lens. The red horizontal line represents the scale bar of 5 μm.
Identification of AhATL1 interaction proteins
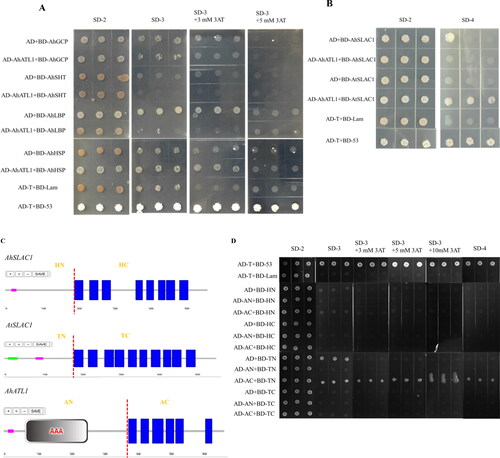
The yeast two-hybrid (Y2H) assay was carried out to investigate whether AhATL1 interacts with AhLBP, AhGCP, AhSHT, AhHSP, AhSLAC1 and AtSALC1. The Y2H results showed that AtSALC1 but not AhSLAC1, nor the candidate interacting proteins, physically interacts with AhATL1 (). To determine which regions of ATL1 and SALC1 are responsible for their interactions, the truncated versions of AhSLAC1, AtSLAC1 and AhATL1 were constructed for further Y2H assays according to the characteristics of their protein domain. Deletion analysis displayed that the C-terminal region (amino acids 378–667) of AhATL1 and the N-terminal region (amino acids 1–183) of AtSLAC1 were required for their interaction ().
Figure 4. Confirmation of the interaction between AhATL1 and its interactive proteins by yeast two-hybrid and BiFC assays. (A) Interactions between AhATL1 and candidate interacting proteins were identified by Y2H. Yeast cells harboring different fusion protein combinations (listed on the left) in pGBK-T7 (BD) and pGAD-T7 (AD) vectors were plated on the medium lacking Leu and Trp (SD-2) or the medium lacking Leu, Trp, and His (SD-3) but supplemented with 0, 3 and 5 mmol L−1 3-AT (3-amino-1,2,4-triazole). (B) Y2H analysis of AhATL1-AhSLAC1 and AhATL1-AtSALC1 interactions. The strains harboring the constructs listed on the left were grown on the medium lacking Leu and Trp (SD-2) or the medium lacking Leu, Trp, His and Ade (SD-4). (C) Diagrams of AhATL1, AhSALC1 and AtSALC1 truncated protein regions used in yeast two-hybrid constructs. HN: amino acids 1-193 from N-terminus of AhSALC1, HC: amino acids 194-565 from C-terminus of AhSALC1, TN: amino acids 1-183 from N-terminus of AtSALC1, TC: amino acids 184-557 from C-terminus of AtSALC1, AN: amino acids 1-377 from N-terminus of AhATL1, AC: amino acids 378-667 from C-terminus of AhATL1. (D) Analysis of the interaction regions between ATL1 and SALC1 shown in (C). The strains harboring the constructs listed on the left were grown on the medium SD-2, SD-3 supplemented with 0, 3, 5 and 10 mM 3-AT or SD-4. A yeast colony formed on an SD-3/4 plate indicated a positive protein interaction. (E)AhATL1 interacted with AhSALC1 and AtSALC1 in Arabidopsis protoplasts by BiFC. Fluorescence images were captured with a confocal microscope by using 63x oil lens. The red horizontal line represents the scale bar of 5 μm.
The interaction between AhATL1 and SALC1 was further confirmed using the Bimolecular Fluorescent Complimentary (BiFC) assay. Full-length fragments of AhATL1, AtSLAC1 and AhATL1 were fused to the C-terminal and N-terminal halves of yellow fluorescent protein (YFP) respectively. The corresponding fused constructs were transiently co-expressed in Arabidopsis protoplasts and then observed by a confocal microscope. It was found that the YFP signal was obtained on the plasma membrane of Arabidopsis protoplasts only when AhATL1 and AtSLAC1 or AhSLAC1 were co-transformed into protoplasts (), suggesting that there was an interaction between AhATL1 and SLAC1.
Discussion
Physiological processes such as intracellular immune response and signal transduction are all realized through proteins. When proteins perform these activities, they are usually completed by the interaction between protein and protein or protein and nucleic acid. Therefore, the interaction between protein and protein plays a very important role in the process of signal transduction, post-transcriptional modification and metabolic regulation in the cell.
Previous studies have shown that the amino acid sequence of peanut AhATL1 contains a transmembrane domain, an ABC carrier conserved domain, and an ATP binding site, which is homologous to Arabidopsis AtABCG11. It is further speculated that AhATL1 protein may be used as a transport carrier to transport ABA [Citation37]. ATP binding (ABC) transporters play different roles in plants, including lipid transport. The ABCG subfamily transporters encoding half transporters require dimerization to form functional ABC transporters [Citation40]. The cuticle includes wax and cutin. It is a barrier covering plant aerial organs and protecting internal tissues. Arabidopsis AtABCG11/WBC11 and AtABCG12/CER5 have been identified as waxy export proteins [Citation41]. Mutation analysis showed that AtABCG11 and AtABCG12 were necessary for lipid export from the epidermis to the protective cuticle [Citation40]. Through in vivo Bimolecular Fluorescence Complementation (BiFC), it was found that ABCG11/ABCG12 can form a heterodimer, and ABCG11 can form a homodimer. In the absence of ABCG12, ABCG11 can be transported to the plasma membrane. On the contrary, in the absence of ABCG11, ABCG12 remains in the endoplasmic reticulum, indicating that some ABCG proteins can interact with multiple proteins, while other ABCG transporters require to form a proprietary heterodimer to achieve specific functions [Citation40]. This also provides a reference for the possible interactions between AhATL1 and other proteins and the ways in which they interact.
The stomata are the window through which plants and the external environment exchange materials and information. Stomata mediate the adaptation process of plants to the external environment by sensing and decoding a variety of external environmental signals such as drought, CO2 and ozone. The stomata are formed by specialized guard cells, which can regulate the opening and closing of the stomata by decoding various external environmental signals and integrating them into the turbulence changes of the guard cells. Stomata are the main structure that controls plant gas exchange and water balance. ABA activates the OST1 kinase and further initiates the up-regulation of downstream ion channel genes (ALMT12, SLAC1) expression to cause stomata closure [Citation42–44]. SLAC1 is mainly expressed in the stomata guard cell membrane and participates in anion outflow guard cells. The slac1 mutant plants showed stomata closure barriers to ABA, CO2, Ca2+ and ozone treatments. In addition, the guard cells in the slac1 mutant blocked the activation of S-type anion channels by Ca2+ and ABA, indicating that SLAC1 is the main S-type anion channel in guard cells [Citation20]. There is an interaction between the protein kinase SnRK2.6/OST1 (Sucrose non-fermenting-1 Related protein Kinase 2/Open stomata 1) and the anion channel SLAC1. PP2CA is one of the members of the PP2C phosphatase family. It inhibits the activity of SLAC1 through two mechanisms: (1) directly interacts with SLAC1 itself; (2) physical interaction with OST1 leads to kinase inhibition, independent of phosphatase activity [Citation24]. SnRK2s target and regulate the two downstream ion channels, slow anion channel 1 (SLAC1) and inward rectifier potassium channel (KAT). Specifically, SnRK2-mediated phosphorylation activates SLAC1, inactivating KAT, leading to plasma membrane depolarization and activation of outward K+ channels. The leakage of anions and K+ leads to the loss of swelling in the guard cells, which leads to the closure of the stomata [Citation20,Citation45,Citation46]. In this study, the interaction between AhATL1 and Ah/AtSLAC1 was found in the BiFC experiment, but only the interaction between AhATL1 and AtSLAC1 was found in the yeast two-hybrid experiment. One possible explanation is that the weak or short-lived interaction between AhATL1 and AhSLAC1 was not revealed in the yeast system due to the lack of unknown mediators. Aligning the amino acid sequence revealed that the homology of AtSLAC1 and AhSLAC1 is 68.07%. Therefore, the evolutionary differences of each species may lead to the differences of their homologous proteins. For example, Arabidopsis POCO1 belongs to the PPR family of proteins, which is located in the mitochondria and affects flowering, but it is different from other proteins in the pentapeptide repeat (PPR) protein family and participates in different RNA processing steps [Citation47]. Studies have shown that SLAC1 from different plants exhibits different characteristics. For example, SLAC1 from Arabidopsis, Brassica pekinensis and Solanum lycopersicum are permeable to both nitrate and chlorine, while PdSLAC1 cloned from the desert plant Phoenix is a nitrate-activated chloride ion permeable channel [Citation23,Citation24,Citation48,Citation49]. In addition, studies have shown that AhATL1 was probably closely associated with SLAC1 [Citation8,Citation38]. We speculate that AhATL1 acts as an ABA transport carrier. The carrier can transport ABA from vascular tissues and other synthetic sites to stomatal guard cells. And the interaction between AhATL1 and SLAC1 may have crossed the activation of SLAC1 by protein kinase OST1. Another possibility is that AhATL1 and SLAC1 form a structure similar to a co-transporter. That structure allows it to transport ions faster, triggering more rapid changes in stomatal opening and closing, and then responding to drought stress training. The specific mechanism needs to be further studied.
Plants will experience repeated dehydration stress throughout the growth cycle, and there will be intermittent water recovery periods during the process. After repeated stress, pre-exposure to environmental stress will change the response to subsequent stress, and the phenotypic plasticity response that occurs during this process may increase the long-term survival rate and yield of plants [Citation50,Citation51]. These results indicate that plants have some form of ‘memory’ that can change their response to subsequent stress [Citation2,Citation52]. Compared with plants that experienced dehydration stress for the first time, plants that were repeatedly subjected to dehydration stress and alternately entered the water recovery period exhibited transcriptional and physiological memory responses during the subsequent dehydration stress, and then proposed the standard of transcriptional memory [Citation3,Citation53]. This study found that during drought training, the overall transcription levels of AhLBP, AhGCP and AhHSP identified by MS were significantly different from the initial treatment, which was related to the repeated dehydration stress of peanuts, while the overall transcription level of AhSHT was similar to the initial treatment. According to the standard of transcriptional memory, the gene expression patterns of AhLBP, AhGCP, AhHSP and AhSHT are [+/+], [+/−], [+/−], [+/=], and AhLBP, AhGCP, AhHSP are memory genes, but AhSHT is a non-memory gene. The difference in gene transcription patterns, similar to the activation of defense genes, can provide a stronger or altered stress response, while reducing the consumption of preparedness [54]. In addition, studies have shown that the mode transformation between memory genes might be more helpful for plant survival under repeated drought stress, especially in the case of the overexpression or knockout of a certain gene [Citation38].
Conclusions
In this study, the AP-MS assay was carried out using AhATL1 as a bait to screen its potential interacting proteins. According to the protein profiles, four proteins (AhLBP, AhGCP, AhSHT and AhHSP) were selected. The transcriptional responses suggested that AhLBP, AhGCP and AhHSP may play a role in peanut drought stress memory that was related to ABA transport. Yeast two-hybrid results showed that AtSLAC1 but not AhLBP, AhGCP, AhSHT, AhHSP or AhSLAC1 interacted with AhATL1 in yeast. The interaction between AhATL1 and Ah/AtSLAC1 was further identified by BiFC assay. In addition, AhATL1 was co-localized with Ah/AtSALC1, indicating that there was a close connection between AhATL1 and SLAC1. Overall, the findings provided a molecular perspective for further exploring the role of AhATL1 in the response to drought stress or drought stress memory.
Author contributions
B.H. designed the research. X.W. and X.L. performed the research. X.W., X.L. and B.H. analyzed the data and prepared figures. X.W. wrote the manuscript in consultation with B.H., Y.H. and B.H. contributed reagents/materials/analysistools.
Supplemental Material
Download PDF (277.9 KB)Disclosure statement
No potential conflict of interest was reported by the authors.
Data availability statement
All data that support the findings reported in this study are available from the corresponding author upon reasonable request.
Funding
This work was supported by The Natural Science Foundation of Guangdong Province (Grant No. 2021A1515010448, No. 2018A030313629 granted to BH).
References
- Hilker M, Schmülling T. Stress priming, memory, and signalling in plants. Plant Cell Environ. 2019;42(3):753–761.
- Avramova Z. Transcriptional ‘memory’ of a stress: transient chromatin and memory (epigenetic) marks at stress-response genes. Plant J. 2015;83(1):149–159.
- Ding Y, Fromm M, Avramova Z. Multiple exposures to drought ‘train’ transcriptional responses in arabidopsis. Nat Commun. 2012;3(1):9.
- Liu N, Avramova Z. Molecular mechanism of the priming by jasmonic acid of specific dehydration stress response genes in arabidopsis. Epigenet. Chromatin. 2016;9(1):8.
- Ding Y, Liu N, Virlouvet L, et al. Four distinct types of dehydration stress memory genes in Arabidopsis thaliana. BMC Plant Biol. 2013;13(1):229.
- Tabassum T, Farooq M, Ahmad R, et al. Terminal drought and seed priming improves drought tolerance in wheat. Physiol Mol Biol Plants. 2018;24(5):845–856.
- Li P, Yang H, Wang L, et al. Physiological and transcriptome analyses reveal short-term responses and formation of memory under drought stress in rice. Front Genet. 2019;10:55.
- Chen RQ, Shu W, Ge K, et al. Effect on growth and expressions of stress-related genes in peanut under drought stress training. Plant Physiol J. 2017;53(10):1921–1927.
- Kim YK, Chae S, Oh NL, et al. Recurrent drought conditions enhance the induction of drought stress memory genes in Glycine max L. Front. Genet. 2020;11:1248.
- Virlouvet L, Avenson TJ, Du Q, et al. Dehydration stress memory: gene networks linked to physiological responses during repeated stresses of zea mays. Front Plant Sci. 2018;9:1058.
- Lämke J, Bäurle I. Epigenetic and chromatin-based mechanisms in environmental stress adaptation and stress memory in plants. Genome Biol. 2017;18(1):1–11.
- Friedrich T, Faivre L, Bäurle I, et al. Chromatin-based mechanisms of temperature memory in plants. Plant Cell Environ. 2019;42(3):762–770.
- Heard E, Martienssen RA. Transgenerational epigenetic inheritance: myths and mechanisms. Cell. 2014;157(1):95–109.
- Iwasaki M, Paszkowski J. Identification of genes preventing transgenerational transmission of stress-induced epigenetic states. Proc Natl Acad Sci USA. 2014;111(23):8547–8552.
- Zheng X, Chen L, Xia H, et al. Transgenerational epimutations induced by multi-generation drought imposition mediate rice plant’s adaptation to drought condition. Sci Rep. 2017;7(1):39843.
- Osakabe Y, Osakabe K, Shinozaki K, et al. Response of plants to water stress. Front Plant Sci. 2014;5:86.
- Lim CW, Baek W, Jung J, et al. Function of ABA in stomatal defense against biotic and drought stresses. Int J Mol Sci. 2015;16(7):15251–15270.
- Schroeder JI, Allen GJ, Hugouvieux V, et al. Guard cell signal transduction. Annu Rev Plant Physiol Plant Mol Biol. 2001;52:627–658.
- Li JL, Han L, Su YH, et al. Functional identification of ammopiptanthus mongolicus anion channel AmSLAC1 involved in drought induced stomata closure. Plant Physiol Biochem. 2019;143:340–350.
- Vahisalu T, Kollist H, Wang YF, et al. SLAC1 is required for plant guard cell S-type anion channel function in stomatal signalling. Nature. 2008;452(7186):487–491.
- Geiger D, Scherzer S, Mumm P, et al. Guard cell anion channel SLAC1 is regulated by CDPK protein kinases with distinct Ca2+ affinities. Proc Natl Acad Sci USA. 2010;107(17):8023–8028.
- Huang SG, Waadt R, Nuhkat M, et al. Calcium signals in guard cells enhance the efficiency by which abscisic acid triggers stomatal closure. New Phytol. 2019;224(1):177–187.
- Geiger D, Scherzer S, Mumm P, et al. Activity of guard cell anion channel SLAC1 is controlled by drought-stress signaling kinase-phosphatase pair. Proc Natl Acad Sci USA. 2009;106(50):21425–21430.
- Lee SC, Lan WZ, Buchanan BB, et al. A protein kinase-phosphatase pair interacts with an ion channel to regulate ABA signaling in plant guard cells. Proc Natl Acad Sci USA. 2009;106(50):21419–21424.
- Lee KH, Piao HL, Kim HY, et al. Activation of glucosidase via stress-induced polymerization rapidly increases active pools of abscisic acid. Cell. 2006;126(6):1109–1120.
- Hu B, Cao J, Ge K, et al. The site of water stress governs the pattern of ABA synthesis and transport in peanut. Sci Rep. 2016;6:32143.
- Seo M, Koshiba T. Transport of ABA from the site of biosynthesis to the site of action. J Plant Res. 2011;124(4):501–507.
- Sauter A, Davies WJ, Hartung W. The long-distance abscisic acid signal in the droughted plant: the fate of the hormone on its way from root to shoot. J Exp Bot. 2001;52(363):1991–1997.
- Matsuda S, Funabiki A, Furukawa K, et al. Genome-wide analysis and expression profiling of half-size ABC protein subgroup G in rice in response to abiotic stress and phytohormone treatments. Mol Genet Genom. 2012;287(10):819–835.
- Nguyen VNT, Moon S, Jung K-H. Genome-wide expression analysis of rice ABC transporter family across spatio-temporal samples and in response to abiotic stresses. J Plant Physiol. 2014;171(14):1276–1288.
- Zhang XD, Zhao KX, Yang ZM. Identification of genomic ATP binding cassette (ABC) transporter genes and cd-responsive ABCs in brassica napus. Gene. 2018;664:139–151.
- Suh SJ, Wang Y-F, Frelet A, et al. The ATP binding cassette transporter AtMRP5 modulates anion and calcium channel activities in arabidopsis guard cells. J Biol Chem. 2007;282(3):1916–1924.
- Kuromori T, Sugimoto E, Shinozaki K. Arabidopsis mutants of AtABCG22, an ABC transporter gene, increase water transpiration and drought susceptibility. Plant J. 2011;67(5):885–894.
- Matsuda S, Takano S, Sato M, et al. Rice stomatal closure requires guard cell plasma membrane ATP-binding cassette transporter RCN1/OsABCG5. Mol Plant. 2016;9(3):417–427.
- Zhang ZM, Wan SB, Dai LX, et al. Estimating and screening of drought resistance indexes of peanut. Chin J Plant Ecol. 2011;35(1):100–109.
- Qin FF, Ci DW. Previous drought alters physiological responses to subsequent drought stress in peanut seedlings. Acta Ecol Sin. 2017;37(24):8486–8498.
- Ge K, Liu X, Li XY, et al. Isolation of an ABA transporter-like 1 gene from Arachis hypogaea that affects ABA import and reduces ABA sensitivity in arabidopsis. Front Plant Sci. 2017;8:1150.
- Qin M, Li X, Tang S, et al. Expression of AhATL1, an ABA transport factor gene from peanut, is affected by altered memory gene expression patterns and increased tolerance to drought stress in arabidopsis. IJMS. 2021;22(7):3398.
- Liu S, Li M, Su L, et al. Negative feedback regulation of ABA biosynthesis in peanut (Arachis hypogaea): a transcription factor complex inhibits AhNCED1 expression during water stress. Sci Rep. 2016;6(9):1–11. McFarlane HE, Shin JJ, Bird DA, et al. Arabidopsis ABCG transporters, which are required for export of diverse cuticular lipids, dimerize in different combinations. The Plant Cell. 2010;223066:–3075.
- Luo B, Xue XY, Hu WL, et al. An ABC transporter gene of Arabidopsis thaliana, AtWBC11, is involved in cuticle development and prevention of organ fusion. Plant Cell Physiol. 2007;48(12):1790–1802.
- Negi J, Matsuda O, Nagasawa T, et al. CO2 regulator SLAC1 and its homologues are essential for anion homeostasis in plant cells. Nature. 2008;452(7186):483–486.
- Acharya BR, Jeon BW, Zhang W, et al. Open stomata 1 (OST1) is limiting in abscisic acid responses of Arabidopsis guard cells. New Phytol. 2013;200(4):1049–1063.
- Munemasa S, Hauser F, Park J, et al. Mechanisms of abscisic acid-mediated control of stomatal aperture. Curr Opin Plant Biol. 2015;28:154–162.
- Hubbard KE, Nishimura N, Hitomi K, et al. Early abscisic acid signal transduction mechanisms: newly discovered components and newly emerging questions. Genes Dev. 2010;24(16):1695–1708.
- Umezawa T, Sugiyama N, Mizoguchi M, et al. Type 2C protein phosphatases directly regulate abscisic acid-activated protein kinases in arabidopsis. Proc Natl Acad Sci USA. 2009;106(41):17588–17593.
- Emami H, Kempken F. PRECOCIOUS1 (POCO1), a mitochondrial pentatricopeptide repeat protein affects flowering time in Arabidopsis thaliana. Plant J. 2019;100(2):265–278.
- Müller HM, Schäfer N, Bauer H, et al. The desert plant phoenix dactylifera closes stomata via nitrate-regulated SLAC1 anion channel. New Phytol. 2017;216(1):ww150–162.
- Qi GN, Yao FY, Ren HM, et al. The S-type anion channel ZmSLAC1 plays essential roles in stomatal closure by mediating nitrate efflux in maize. Plant Cell Physiol. 2018;59(3):614–623.
- Nicotra AB, Davidson A. Adaptive phenotypic plasticity and plant water use. Funct Plant Biol. 2010;37(2):117–127.
- Cavanagh AP, Kubien DS. Can phenotypic plasticity in rubisco performance contribute to photosynthetic acclimation. Photosynth Res. 2014;119(1-2):203–214.
- Bruce TJA, Matthes MC, Napier JA, et al. Stressful “memories” of plants: evidence and possible mechanisms. Plant Sci. 2007;173(6):603–608.
- Ding Y, Virlouvet L, Liu N, et al. Dehydration stress memory genes of zea mays; comparison with Arabidopsis thaliana. BMC Plant Biol. 2014;14(1):1–15.
- Fleta-Soriano E, Munné-Bosch S. Stress memory and the inevitable effects of drought: a physiological perspective. Front Plant Sci. 2016;7:143.