?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
The association between the periodontal conditions and smoking is attracting increasing attention. There is little information about the significance of the gene expression levels of interleukin 6 (IL-6) and tumor necrosis factor alpha (TNF-α) for the severity of attachment loss in patients with smoking habits. The aim of this study was to evaluate the relationship between the severity of periodontitis and the gene expression levels of IL-6 and TNF-α in the presence of a main risk factor – smoking. A total of 20 individuals (periodontitis patients) participated in this study. The following clinical parameters were studied: hygiene index, gingival bleeding index, probing depth, clinical attachment loss, furcation involvement and tooth mobility, in patients of mean age of 50.25 ± 8.45 years with untreated periodontitis. Gingival biopsies were taken adjacent to shallow (<4 mm), moderate (4–6 mm) and deep (> 6 mm) periodontal pockets and similar attachment loss for Real-Time polymerase chain reaction (PCR) assessment of IL-6 and TNF-α gene expression levels. The obtained results demonstrated higher values for attachment loss and periodontal pocket depth in the presence of smoking. Furthermore, the results indicated smoking as an important risk factor which could greatly influence the studied gene expression levels. Smoking affected the gene expression mainly in conjunction with deep periodontal pockets. Our results suggest that smoking is an important risk factor for attachment loss in periodontitis patients demonstrated by clinical measurements and IL-6 and TNF-α gene expression.
Introduction
The mechanisms of modifying the body’s response to certain environmental factors are widely discussed in the literature. Many epidemiological studies have been conducted to assess potential risk factors for periodontal disease and have found an increased clinical attachment loss in their presence. Several risk factors that reportedly affect the risk statistically significantly are the presence of increased levels of Porphiromonas gingivalis, male gender, smoking and age [Citation1–4].
Studies on the risk factors for periodontitis demonstrate smoking as a potential risk factor for periodontal disease [Citation1,Citation2]. The physiological effects of smoking include the influence on vasoconstriction and revascularization, the production of collagen and collagenase, oxygen transport and wound healing [Citation3,Citation5]. In 1995 Schenkein et al. [Citation5] studied the effects of smoking in patients with aggressive periodontitis and suggested, like Thangata et al. [Citation6], that smoking is a risk factor that may modify the body’s immune response and therefore influence the clinical expression of the disease. A number of other authors also conclude that smoking can affect various aspects of the response, such as chemotaxis and phagocytosis [Citation7–9].
According to Schenkein et al. [Citation9], smoking can stimulate the production of proinflammatory cytokines, such as interleukin (IL)-1, IL-6, IL-8, tumor necrosis factor (TNF)-α and TGF-β, and interferes by increasing bone resorption and decreasing bone formation.
The aim of this study was to investigate the main clinical parameters of periodontitis (the periodontal pocket depth and the clinical attachment loss), as well as the local gene expression of IL-6 and TNF-α depending on smoking as a risk factor-.
Subjects and methods
Ethics statement
All subjects signed informed consent forms. The research complied with the Rules of Procedure of the Research Ethics Committee at Medical University of Sofia (KENIMUS).
Patients
The selection of patients was made on the basis of clinical and radiological diagnostic methods. Patients with generalized moderate or severe periodontitis (Stage III and IV) were included in the study. Ten of the patients were smokers and ten non-smokers.
Inclusion criteria were: periodontitis (Stage III and IV), clinical attachment loss ≥5 mm, periodontal probing depth ≥6 mm, presence of at least 20 teeth of the dentition, lack of periodontal therapy in the last 6 months, including systemic antimicrobial treatment.
Exclusion criteria were: pregnant women, patients with systemic diseases and systemic medication.
Clinical methods
Patients were examined for the following clinical parameters: Hygiene index (HI); Gingival Bleeding Index (BOP); Periodontal pocket depth in mm (PPD), Clinical attachment loss in mm (CAL); Furcation involvement (F); Tooth mobility (grades I to III).
Molecular methods
The gene expression of IL-6 and TNF-α in gingival tissue in periodontitis was determined by TaqMan Real-Time polymerase chain reaction (PCR). The gingival tissue biopsies (3 mm/3 mm) were taken under local anesthesia with infiltration of Ubistesin from the areas adjacent to the shallow, medium and deep periodontal pockets one single time during the diagnosis.
Molecular analysis: The substrate for the study was gingival tissue (approximately 3 mm/3 mm), which was excised after a local anaesthesia, and placed in a sterile plastic tube (1.5 mL). Three gingival samples were taken in each patient – near three different probing depths. The samples were stored at −80 °C until analysis. After the samples’ defrosting and the homogenization of the tissue (rotor-stator homogenizer), the laboratory procedures were performed in the following order:
RNA was isolated by mi-Total RNA Isolation Kit (mi-CZ100, Metabion) according to the manufacturer’s instructions. Using 500 μL of mi-Total reagent for extraction, the RNA pellet was resuspended with 30 μL DEPC-treated (diethylpyrocarbonate) water.
Reverse transcription was performed using the First Strand cDNA Synthesis Kit (K1612, Thermo Scientific) according to the manufacturer’s instructions using 10 μL RNA and oligo- (dT) 18 primer.
Amplification was made by using Mastercycler Relex2 (Eppendorf) with primers and probes - Custom designed real-time PCR assay with Double-Dye probe (Primerdesign) for each gene, and Mastermix for Real-time PCR Maxima Probe qPCR Master Mix (2X) (K0261, Thermo Scientific), using the volumes and conditions for the IL-6 and TNF gene (according to the manufacturer Primerdesign).
Statistical analysis
We used descriptive analysis, Fisher test and Shapiro-Wilk test. SPSS statistical program was used to analyze the data (ANOVA-IBM SPSS Statistic Version 19). Differences were considered statistically significant at the p < 0.05 level. Student’s t-test was used to establish significant differences between the individual indicators, and in case of abnormal distribution and/or lack of equality of variances - Mann-Whitney U-test. A paired t-test was used to establish significant differences between dCt and ddCt of IL6 and TNF at different probing depths. The dCt is related with the comparison of the Ct of the detected gene and the Ct of a referent gene, ACTB (β-actin), where the Ct value is the cycle threshold, i.e. a relative measure of the concentration of the target mRNA. The ddCt compares two different dCts of each assayed gene.
Results
In our study, the impact of smoking on the severity of periodontitis was assessed using main clinical parameters such as periodontal pocket depth and clinical attachment loss. The research included 20 patients of a mean age of 50.25 ± 8.45 years with periodontitis. The patients were divided into two groups: group I (smokers, n = 12; 5 men and 7 women) and group II (non-smokers, n = 8; 3 men and 5 women). The average age of the participants in the study was 47.55 ± 6.42 years.
The data obtained in this study demonstrated higher values for the clinical attachment loss and periodontal pocket depth in the presence of smoking compared with non-smokers ().
Table 1. Comparative analysis of pocket depth and attachment loss in smokers and non-smokers.
The results presented in show that smoking had an effect with marginal significance (p < 0.1) for the parameter periodontal probing depth (average value in mm). The presence of the smoking factor was associated with higher values of the main studied parameters related to the severity of periodontitis - probing depth and attachment loss.
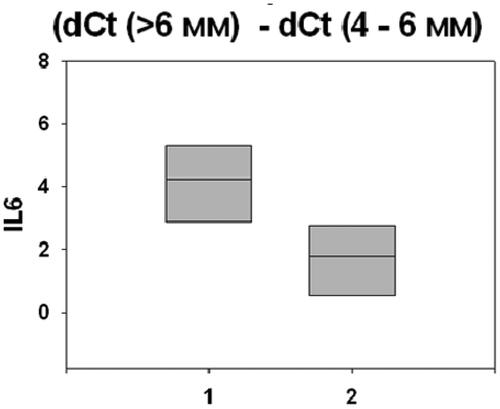
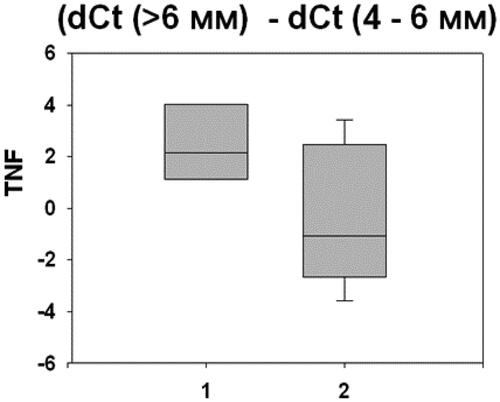
In relation to the objectives of the study, we investigated the effect of smoking on the gene expression levels of two important biological mediators of inflammation - Interleukin 6 (IL-6) and Tumor necrosis factor alpha (TNF-α). The outcomes of this research () showed that the smoking factor concerned deeper periodontal sites.
Table 2. Comparison between gene expression levels and smoking.
Our results demonstrate that smoking was an important risk factor and greatly influenced the studied gene expression levels. Smoking was associated with altered gene expression mainly in conjunction with deep (>6mm) periodontal pockets. There were statistically significant differences between different pocket depths: (> 6 mm) and (4–6 mm), and (> 6 mm) and (<4mm). and present the statistically signifficant differences of the gene expressions of IL-6 and TNF-α in the studied patients with periodontitis (p < 0.05).
Discussion
The main important risk factors for periodontitis cited in literature data are age, gender, socioeconomic status, smoking, genetic susceptibility, periodontal bacteria, several systemic conditions. Referred literature shows smoking as a main risk factor associated with destructive periodontal disease [Citation2,Citation4,Citation6,Citation10].
Some studies have found a relationship between the effect of smoking and periodontal attachment loss. Zambon et al. [Citation11] in 1997, demonstrated that heavy smokers were more than twice affected by attachment loss and alveolar bone destruction compared with light smokers. Machuca et al. [Citation12] reported also increased attachment loss in a young male population - heavier smokers.
Heasman et al. [Citation13] show that the cigarette smoke contains more than 4,000 different toxic substances:
related to periodontal pathogens like Porphyromonas gingivalis and Treponema denticola;
causing peripheral vasoconstriction and, consequently, reduced bleeding on probing in smokers;
reduced oxygen saturation in the periodontal pockets and favorable growth of anaerobic bacteria;
damaging the neutrophil function, affecting the granulocyte degranulation, and the secretion of elevated levels of TNF-α;
increased cytokine expression (IL-1ß);
diminished healing processes.
Authors [Citation13] studied the association between tobacco smoking and periodontal bone height as well as the assessment of oral hygiene, gingival inflammation and probing depth. The association between smoking and probing depth that they found was statistically significant (p < 0.001) [Citation13]. Moreover, there was shown an association between lifetime smoking exposure and mean probing depth (p < 0.001). Authors suggest that the relative risk for periodontal disease increases by 3.8-fold in cigarette smokers, compared to non-smokers (p < 0.05) [Citation14].
Bergström [Citation15] suggests that the main features of smoking-associated periodontal disease are: destruction of tooth-supporting tissues, clinical attachment loss, bone loss, periodontal pocket formation and, finally, tooth loss.
As can be seen in and , the results obtained in our study are in agreement with the results and experience of recent researches that show a strong relation of smoking as a risk factor with the destructive periodontal disease.
Many studies present controversial data on the potential role of smoking in regard to the action of inflammatory mediators. Smokers were found to have a lower response to periodontal treatment than non-smokers [Citation5,Citation11,Citation16–19]. The presence of the smoking factor may influent negatively the prognosis of the disease. Therefore, smoking cessation in mild and moderate periodontitis could improve the prognosis of periodontitis. Authors show also that smoking influences the response to treatment, and smokers are more resistant to conventional periodontal therapy. Furthermore, implant failure and risk of peri-implantitis are higher among smokers [Citation20].
Several inflammatory biomarkers present in saliva, gingival crevicular fluid, blood serum and gingival tissue were found to be associated with periodontitis in smokers [Citation21–24]. Literature data show that both smoking and gene expression profiles may reflect and increase the individual sensitivity of periodontitis patients. Studies showed elevated levels of IL-6 and TNF gene expression in smokers with periodontitis, but lower levels of IL-4 and IL-10 gene expression [Citation25–30]. TNFα was significantly associated with the presence of smoking. However, there was or was not an association between smoking and IL-6 levels [Citation25]. Authors demonstrated 830 significantly (p < 0.05) differentially expressed genes, including genes encoding interleukins, proteins, prostanoid synthesis enzymes and receptors, matrix metalloproteinases and oxygen radical scavengers. [Citation21,Citation25]. They concluded that smoking seems to affect important genes in relation with immune defense and immune response, and thus, may lead to an increased periodontitis sensitivity. Recently, Gholami et al. [Citation31] suggested that smoking effects a number of biologic mediators, immune/inflammatory cells, immunoglobulins, and can modify the host response and the treatment outcomes due to the changed connective tissue and bone metabolism [Citation31]. Moreover, Sayad et al. [Citation32] assessed cytokine coding genes (IL-1α, IL-1β, IL-6, IL-10, TNFα) and found these genes to be associated with the risk of destructive periodontal disease.
Both biomarkers, IL-6 and TNF-α, were detected to be elevated (p < 0.001) in smokers when compared with non-smokers [Citation28]. Furthermore, Elisia et al. [Citation33] also showed an association of smoking with the elevated levels of IL-6 in plasma simples in 30 periodontitis patients (p = 0.049).
In their research, Ojiima and Hanioka [Citation25] found that the relationships ‘genes–smoking’ are suggestive and based on different pathways: microcirculatory and host immune systems, connective tissue and bone metabolism [Citation25]. Smoking can affect indirectly the periodontitis status by relative gene expression changes. It is considered that the differences between the results reported in different studies evaluating the smoking/periodontitis outcomes, can be explained by changes in gene expression caused by other gene–related variations and/or individual situations (stress factors, gender, age, social factors, behavior, duration of smoking habit, and others). We agree with other authors who noted that smoking may affect negatively the healing potential of periodontal wounds and may also accelerate the periodontal destruction [Citation22] through its effects on host reactivity.
Cho et al. [Citation22], similar to our study, assessed the interleukins’ gene expression in only 20 periodontitis patients (10 smokers and 10 non-smokers). They also used gingival biopsies and obtained results similar to ours (p < 0.05) [Citation22]. A limitation of our study is the small sample size (only 20 periodontitis subjects). Because of this difficulty, the results of our study should be interpreted carefully and preferably as а tendency. However, further studies with a larger sample size are needed to validate the data.
Conclusions
The results of this study confirm previous literature data on the importance of smoking as an environmental risk factor in periodontitis. There was evidence that smoking has an influence on the expression levels of the Il-6 and TNF-α genes in gingival tissues. It is important for clinical practice to inform patients that smoking affects both the severity of periodontitis and the healing potential of periodontal tissues.
Disclosure statement
No potential conflict of interest was reported by the authors.
Data availability statement
The data that support the findings of this study are available upon reasonable request from the authors.
Funding
The author(s) reported there is no funding associated with the work featured in this article.
References
- Albandar JM. Global risk factors and risk indicators for periodontal disease. Periodontol 2000. 2002;29:177–206. [PubMed], [Google Scholar]
- Tonetti MS. Cigarette smoking and periodontal diseases: etiology and management of disease. Ann Periodontol. 1998;3(1):88–101. [Google Scholar]
- Calsina G, Ramón JM, Echeverría JJ. Effects of smoking on periodontal tissues. J Clin Periodontol. 2002;29(8):771–776. [PubMed], [Google Scholar]
- Genco JR, Borgnakke WS. Risk factors for periodontal disease. Periodontol 2000. 2013;62(1):59–94. [Crossref], [PubMed], [Web of Science ®], [Google Scholar]
- Schenkein HA, Gunsolley JC, Koertge TE, et al. Smoking and its effects on early-onset periodontitis. J Am Dent Assoc. 1995;126(8):1107–1113. PMID: 7560567. [Google Scholar]
- Tangada SD, Califano IV, Nakashima K, et al. The effect of smoking on serum IgG2 reactive with actinobacillus actino- mycetemcomitans in early-onset periodontitis patients. J Periodontol. 1997;68(9):842–850. [PubMed], [Google Scholar]
- Barbour SE, Nakashima K, Zhang JB, et al. Tobacco and smoking: environmental factors that modify the host response (immune system) and have an impact on periodontal health. Crit Rev Oral Biol Med. 1997;8(4):437–460. [Google Scholar]
- Kumar V, Faizuddin M. Effect of smoking on gingival microvasculature: a histological study. J Indian Soc Periodontol. 2011;15(4):344–348. [PubMed]
- Schenkein H. Host responses in maintaining periodontal health and determining periodontal disease. Periodontol 2000. 2006;40:77–93. [PubMed], [Google Scholar]
- Jepsen S, Caton JG, Albandar JM, et al. Periodontal manifestations of systemic diseases and developmental and acquired conditions: consensus report of workgroup 3 of the 2017 world workshop on the classification of periodontal and peri-implant diseases and conditions. J Periodontol. 2018;45(Suppl 20):219–229. [Crossref], [PubMed], [Web of Science ®], [Google Scholar]
- Zambon JJ, Grossi S, Machtei EE, et al. Cigarette smoking increases the risk for subgingival infection with periodontal pathogens. J Periodontol. 1996;67(10s):1050–1054. [PubMed] [Google Scholar]
- Machuca G, Rosales I, Lacalle JR, et al. Effect of cigarette smoking on periodontal status of healthy young adults. J Periodontol. 2000;71(1):73–78. https://doi.org/10.1902/jop.2000.71.1.73[Crossref]. [PubMed], [Web of Science ®], [Google Scholar]
- Heasman L, Stacey F, Preshaw PM, et al. The effect of smoking on periodontal treatment response: a review of clinical evidence. J Clin Periodontol. 2006;33(4):241–253. [PubMed], [Google Scholar]
- Natto S, Baljoon M, Bergström J. Tobacco smoking and periodontal health in a saudi arabian population. J Periodontol. 2005;76(11):1919–1926. [Crossref], [PubMed], [Web of Science ®], [Google Scholar]
- Bergström J. Tobacco smoking and chronic destructive periodontal disease. Odontology. 2004;92(1):1–8.
- Freitag-Wolf S, Munz M, Wiehe R, et al. Smoking modifies the genetic risk for early-onset periodontitis. J Dent Res. 2019;98(12):1332–1339. [PubMed], [Google Scholar]
- Schwendicke F, Dörfer CE, Meier T. Global smoking-attributable burden of periodontal disease in 186 countries in the year 2015. J Clin Periodontol. 2018;45(1):2–14. [Crossref], [PubMed], [Web of Science ®], [Google Scholar]
- Leite FRM, Nascimento GG, Scheutz F, et al. Effect of smoking on periodontitis: a systematic review and Meta-regression. Am J Prev Med. 2018;54(6):831–841.
- Palmer RM, Wilson RF, Hasan AS, et al. Mechanisms of action of environmental factors – tobacco smoking. J Clin Periodontol. 2005;32(s6):180–195. [Crossref], [PubMed], [Web of Science ®], [Google Scholar]
- Selva Süme Keşir S, Ebru Olgun H. Smoking and periodontal health. Curr Oral Health Rep. 2018;5(1):50–62. https://doi.org/10.1007/s40496-018-0170-6. [PubMed], [Google Scholar]
- Wang Y, Anderson EP, Tatakis DN. Whole transcriptome analysis of smoker palatal mucosa identifies multiple downregulated innate immunity genes. J Periodontol. 2020;91(6):756–766. https://doi.org/10.1002/JPER.19-0467.
- Cho YD, Kim PJ, Kim HG, et al. Transcriptomics and methylomics in chronic periodontitis with tobacco use: a pilot study. Clin Epigenet. 2017;9:81. https://doi.org/10.1186/s13148-017-0381-z.
- Heikkinen AM, Mäntylä P, Leppilahti J, et al. Oral fluid biomarkers in smoking periodontitis patients and systemic inflammation. In (Mandeep Singh V, editor. Emerging trends in oral health sciences and dentistry. Croatia: IntechOpen; 2015. https://doi.org/10.5772/59813.
- Haytural O, Yaman D, Ural EC, et al. Impact of periodontitis on chemokines in smokers. Clin Oral Investig. 2015;19(5):979–986.
- Ojima M, Hanioka T. Destructive effects of smoking on molecular and genetic factors of periodontal disease. Tob Induc Dis. 2010;8(1):4.
- Blanco-Pintos T, Regueira-Iglesias A, Balsa-Castro C, et al. Update on the role of cytokines as oral biomarkers in the diagnosis of periodontitis. Adv Exp Med Biol. 2022;1373:283–302. PMID: 35612804.
- Noh MK, Jung M, Kim SH, et al. Assessment of IL-6, IL-8 and TNF-α levels in the gingival tissue of patients with periodontitis. Exp Ther Med. 2013;6(3):847–851. Epub 2013 Jul 15. PMID: 24137277; PMCID: PMC3786859.
- Al-tameemi S, Hameed N, Gomes K, et al. Cigarette smoking increases plasma levels of IL-6 and TNF-α. Baghdad J Biochem Appl Biol Sci. 2022;3(01):60–68. https://doi.org/10.47419/bjbabs.v3i01.108.
- Boström L, Linder LE, Bergström J. Clinical expression of TNF-alpha in smoking-associated periodontal disease. J Clin Periodontol. 1998;25(10):767–773.
- Giannopoulou C, Kamma JJ, Mombelli A. Effect of inflammation, smoking and stress on gingival crevicular fluid cytokine level. J Clin Periodontol. 2003;30(2):145–153.
- Gholami L, Badrlou E, Nazer N, et al. Assessment of expression of a number of immune-related genes in the periodontitis. Ecol Genet Genom. 2022;22:100106.
- Sayad A, Gholami L, Mirzajani S, et al. Genetic susceptibility for periodontitis with special focus on immune-related genes: a concise review. Gene Rep. 2020;21:100814.
- Elisia I, Lam V, Cho B, et al. The effect of smoking on chronic inflammation, immune function and blood cell composition. Sci Rep. 2020;10(1):19480. https://doi.org/10.1038/s41598-020-76556-7.