Coronaviruses (CoV) are a family of viruses that cause the common cold and severe diseases such as the Middle East Respiratory Syndrome (MERS-CoV) and the Severe Acute Respiratory Syndrome (SARS-CoV) [Citation1–Citation3]. Near the end of 2019, a novel strain named SARS-CoV-2 [Citation4], and previously not identified in humans caused the outbreak of a new disease (commonly known as COVID-19) that started in China and is rapidly spreading in Asia, Europe, the USA, Australia and the rest of the world. Like SARS-CoV and MERS-CoV, SARS-CoV-2 is a zoonosis in which bats are probably the source of the virus, and other mammals were intermediary hosts that subsequently infected humans. For example, the intermediary hosts for SARS, MERS and COVID-19 were respectively civets [Citation5], camels [Citation6] and likely pangolins [Citation7]. The initial coverup in the country where the outbreak initially started, coupled with delays from World Health Organization (WHO) in recognizing the potential for the spread of COVID-19 to result in a global pandemic has been attributed to the severity of the current situation, which has not been encountered by humankind for at least the last 100 years.
Currently, no drugs or vaccines are available to treat COVID-19, and a significant number of deaths are reported mostly in elderly patients with comorbidity. By 21 April 2020, more than 2.7 million cases were recorded worldwide, with more than 194,000 deaths, which continue to increase, mainly in Europe and the USA, which are countries with a larger fraction of elderly population who have acquired the infection. The only effective measure to counteract the spreading of COVID-19 involves isolating the population, quarantining infected people, suspending most businesses and commercial activities, coupled with intensive clinical therapy for patients with severe symptoms, as underlined by the successful example in China, which reported no new internal cases per day as of 20 March 2020. However, the adoption of such containment measures dramatically impacted the economy and social life of all countries currently grappling with this pandemic. Moreover, the risk of a deep and lengthy global recession is elevated. Some studies are currently recruiting patients for Phase I to Phase IV trials with drugs used in the therapy of various diseases (e.g., Tocilizumab, Chloroquine, Remdesivir, etc.) but no specific drug candidates against COVID-19 are being investigated yet in clinical trials. For these reasons, novel drugs that are capable of impairing the replication of CoV that can be used in the current outbreak as well as in future occurrences are in exceedingly high demand but are not available.
How could this happen, considering the horrifying warning signals from the previous two outbreaks, SARS in 2002–2003 and MERS shortly thereafter? Especially considering the publication in 2012 of the excellent book by David Quammen, Spillover: Animal Infections and the Next Human Pandemic [Citation8], in which the current global crisis was predicted. The answer is unfortunately rather simple: all of us, including scientists, governments, pharmaceutical companies, and laypeople, have a tremendous responsibility.
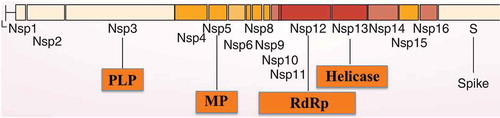
Scientists have continued to investigate CoV despite having limited resources. Funding has been limited in CoV research likely because the previous two outbreaks were resolved with few casualties [Citation5,Citation6]. However, based on such pioneering work, we now know a lot about genetics, molecular biology, and the lifecycles of these pathogens. Indeed, SARS-CoV-2 is an enveloped, positive-sense, single-stranded RNA beta-coronavirus. These viruses mutate very rapidly since RNA viruses make mistakes during RNA replication for the lack of the error-correction mechanisms of the cells involved in copying DNA. Any of these mutations can confer new properties, including the ability to infect new types of cells, or even new organisms and thus to determine the spillover. Like SARS-CoV and MERS-CoV, its genome encodes for non-structural proteins (including 3-chymotrypsin-like protease, also known as main protease, MP; papain-like protease, PLP; helicase; and RNA-dependent RNA polymerase, RdRp) (). Some structural proteins (such as the spike glycoprotein) and accessory proteins are also present in the genomes of all CoVs. In this genomic area, coronaviruses are subjected to many mutations, which allow the virus evolution, as well as their potential in evading the response of the immune system, and the jumping from animal to human species.
Figure 1. Open reading frame 1 (ORF1) genomic organization of SARS-CoV-2. In the orange boxes, the four enzymes that might be targeted by antiviral drugs: papain-like protease (PLP), main protease (MP), RNA-dependent RNA polymerase (RdRp) and helicase.
The genetic material of the virus is transcribed into two viral polypeptides, of 490 kDa and 790 kDa, respectively, which are subsequently co-translationally cleaved into mature nonstructural proteins (Nsps) through the activity of two proteases encoded in the 5′ region of ORF1: PLP and MP (). The latter protein has a dominant role in the post-translational processing of the replicase polyprotein. The MPs of various CoV contain similar substrate-binding pockets, usually with the requirement for Gln at position P1 and a preference for leucine/methionine at the P2 sub-pocket (P1 and P2 are the amino acid residues in the substrate undergoing cleavage in the N-terminal direction from the cleaved bond). Both PLP and MP are cysteine proteases: MP is a dimer with a Cys-His dyad at the active site, whereas PLP is a monomer with a Cys-His-Glu canonical catalytic triad. The MP of SARS-CoV-2 has been crystallized recently (and PDB codes released) [Citation9–Citation12]. Due to the crucial role that these proteases play in the life cycle of the virus, their inhibition may have a potent antiviral effect [Citation13–Citation16].
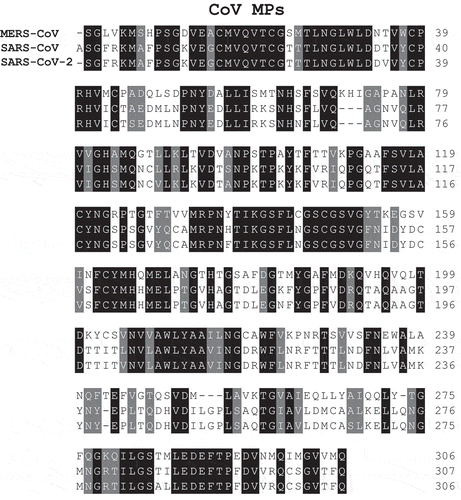
Furthermore, there is a rather relevant homology between the pair of the two proteases in the three CoV mentioned above, which produced epidemic outbreaks in humans (–), which could be used to target such proteins by designing specific inhibitors. Both PLP and MP of SARS-CoV-2, SARS-CoV, and MERS-CoV have, in fact, a quite conserved amino acid sequence, which means that presumably effective inhibitors for one of them may act efficaciously for all three.
Figure 2. Alignment of the PLPs from the three CoV. Multiple amino acid sequence alignment was performed with the program ClustalW, version 2.1. The alignment was formatted highlighting in black the identical residues and the conservative substitutions in gray.

Figure 3. Multiple amino acid sequence alignment of the MPs from SARS-CoV, MERS-CoV, and SARS-CoV-2 carried out with ClustalW, version 2.1. The formatted alignment shows the identical amino acid residues (black) and the conservative substitutions (gray).
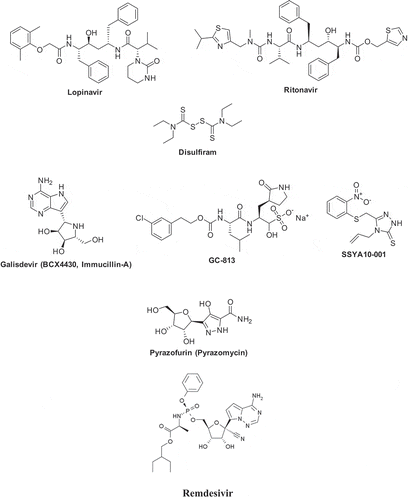
Thus, our knowledge regarding the biochemical machinery of these viruses (exemplified here with the proteases, but the same is valid for the helicase and the polymerase) is rather detailed. Still, it was not translated to the clinics due to the lack of interest from the drug companies, as discovering drugs for a disease that affects a limited number of people (as was the case with SARS and MERS) is not cost-effective. However, the current outbreak proves how wrong we were. Antivirals, which could be used to treat COVID-19 patients, would probably make the crucial difference in the current pandemic. The development of strategic drugs for such pandemic outbreaks should, from now on, not be left only in the hands of big pharma, but industrial countries (or WHO) should fund programs to develop such therapies in the interests of public health and the global economy. There are some recent anecdotal reports on the repurposing of some antivirals developed for other viruses, such as the human immunodeficiency virus-1 (HIV-1) protease inhibitors lopinavir and ritonavir [Citation17], disulfiram, which is known to inhibit SARS-CoV proteases [Citation18] as well as some newer agents, which are shown in [Citation19]. Among them, Remdesivir is a nucleoside analog acting as a polymerase inhibitor that is in clinical development for the treatment of Ebola [Citation20], which seems to have some efficacy for the management of COVID-19 [Citation21]. However, all these are relatively desperate options due to the lack of specific agents targeting CoV.
There are several proposals for vaccine candidates [Citation22] based on the mechanism used by SARS-CoV-2 to infect T lymphocytes [Citation23] or approaches which contemplate the use of fusion inhibitors [Citation24], targeting the spike protein of the virus, but the development of such therapeutics may take longer compared to the classical targets mentioned above. All these data show that the scientific community responded in an exemplary manner to the emergency, but as usual, a voice out of the chorus emerged [Citation25] with irrelevant comments and exaggerations on statistics, as was the case with a dubious analysis of citations and impact factors of the major journals.
Overall, more than half of the world’s population is locked down, and a massive number of new cases are reported each day, many more cases remain undiagnosed, and no effective drugs are available. For a species that chooses to name itself Homo sapiens (‘wise humans’ in Latin), this is probably a time when it is obvious that a more appropriate name should be ‘Homo insapiens’ (unwise humans). At the end of this emergency, we should dramatically rethink how to manage the environment, the extinction of a considerable number of species with a consequent impoverishment of the biosphere and, last but not least, global warming. The COVID-19 outbreak is another ringing bell announcing how much the decisions our species are currently making are insapiens.
Declaration of interest
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
Reviewer disclosures
A reviewer on this manuscript has disclosed that they acted as a consultant for Abbvie, MSD, Correvio, Pfizer and Angelini, and managed department grants from Gilead Sciences. All other peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Correction Statement
This article has been republished with minor changes. These changes do not impact the academic content of the article.
Additional information
Funding
References
- Ksiazek TG, Erdman D, Goldsmith CS, et al. A novel coronavirus associated with severe acute respiratory syndrome. N Engl J Med. 2003;348:1953–1966.
- Hui DS, Azhar EI, Kim YJ, et al. Middle East respiratory syndrome coronavirus: risk factors and determinants of primary, household, and nosocomial transmission. Lancet Infect Dis. 2018;18:e217–e227.
- Guarner J. Three emerging coronaviruses in two decades. Am J Clin Pathol. 2020;153:420–421.
- Zhu N, Zhang D, Wang W, et al. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020;382:727–733.
- Woo PC, Huang Y, Lau SK, et al. Coronavirus genomics and bioinformatics analysis. Viruses. 2010;2:1804–1820.
- Killerby ME, Biggs HM, Midgley CM, et al. Middle East respiratory syndrome coronavirus transmission. Emerg Infect Dis. 2020;26:191–198.
- Li X, Zai J, Zhao Q, et al. Evolutionary history, potential intermediate animal host, and cross-species analyses of SARS-CoV-2. J Med Virol. 2020. In press. DOI:10.1002/jmv.25731.
- Quammen D. Spillover: animal infections and the next human pandemic. New York: W.W. Norton and Co.; 2012. p. 1–608.
- Jin Z, Du X, Xu Y, et al. Structure of Mpro from COVID-19 virus and discovery of its inhibitors. Nature. 2020. in press. DOI:10.1038/s41586-020-2223-y.
- Niemeyer D, Mösbauer K, Klein EM, et al. The papain-like protease determines a virulence trait that varies among members of the SARS-coronavirus species. PLoS Pathog. 2018 Sep;24(14):e1007296.
- Wang F, Chen C, Tan W, et al. Structure of main protease from human coronavirus NL63: insights for wide spectrum anti-coronavirus drug design. Sci Rep. 2016;6(1):22677.
- Zhang L, Lin D, Sun X, et al. Crystal structure of SARS-CoV-2 main protease provides a basis for design of improved α-ketoamide inhibitors. Science. 2020;pii:eabb3405. In press.
- Zhang L, Lin D, Kusov Y, et al. α-ketoamides as broad-spectrum inhibitors of coronavirus and enterovirus replication: structure-based design, synthesis, and activity assessment. J Med Chem. 2020. In press. DOI:10.1021/acs.jmedchem.9b01828.
- Ma-Lauer Y, Carbajo-Lozoya J, Hein MY, et al. p53 down-regulates SARS coronavirus replication and is targeted by the SARS-unique domain and PLpro via E3 ubiquitin ligase RCHY1. Proc Natl Acad Sci U S A. 2016;113:E5192–5201.
- Wang L, Bao BB, Song GQ, et al. Discovery of unsymmetrical aromatic disulfides as novel inhibitors of SARS-CoV main protease: chemical synthesis, biological evaluation, molecular docking and 3D-QSAR study. Eur J Med Chem. 2017;137:450–461.
- Rabaan AA, Alahmed SH, Bazzi AM, et al. a review of candidate therapies for middle East respiratory syndrome from a molecular perspective. J Med Microbiol. 2017;66:1261–1274.
- Yao TT, Qian JD, Zhu WY, et al. a systematic review of lopinavir therapy for SARS coronavirus and MERS coronavirus-a possible reference for coronavirus disease-19 treatment option. J Med Virol. 2020. In press. DOI:10.1002/jmv.25729.
- Lin MH, Moses DC, Hsieh CH, et al. Disulfiram can inhibit MERS and SARS coronavirus papain-like proteases via different modes. Antiviral Res. 2018;150:155–163.
- Li G, De Clercq E. Therapeutic options for the 2019 novel coronavirus (2019-nCoV). Nat Rev Drug Discov. 2020;19:149–150.
- Warren TK, Jordan R, Lo MK, et al. Therapeutic efficacy of the small molecule GS-5734 against Ebola virus in rhesus monkeys. Nature. 2016;531:381–385.
- Al-Tawfiq JA, Al-Homoud AH, Memish ZA. Remdesivir as a possible therapeutic option for the COVID-19. Travel Med Infect Dis. 2020: 101615. In press. doi:10.1016/j.tmaid.2020.101615.
- Wang N, Shang J, Jiang S, et al. Subunit vaccines against emerging pathogenic human Coronaviruses. Front Microbiol. 2020;11:298.
- Wang X, Xu W, Hu G, et al. SARS-CoV-2 infects T lymphocytes through its spike protein-mediated membrane fusion. Cell Mol Immunol. 2020. In press. DOI:10.1038/s41423-020-0424-9.
- Xia S, Liu M, Wang C, et al. Inhibition of SARS-CoV-2 (previously 2019-nCoV) infection by a highly potent pan-coronavirus fusion inhibitor targeting its spike protein that harbors a high capacity to mediate membrane fusion. Cell Res. 2020;30:343–355.
- Ioannidis JPA. Coronavirus disease 2019: the harms of exaggerated information and non-evidence-based measures. Eur J Clin Invest. 2020;e13223. DOI:10.1111/eci.13223