ABSTRACT
The mega-tsunami generated by the Great East Japan Earthquake on 11 March 2011 devastated coastal forests on the Pacific seaboard in northeastern Japan, mainly composed of Pinus thunbergii and P. densiflora. After the tsunami hit, decline and resulting death occurred in the remaining pine trees, which arouse concern about the availability of pine trees in restoring the devastated coastal forests, although the extent and factors of the tree decline were unclear. We monitored the decline and death of P. thunbergii and P. densiflora trees individually at the study plots set in six locations showing different types of tsunami damage for two years. During the monitoring period, mature P. thunbergii trees showed good survival, suggesting their resistance to the tsunami damage, unless they seemed to suffer from strong stress from shading before the disaster, physical damage by the tsunami or poor drainage condition of the soil. By contrast, P. densiflora was more vulnerable to seawater inundation. From those results, we could present aconcrete argument for the relevance of P. thunbergii for use in coastal plantations, as is historically approved in Japan, and the importance of securing drainage to make coastal forests more resistant to tsunami damage.
Introduction
A violent earthquake measuring 9.0 on the Richter scale, named the Great East Japan Earthquake, hit the Japanese Pacific Northeast (Tohoku District) on 11 March 2011. The mega-tsunami following this earthquake caused catastrophic damage to the coastal forests on the Pacific coast in northeastern Japan (Hoshino Citation2012), as well as massive devastation and loss of life to the area. Japanese coastal forests, particularly those established on sand dunes, are dominated by the Japanese black pine (Pinus thunbergii Parl.), mostly originating from the plantation for wind- and sand-break (Honda Citation1919; Murai et al. Citation1992). The Japanese red pine (P. densiflora Sieb. et Zucc.) is widely distributed in secondary forests in Japanese suburban and rural areas (Yoshioka Citation1948; Nakagoshi Citation1995) but is also common on the Pacific seaboard in northeastern Japan (Sato Citation1961). This species often forms a pure stand on coastal cliffs or co-occur with P. thunbergii in dune forests. Because these pine species are dominant components of coastal forests in the disaster area, the tsunami damage in coastal forests can be regarded as that to pine trees.
The tsunami damage in coastal forests varied depending on the location (Nakamura et al. Citation2012; Sakamoto Citation2015); trees were mostly broken off or washed out from the roots and the land became almost bare at some pocket beaches in the Ria shoreline area; uprooted pine trees with shallow root systems resulting from the high groundwater level (Tamura Citation2012; Noguchi et al. Citation2014) were drifted out in lower parts of the alluvial plain; frontline pine trees, mostly small and with arelatively flexible stem either fell or became slanted under the water pressure; or the tsunami flowed through hinterland forests and trees remained there although some were inflicted wound(s) by drifting objects. In some cases, tree roots submerged in seawater, due to ground subsidence and destruction of seawalls. Consequently, there were not a few pine trees remaining in the tsunami-impacted area, including those leaning, injured, or inundated, which kept their green foliage after the tsunami. In such pine trees, however, the signs of decline like droop and/or discoloration of the foliage became visible subsequently, sometimes followed by the death of the tree (Nakamura et al. Citation2012; Kimura Citation2014). The extensive damage in the seacoast pine forests after the tsunami disaster aroused adubiety that pine trees may not suitable to use in coastal plantations. However, we had had little information about the response of the trees under a severe condition brought by an infrequently occurring mega-tsunami, and thus not prepared to evaluate the tolerance/resistance of a tree species to such an exceptional event appropriately.
To clarify the effect of tsunami damage on pine trees in coastal forests, we conducted a 2-year monitoring survey in the study plots set at different areas, to cover the different types of tsunami damage. In this article, we presented the occurrence pattern of decline and mortality in P. thunbergii and P. densiflora trees under various situations brought by the tsunami, and the contributing factor of the difference in the occurrence pattern was explored. Then we present the proper uses of pine trees in coastal plantations with consideration for the resistance to tsunami-disaster.
Materials and methods
Study plots
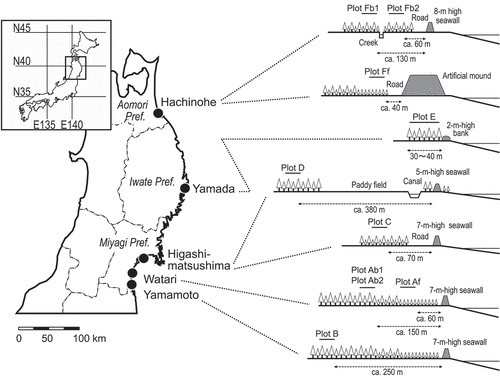
Study plots used to monitor the decline of P. thunbergii and P. densiflora trees were established in six locations within the tsunami-impacted area (, ) from May to June in 2011. Each plot included about 100 pine trees in a rectangular section in the stand except for the two plots (plots E and Fb2, as described below) where an irregular polygonal section was set because of the tree distribution after the tsunami damage.
Table 1. Description of the study plots and outline of the pine tree populations
Watari and Yamamoto were located on the coastline of the Sendai Plane in Miyagi Prefecture (), where the tsunami measured more than 8 m (The 2011 Tohoku Earthquake Tsunami Joint Survey Group Citation2011-2019), reaching up to 7 km inland behind from the shoreline. In Watari, a set of plots of frontline and hinterland pine stands was established. The frontline plot was set in the P. thunbergii stand 60 m away from the 7-m seawall in height (plot Af; ). In this plot, most trees had either felled or were leaning due to the water pressure caused by the tsunami, as was commonly seen in the frontline pine forest in this area (Sakamoto Citation2015). Plot Ab1 was set in the P. thunbergii forest 150 m inland from plot Af, mixed with a small number of P.densiflora (). The tsunami flowed through the stand and pushed down smaller-sized trees but the canopy trees were mostly intact. Due to the construction of a rubble-disposing facility for the earthquake disaster reconstruction, this section of the pine forest was destroyed before November2011 (). Therefore, we added a study plot in the adjacent stand in June 2012 (plot Ab2; ). Although the stand lacked the layer of small-sized pine trees, the size and density of the canopy trees were very similar to plot Ab1. In Yamamoto, we set astudy plot in a mixed stand of P.thunbergii and P.densiflora, which was 250 m away from the 7-m seawall in height (plot B; ).
Plots C and D were both located in Higashi-Matsushima, Miyagi Prefecture (). In this area, the tsunami measured 8–9 m (The 2011 Tohoku Earthquake Tsunami Joint Survey Group Citation2011-2019) and flooded over the 1–3km width of lowland between the sea and hills. Plot C was set in a ca. 100-m-wide tree belt composed of middle-sized P. thunbergii behind a 7-m seawall in height along the shoreline (). The tsunami broke the trees in the front half of the stand, but the latter part (where we set the study plot) was less damaged. Plot D was set in a mature P. densiflora stand with understory broad-leaved trees such as Prunus lannesiana (Carr.) Wils. and Morus bombycis Koidz. There was a 5-m seawall in height with thin belts of P. thunbergii on both sides, water canal, and paddies of ca. 100m in width between the seashore and the pine forest (). The tsunami flowed through the stand, breaking down broad-leaved trees in the process, while large P.densiflora trees remained standing.
Plot E was located in an isolated P. thunbergii stand () at the closed-off section of abay in Yamada, Iwate Prefecture (). The tsunami in this area was 14.5-mhigh (The 2011 Tohoku Earthquake Tsunami Joint Survey Group Citation2011-2019), partly destroying the 2-m-high bank in front of the stand () and breaking branches and stems of pine trees or felling them from the roots. The forest floor was partly excavated and some of the holes were filled by seawater flowing from the broken parts of the bank due to ground subsidence. As the stand was isolated and the tsunami damage was not uniformly distributed, we regarded the whole stand as a single study plot, and this made the number of trees to be monitored greater than the other plots ().
Another tsunami-damaged P. thunbergii forest composed of different aged stands was located near a fishing port in Hachinohe, Aomori Prefecture (). Although the area was protected by an 8-m high seawall in height or a 9.5–12.5-m high artificial mound on the shoreline (; Sakamoto etal. Citation2012), the 7–8 m tsunami rushed from the port (The 2011 Tohoku Earthquake Tsunami Joint Survey Group Citation2011-2019) and pushed down the pine trees around there. In this area, we initially set up two study plots (); one in a small-sized P. thunbergii stand just behind the artificial mound (plot Ff; ) and the other in a middle-sized stand 130 m away from the seawall (plot Fb; ). Both stands were located out of the tsunami-devastated area, hence few trees had felled or were leaning. While adecline in pine trees in plot Fb became evident several months after the tsunami, it was not apparent in the surrounding stands. Thus, we set an additional study plot in the vicinity of plot Fb1 in November2011 (plot Fb2; ), to compare the prevalence of tree decline in P. thunbergii in the proximity locations.
Field survey
We recorded species, DBH, suppression condition (canopy– shaded), the status of the trees after the tsunami, occurrence of a newly-deposited sand layer at the root, and foliage discoloration for each P. thunbergii and P. densiflora tree exceeding a height of 2 m in the study plots in mid-May through early July 2011, or 2–4 months after the tsunami. Excavation and/or seawater submergence at the root was also recorded in plot E. In the additional plots (Ab2 and Fb2), the same procedure was performed at the time of plot establishment, July 2012 and November 2011, respectively (). When some of the trees were broken and lost their trunk, we estimated the DBHs of such trees using correlation of the diameters at trunk base and chest height of the remaining trees in the plot concerned. The status of the trees after the tsunami was categorized as follows: broken at the trunk, felled or leaning, and standing. Foliage discoloration was measured as the percentage of foliage that had turned yellow, reddish-brown, or gray-brown. When a tree sheds its leaves after complete foliage discoloration, it was classified as “defoliated”. Trees that had died before the tsunami were omitted from the data, and those broken at the upper part of the trunk and lost the crown were counted in the first census but were discarded in the following survey(s).
In some of the plots, a substantial number of pine trees were injured on their trunk by collisions with drifting objects. When scars were found on the trunk, we counted those with the longest span over 10 cm (major injury) or less (minor injury), separately, up to 2 m above ground. Then, we tentatively classified the trees as “severe” (with one or more major injuries or more than five minor injuries), “light” (with less than for minor injuries) and “none” regarding damage level of trunk injury.
We repeated the survey for foliage discoloration three times; the first from late-September to mid-October 2011, the second from mid-June to mid-July, and the third from late October to early November2012. When a tree showed 100% foliage discoloration and did not recover throughout the monitoring period, the death of the tree was recorded at the time when complete discoloration was first observed. We could not complete the census for some trees in plots Af and C (), due to the post-quake reconstruction. Most of the trees were removed in plots D and E before the summer 2012 and the stand was completely demolished in plot Ab1 in December2011 (), which undermined the consistency of monitoring efforts (). We basically estimated the tree mortality in each plot as the percentage of dead trees in the final census time in autumn 2012, but had to use the data taken in autumn 2011 for plots Ab1, D and E, because of the destruction of pine forest before 2012 ().
Pinewood nematode infections
Pine tree mortality from pine wilt disease is very common in Japan (Futai Citation2008); it thus needs to be estimated to avoid wrongly attribute tsunami-induced tree decline. This disease is a contagious disease of pine trees caused by the pinewood nematode, Bursaphelenchus xylophilus Steiner et Buhrer (Kiyohara and Tokushige Citation1971; Mamiya Citation1983), that is vectored by cerambycid beetles of the genus Monochamus (Akbulut and Stamps Citation2012; Togashi Citation2008).
We examined infections of B.xylophilus in the dead trees in the study plots, which were located within the known distribution range of pine wilt disease in Japan (Forest Conservation Departmental Meeting of Tohoku Forestry Research Institute Liaison Council Citation2014). When atree showed systemic foliage discoloration, we collected ca. 5 g of wood chips from the trunk using a battery-powered drill with a blade of 15 mm in diameter. We tried to obtain wood samples from all of the dead trees in each plot, however, as some trees had already been cut for the post-quake reconstruction, we were unable to do so. As there were too many dead trees in plot Af during the first census to handle them in time, we arbitrarily selected about one-third of the trees and obtained wood samples from them. The wood sample was placed in a sealed plastic bag and kept at 5°C until examined.
A commercial kit for detecting B.xylophilus (Nippon Gene Co. Ltd.) (Aikawa et al. Citation2010), based on a highly sensitive detection method using the LAMP technique (Kikuchi et al. Citation2009), was adopted to examine the wood samples collected. Two semicircular-shaped wood chips per sample were used for a test. When the result was negative, the sample was examined again. If the result was positive in the first or second trial, the tree was considered to have been infected by B.xylophilus.
Statistical analysis
As regards the mortalities of standing trees, that is the trees showing no apparent structural damage such as broken, felled, and leaning, the contribution of the possible explanatory factors was assessed using generalized linear models (GLMs). As described in the “Study plots” section, site conditions and the mode of tsunami damage materia1ly differed among the plots. Hence we could not construct a model from the pooled data of all of the plots. Alternatively, we grouped the plots (the additional plots, Ab2 and Fb2, were excluded in this analysis) according to the location, pine tree population, site condition, and tsunami damage, to access the generalized trend among the plots. The groups of the plots were Ab1 + B, C+ D, E, Ff, and Fb. Plot Af was not chosen because there was not enough number of standing trees remained ().
Table 2. Pine tree mortality with reference to the status of the trees and their species in the plots set in the first census time after the tsunami disaster
In the models, tree mortality (dead or live) was used as the response variable. Tree mortality was estimated from the final census data taken in autumn 2012 in plots Ff and Fb, and those in autumn 2011 in the other (groups of) plots, as the plots Ab1, Dand E were demolished before 2012 (). The explanatory variables were selected from the followings according to the actual conditions of the plots; tree species, DBH, suppression status (shaded or not), trunk injury (heavy, light, none), sand deposition (exist or not), excavation at the root (exist or not) and seawater submergence of the root (exist or not) (). The trees died of B. xylophilus infection and those we could not determine the suppression status because of the broken trunk or branch in the crown were discarded in the analysis. Given that the response variable followed a binomial distribution, we used a log-link function. Multicollinearity in the factors in each plot was assessed using generalized variance inflation factor (GVIF). A stepwise variable reduction procedure was conducted for each of the initially constructed models (full models) to obtain the minimal model.
Table 3. Detection of Bursaphelenchus xylophilus in declined or dead pine trees
Table 4. Factors potentially contributing to the death in the trees remained standing after the tsunami disaster in each plot
All statistical analysis was performed by Rsoftware (Ver.3.5.3).
Results
Patterns in tree decline
The patterns in tree decline, that is expressed as the temporal change of the number of trees with discolored foliage and the mortality of the trees, showed a considerable difference among the plots (, ).
Figure 2. Development of foliage discoloration of the pine trees in each study plot. Trees were classified as follows: 
 < 50% foliage discolored;
< 50% foliage discolored;  50–99% foliage discolored;
50–99% foliage discolored;  100% foliage discolored;
100% foliage discolored;  defoliated. Letters following the stand name on each graph indicate the stand type: Pt; Pinus thunbergii-dominated stand, Pd; P. densiflora-dominated stand, mixed: mixed stand of P. thunbergii and P. densiflora.
defoliated. Letters following the stand name on each graph indicate the stand type: Pt; Pinus thunbergii-dominated stand, Pd; P. densiflora-dominated stand, mixed: mixed stand of P. thunbergii and P. densiflora.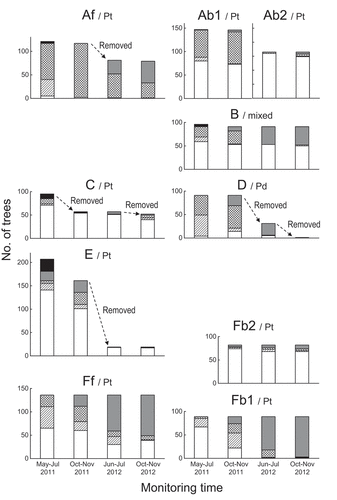
In plot Af, consisted of small trees of P. thunbergii (), most of the trees showed foliage discoloration in June 2011, regardless of tree status (felled/leaning and standing) ((Af)). Although some trees retained a small amount of green foliage and/or had newly developed shoots at the top of branches, most reached complete foliage discoloration by the end of summer 2011. About one-third of the trees in this plot were cut and removed relating to the post-quake reconstructions ((Af)). Tree mortalities in the remaining trees at the final census time (November 2012) accounted for 100% in the felled/leaning trees and 93% in the standing ones (). In plots Ab1 and B, both mature stands of P. thunbergii mixed with P. densiflora and located at the hinterland of the coastal forest, smaller trees were tended to be broken or felled by the tsunami, and resultantly died (). On the other hand, a substantial number of standing trees remained alive until the end of the census period (, (Ab1,B)e). Among them, the tree mortality was greater in P. densiflora than in P. thunbergii in plot B, set in the only P. thunbergii stand mixed with a substantial number of P. densiflora (). In plot Ab2, the average DBH was thicker than in plot Ab1 (), probably because smaller trees had died directly after the tsunami and eliminated before the setting of the plot, and the tree decline hardly progressed ((Ab2)).
In plot C, set in a pure P. thunbergiistand just behind the seawall,10 trees were broken and defoliated while 14 had discolored foliage during the first census (). These trees and some of the adjacent trees were cut and removed from the stand before the second census in September2011. Foliage discoloration in the remaining trees (all standing trees) did not develop sharply till the end of the monitoring (). In contrast, plot D was situated in a P. densiflora dominated stand and almost all of the trees showed foliage discoloration during the first census in June 2011, while more than half of those were spared from complete discoloration (). As a whole, foliage discoloration and resulting defoliation in those trees had developed by the end of summer 2011, but some of the trees either recovered or kept their status (). The stand was demolished by the post-quake reconstruction activity, which prevented further monitoring.
In plot E, the tsunami destroyed or uprooted 26 out of the 207 P. thunbergii trees (). Foliage discoloration in the remaining trees was not prevalent in June 2011 but moderately developed () and 89% of the felled or leaning trees was died by October 2011 (). Most of the trees were cut and removed by the government for the post-quake reconstruction before June 2012, and there were only 19 trees remained in the plot ().
Plot Ff was composed of small-sized P. thunbergii trees () but has no trees felled or leaning (), probably because the artificial mound in front of the stand () intercepted the direct impact of the wave. Foliage discoloration was observed in more than half of the trees during the first census in May 2011, and developed through July 2012 ((Ff)). Trees with less severely discolored foliage in July 2012 recovered by October 2012. Finally, 70.6% of the trees died (). The foliage discoloration in a mature P. thunbergii stand, plot Fb1, looked not so severe during the first census in May 2011; we only found 13 trees with severely discolored foliage and 25 with slight discoloration among the 89 monitored trees ((Fb1)). The number of declining trees progressively increased throughout the monitoring period and most trees were defoliated during the final census in October2012 (). In the adjacent plot Fb2, the prevalence of tree decline was completely different; foliage discoloration was found in only 10–23% of the trees throughout the monitoring period, and 67 out of 80 trees had green foliage by the final census in October 2012 ((Fb2)).
Pinewood nematode infection
Plots E, Ff, Fb1, and Fb2 were out of the range of known distribution of B.xylophilus (Forest Conservation Departmental Meeting of Tohoku Forestry Research Institute Liaison Council Citation2014), thus we did not obtain samples for detecting B. xylophilus.
No or only a few trees were found to be infected by B.xylophilus in the plots except for plot C where the detected trees accounted for as high as 64% (), indicating that pine wilt disease was not a primary factor for the observed tree decline and mortality in the tsunami-impacted pine forests. In plot C, however, we confirmed B. xylophilus infection in nine trees found to be dead in September2011. There were dead or dying trees of P. densiflora sparsely distributed on the hills where trees could have escaped from the direct impact of the tsunami, suggesting that pine wilt disease had been spreading in this area before the tsunami hit.
Factors relating to the tree mortality
The potential factors contributing to the death of the trees that remained standing after the tsunami were listed in . Among the listed factors, trunk injury did not occur in Af1, Ab2, Ff and Fb1. On the other hand, root excavation and root submergence were only found in plot E. In some of the plots (Af1, D, Ff and Fb1), sand deposition was recorded in all trees. In the grouping of the plots for the GLM, factor(s) showing no difference in the combined data was eliminated in constructing the full model.
We regarded the explanatory factors adopted in GLMs for each plot did not correlate with each other, as the GVIFs for the factors were small enough (less than 3.0). The absolute value of a coefficient in the GLM reflexes the relative power of the effect of the factor. In plots Ab1 + B and C+ D, where P. thunbergii and P. densiflora were included, factor “tree species” was selected in the minimal model and with moderate to high values of coefficients (2.03 and 20.74, respectively) (). Factors “DBH” and “suppression” were also selected in the model for Ab1 + B, but the coefficient value was moderate (1.50 for “suppression”) or low (0.006 for “DBH”). In plot E located at the violently damaged stand, the factor “trunk injury”, “root excavation” and “root submergence” was selected in the model. Among them, “root submergence” showed the highest value of coefficient (3.11) (). Factors “DBH” and “suppression” had little or moderate effect in the model. On the other hand, the effect of the selected factors (“DBH” and “suppression”) was not clear in plot Ff and no factor was selected in plot Fb1 (), suggesting that tree mortalities observed in those plots were hardly explained by the factors adopted in the analysis.
Table 5. The generalized linear models (GLMs) for explaining the mortalities found in the remaining standing pine trees in the tsunami-damaged stands
Discussion
Patterns in the occurrence of tree decline in the tsunami-affected trees
There are numerous reports on the damage in the coastal forest brought by tsunamis, and we also have a certain degree of knowledge on the tolerance of trees to seawater inundation both empirically (Honda Citation1919) and through experimental facts (Horie Citation1966; Oda Citation1991; Tateishi et al. Citation2014). Also in the case of the 2011 tsunami, the occurrence of decline and/or death in the pine trees in the early survey of the damaged coastal forest was reported by Nakamura et al. (Citation2012) and Kimura (Citation2014). However, the chronic change of the tsunami-affected trees, that can be translated as the realistic tolerance of the trees to tsunami event, has been hardly explored. In this study, we presented a detailed description of the occurrence of tree decline and resulting death in coastal pine forests under the various situations after the 2011 tsunami disaster by way of the 2-year monitoring covering different types of tsunami damage. Moreover, the contribution of the multiple factors to tree mortalities was estimated in the analysis using GLM.
The patterns of tree decline showed agreat difference among the study plots (), but we did find some common trends. Small trees of P. thunbergii were apt to felled or leaning by the water pressure, especially when they grew in a frontline coastal forest (Af), and died shortly after the disaster (, (Af)). In the standing trees in the groups of the plots Ab1 + B and C+ D, species had a major effect on mortality (), namely P. densiflora was more vulnerable to tsunami damage than P. thunbergii. The low tolerance of P. densiflora to seawater inundation has been confirmed under experimental conditions (Takahashi and Horie Citation1965; Oda Citation1991; Tateishi et al. Citation2014), and our result evidenced it in mature trees under field conditions. Although the majority of P. densiflora trees became dead by autumn in 2011, many of them showed a moderate degree of foliage discoloration in the first census time and some of the trees had recovered from the declined status (). Thus, we consider the decline in P. densiflora underwent seawater inundation developed rather chronically, unlike P. thunbergii trees with structural damage by the power of tsunami observed in plot Af. The destructive effect of the tsunami on the forest floor like root excavation and submergence was only recorded in plot E, and such factors were shown to have some contribution to tree mortality (). A moderate effect of trunk injury was also detected only in this plot (), thus we can consider the trunk injury of the levels observed in this study did not have a fatal effect but accelerated tree decline in co-acting with the other factor(s). Suppression showed a moderate effect in plots Ab1 + B and E, suggesting that it would be a predisposition of the trees to the strong stress brought by the tsunami. On the other hand, the effect of tree size (DBH) was not detected in the analysis using GLMs.
High mortalities of P. thunbergii trees were recorded in Ff and Fb1 (), although the patterns of tree decline differed from each other ((Ff, Fb1)). The GLMs did not designate eminent factor(s) contributing to the tree mortalities in those plots. Since the tree mortality in plot Fb2, adjacent to Fb1, kept alow level ((Fb2)), we cannot infer that P. thunbergii trees in this area were vulnerable to tsunami damage. When we conducted the monitoring, we observed a large puddle that occurred on the forest floor near plot Ff. High underground water level might be an affecting factor of the pine mortality in Plot Ff, and high density and small tree size () was also the promoting factor of the tree decline. There was a concrete creek of 2 m in depth between plots Fb1 and Fb2 (), and plot Fb1 was on the far side to the sea. Thus, the creek may have prevented the underground movement of seawater brought by the tsunami, and the stagnation of seawater in plot Fb1 might pose chronic stress on pine trees.
After all, the results in this study indicate that the well-grown P. thunbergii trees remained to be standing status and having no ill effect from the groundwater seemed to show good survival after undergoing the severe situation after the tsunami.
Possible cause of the mortality in pine trees
Since B. xylophilus was not commonly detected in the tested trees (), pine wilt disease, a major mortality factor of pine trees in Japan (Futai Citation2008), was not concluded to be a determining factor for the observed tree decline and mortality. Plot C was an exceptional case and the tree mortality observed there was attributed in part to pine wilt disease.
Changes in the soil environment caused by the influx of seawater are the most plausible causal factor for the physiological damage of trees in the inundated area (Nakamura et al. Citation2012; Hashimoto et al. Citation2016). Enhanced soil salinity can cause harmful effects in plants through osmotic stress, ion toxicity or an unbalance in soil nutrients (Wainwright Citation1984). Although P. thunbergii is asalinity tolerant tree species and its resistance to seawater inundation has been documented in Japan (Horie Citation1966; Oda Citation1991; Tateishi et al. Citation2014), excessive soil salinity could be fatal. Ono et al. (Citation2013) reported the increasing soil pH and high concentrations of exchangeable cations (e.g. Na+, Ca2+, and Mg2+) were present in the original surface soil buried under the sandy sediment in the stands corresponding to those in Watari, Higashi-matsushima and Hachinohe in this study. Such drastic changes in the original surface soil could have adverse impacts on the pine trees and increase the mortality of trees suffering from physical damage related to breakage of stems, felling or injury on trunk caused by the tsunami, or with a suppression before the disaster. Although the strong effect of increased salinity may wear off by natural desalination resulting from the precipitation (Ono and Hirai Citation2012), it can persist under poor drainage situations (Ono et al. Citation2014; Kubota Citation2017). A follow-up study of the soil properties in Watari (plot Ab) and Hachinohe (plot Fb) by Ono et al. (Citation2014) showed the continuous high concentrations of Na+ and Mg2+ in the buried original surface layer only in the latter plot, which may be responsible for the chronic damage in P. thunbergii trees observed in plot Fb ((Fb)).
Things to be considered in coastal plantations taking heed to the lessons from the 2011 tsunami
Coastal forests are important barriers against strong winds and sand accumulation. They form an essential component of the seaside landscape in Japan and have become a place for various recreation activities (Murai et al. Citation1992). This perceived importance emphasized the wish to restore coastal forests after the tsunami disaster. However, the restoration process should also ensure that the anti-tsunami performance of the coastal forest is improved, to protect the coastline and its inhabitants against potential future tsunamis.
In this study, we described the pattern of tree decline in P. thunbergii and P. densiflora impacted by the mega-tsunami. As aresult, mature P. thunbergii trees were proved to be tolerant to tsunami damage when they were not subjected to extreme physical damage or stress conditions. This is consistent with the historical use of P. thunbergii trees in coastal plantations. On the other hand, small trees of P. thunbergii at the frontline forest were devastated by the tsunami and even mature trees showed a chronic decline that seemed to be due to seawater-induced effect in the soil in some locations. Destruction of the frontline forest by mega-tsunami is unavoidable and the desired property for the trees in such a place should be the wind- and sand break functions in ordinary times. Here, P. thunbergii would present an advantage in its tolerance to a wide range of severe coastal conditions (Honda Citation1919; Oda Citation1991; Murai et al. Citation1992; Konta Citation2013). As a countermeasure to atsunami hit, it is important to prepare the belt of hinterland forest of large-sized mature trees to moderate the damage. The chronic decline of P. thunbergii trees after the seawater inundation could be avoidable with appropriate drainage and/or just planting the trees on the higher ground.
P. densiflora trees showed severe damage in the tsunami-damaged stands (; ). This result concurs with previous reports of the low resistance of P. densiflora to seawater inundation (Takahashi and Horie Citation1965; Oda Citation1991; Tateishi et al. Citation2014). The decline in P. densiflora after the tsunami, however, developed chronically, and some of the trees recovered from the declined status. In the areas surrounding plots C, D, and E, we observed a large number of P. densiflora trees grew on coastal cliffs or slopes that would have been flooded by the tsunami and survived the disaster, suggesting that the seawater-induced damage may not be fatal to P. densiflora when the immersing seawater is promptly drained off. The importance of securing appropriate drainage in restoring coastal pine forests was already pointed out (e.g. Kimura Citation2014), and our results provide a concrete argument to the assertion.
Acknowledgments
We are grateful to Tomoki Sakamoto and Noritoshi Maehara for their help and valuable advice in performing this study. Thanks are also due to Kaoru Niiyama, Takeshi Saito, Keizo Hirai, Kenji Ono, and Eiji Kodani for their help during fieldwork, Eri Oikawa, Ayaka Kinno, Shizuko Matsuzawa and Noriko Kawamura for their dedicated work in nematode detection and data entry. We thank the anonymous coordinating editor and reviewers of JFR for the important suggestions to improve the manuscript. We wish to thank Aomori, Iwate, and Miyagi Prefectures, as well as Miyagi-Hokubu and Sendai District Forest Offices for facilitating our research in the disaster area.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- The 2011 Tohoku Earthquake Tsunami Joint Survey Group. 2011-2019. Results of tsunami surveys. [accessed 2020 Mar 31]. https://coastal.jp/tsunami2011/index.php?Field%20survey%20results.
- Aikawa T, Kanzaki N, Kikuchi T. 2010. A simple diagnostic tool of pine wilt disease using the DNA of pinewood nematode “Bursaphelenchus xylophilus detection kit”. For Pests. 59:60–67. Japanese.
- Akbulut S, StampsWT. 2012. Insect vectors of the pinewood nematode: a review of the biology and ecology of Monochamus species. For Pathol. 42:89–99. doi:https://doi.org/10.1111/j.1439-0329.2011.00733.x.
- Forest Conservation Departmental Meeting of Tohoku Forestry Research Institute Liaison Council. 2014. Changes in the distributions of pine wilt disease and the vector beetle Monochamus alternatus in the Tohoku region of northern Japan between 2007 and 2011. Bull of FFPRI. 13(4):335–343. Japanese.
- Futai K. 2008. Pine wilt in Japan: from first incidence to the present. In: ZhaoBG, FutaiK, SutherlandJR, TakeuchiY, editors. Pine wilt disease. Tokyo: Springer Japan; p. 5–12.
- Hashimoto R, Asaka M, Nonaka M, Akiyama A, Shirahata M, Nakakita O. 2016. Death of trees in the salty wind protection forest of Pinus thunbergii Parl. remaining after the tsunami in Tarou District of Miyako City. Tohoku J For Sci. 21(2):71–77. Japanese.
- Honda S. 1919. A textbook for silviculture volume 1: conifers. Tokyo: Miura Syoten. Japanese.
- Horie Y. 1966. Tolerance to salt water of several plants (2). Bull Gov For Exp Sta. 186:113–133. Japanese.
- Hoshino D. 2012. Costal forest and town damage in Iwate Prefecture, Japan, following the 2011 Tohoku earthquake tsunami. J Jpn For Soc. 94:243–246. Japanese. doi:https://doi.org/10.4005/jjfs.94.243.
- Kikuchi T, Aikawa T, Oeda Y, Karim N, Kanzaki N. 2009. Arapid and precise diagnostic method for detecting the Pinewood nematode Bursaphelenchus xylophilus by loop-mediated isothermal amplification. Phytopathol. 99(12):1365–1369. doi:https://doi.org/10.1094/PHYTO-99-12-1365.
- Kimura K. 2014. Mass mortality of Japanese black pine trees in coastal forests along the Pacific Ocean in Aomori Prefecture. Jpn J For Environ. 56:27–35. Japanese.
- Kiyohara T, TokushigeY. 1971. Inoculation experiments of a nematode, Bursaphelenchus sp. onto pine trees. J Jpn For Soc. 53:210–218. Japanese.
- Konta F. 2013. Why is it Japanese black pine? The disaster prevention of the coastal forest in Japan. J Jpn Soc Coastal For. 12(2):23–28. Japanese.
- Kubota T. 2017. Relationship between drainage conditions in a coastal forest and salt damage of Pinus thunbergii. Tohoku J For Sci. 22(1):9–14. Japanese.
- Mamiya Y. 1983. Pathology of the pine wilt disease caused by Bursaphelenchus xylophilus. Ann Rev Phytopathol. 21:201–220. doi:https://doi.org/10.1146/annurev.py.21.090183.001221.
- Murai H, Ishikawa M, Endo J, Tadaki R. 1992. The coastal forest in Japan: its multiple functions and use. Tokyo: Soft Science. Japanese.
- Nakagoshi N. 1995. Pine forests in east Asia. In: BoxEO, PeetRK, MasuzawaT, YamadaI, FujiharaK, MaycockPF, editors. Vegetation science in forestry. Dordrecht: Kluwer Academic Publishers; p. 85–104.
- Nakamura K, Kodani E, Ono K. 2012. Debilitation and death of trees in tsunami-affected coastal forests. Shinrin-kagaku. 66:7–12. Japanese.
- Noguchi S, Niiyama K, Tamura H, Tanaka S, Kubota T, Yasuda Y. 2014. Variation of the ground water level in a coastal forest in Miyagino-ku, Miyagi Prefecture, Japan. J Jpn For Soc. 96:150–154. Japanese. doi:https://doi.org/10.4005/jjfs.96.150.
- Oda T. 1991. Tolerance of Pinus thunbergii seedlings to inundation by salt- and freshwater. Chisan. 36(6):21–24. Japanese.
- Ono K, Hirai K. 2012. Needle discoloration of Japanese cedar (Cryptomeria japonica) along the Pacific coastline of Sanriku, Japan, after the Heisei Sanriku Massive Tsunami with the 2011 off the Pacific coast of Tohoku Earthquake. Bull of FFPRI. 11(2):33–42. Japanese.
- Ono K, Nakamura K, Hirai K. 2014. Fluctuations in the concentration of exchangeable cations in tsunami-hit forest soils on the northern Pacific coast. J Jpn For Soc. 96:301–307. Japanese. doi:https://doi.org/10.4005/jjfs.96.301.
- Ono K, Nakamura K, Tanaka N, Furusawa H, Hirai K. 2013. Soil conditions in coastal pine forests damaged by the Heisei-Sanriku mega-tsunami following Tohoku Earthquake along the eastern =9Pacific coast of Japan 2011. Bull of FFPRI. 12(1):49–66. Japanese.
- Sakamoto T. 2015. Regeneration of coastal forests affected by tsunami. Tsukuba: Forestry and Forest Research Institute. [accessed 2020 Mar 31]. https://www.ffpri.affrc.go.jp/pubs/chukiseika/documents/3rd-\\\chuukiseika29.pdf.
- Sakamoto T, Niiyama K, Nakamura K, Kodani E, Hirai K, Saito T, Kimura K, Kon J. 2012. Effectiveness of the coastal forest in stopping drifts from entering the residential area. J Jpn Soc Coastal For. 11(2):65–70. Japanese.
- Sato K. 1961. Japanese pines volume 1: artificial afforestation. Tokyo: Zenrinkyo. Japanese.
- Takahashi K, Horie Y. 1965. Tolerance to salt water of several plants (1). Bull Gov For Exp Sta. 183:1132–1151. Japanese.
- Tamura H. 2012. Uprooting damages in coastal forests in the Sendai Plain. Shinrin-kagaku. 66:3–6. Japanese.
- Tateishi M, Maimaiti A, Tsuji M, Inoue M, Taniguchi T, Yamamoto F, Yamanaka N. 2014. Temporal changes in effects of sea water flooding on physiology of Machilus thunbergii, Pinus densiflora and P. thunbergii. J Jpn Soc Reveget Tech. 40(1):54–59. Japanese.doi: https://doi.org/10.7211/jjsrt.40.54.
- Togashi K. 2008. Vector-nematode relationships and epidemiology in pine wilt disease. In: ZhaoBG, FutaiK, SutherlandJR, TakeuchiY, editors. Pine wilt disease. Tokyo: Springer Japan; p. 162–183.
- Wainwright SJ. 1984. Adaptations of plants to flooding with salt water. In: Kozlowski TT, editor. Flooding and plant growth. Orland (FL): Academic Press; p. 295–343.
- Yoshioka K. 1948. Community types and their development of pine forests in Japan. Seitaigaku-kenkyu. 11:204–216. Japanese.