Abstract
Background
Standard influenza vaccines are produced using egg-based manufacturing methods. Through the process, the resulting egg-adapted viral strains may differ from the selected vaccine strain. Cell-derived influenza vaccine manufacturing prevents egg-adaptation of the antigen which can improve vaccine effectiveness. We evaluated the cost-effectiveness of quadrivalent cell-derived influenza vaccine (QIVc) versus an egg-based quadrivalent influenza vaccine (QIVe) in preventing seasonal influenza from German societal and payer perspectives.
Methods
Adapted version of the individual-based dynamic 4Flu transmission model was combined with a decision-tree to calculate the impact of QIVc versus QIVe on influenza over 20 seasons in Germany. Egg-adaptation, resulting in lower effectiveness of QIVe versus QIVc towards the H3N2 influenza strain, is sourced from a US retrospective study and assumed in 100% (base case) or 55% (conservative scenario) of years. Influenza-related probabilities of outpatient visits, hospitalizations, productivity loss, and mortality, with associated (dis)utilities/costs, were extracted from literature. Costs and outcomes were discounted 3.0%/year.
Results
Replacing QIVe with QIVc in subjects aged ≥ 9 years can annually prevent 167,265 symptomatic cases, 51,114 outpatient visits, 2,091 hospitalizations, and 103 deaths in Germany. The annual number of quality-adjusted life-years (QALYs) increased by 1,628 and healthcare costs decreased by €178 M from societal perspective. From payer perspective, the incremental cost-effectiveness ratio was €2,285 per QALY. Scenario analyses confirmed results robustness.
Conclusions
The use of QIVc compared to QIVe, in the German Immunization Program, could significantly prevent outpatient visits and hospitalizations and would enable substantial savings from a societal perspective.
Introduction
There are two main types of influenza viruses that commonly cause seasonal influenza: Types A and B. Influenza A viruses are further classified into subtypes H1N1 and H3N2. Influenza B viruses are broken down into lineages B/Yamagata and B/VictoriaCitation1. The most common symptoms of influenza are chills, fever, sore throat, muscle pains, headache (often severe), coughing, weakness/fatigue, and general discomfortCitation1. Annually, an estimated three to five million influenza-related hospitalizations and about 250,000–500,000 influenza-related deaths are reported globallyCitation1. In Germany, 3.8 million general practitioner (GP) visits and 18,000 hospitalizations were reported in the 2018–2019 season due to influenzaCitation2. Influenza can occasionally lead to pneumonia, and several rare but severe complications such as myocarditis, myocardial infarction, stroke, and gastrointestinal bleedingCitation3. All age groups can be affected by influenza; however, elderly people (>65 years of age), young children (<2 years of age), and patients with comorbidities such as chronic lung, heart, and renal diseases, and immunodeficiency are more prone to develop complications and are considered as high risk groupsCitation4. In Germany, quadrivalent influenza vaccine (QIV) is recommended for all people aged ≥60 years and for high risk groupsCitation5.
The influenza virus needs to bind to a cellular receptor in order to infect a cell. The influenza viruses grow in humans as well as in other mammals. However, nearly all seasonal influenza vaccines are produced using candidate vaccine viruses (CVVs) grown in chicken eggsCitation6. Avian cells have different receptors than those that are on the surface of human or other mammalian cells. For a human influenza virus to grow well in avian cells, it needs to adapt to bind the avian receptor in a process known as egg-adaptationCitation7. This adaptation during the isolation and propagation steps of egg-based vaccines antigen may lead to a mismatch with the World Health Organization (WHO) selected influenza vaccine strainCitation8,Citation9. As a result, the standard egg-adapted trivalent and quadrivalent influenza vaccines (QIVe) have shown lower vaccine effectiveness (VE) in recent years, especially against circulating type A (H3N2) virusesCitation8,Citation10. A manufacturing process that uses cell-culture technology (Madin-Darby Canine Kidney cells) and does not involve the use of fertilized eggs for influenza vaccine production was approved by the United States Food and Drug Administration (US FDA) in 2012Citation7. Cell-derived manufacture prevents changes in the antigenic structures due to egg-adaptation and can minimize the chances of altering the influenza vaccine strain, thereby potentially increasing the VECitation11. Flucelvax Tetra is a cell-culture based quadrivalent influenza vaccine (QIVc) developed by Seqirus and it is the first QIVc approved by the European Medicine Agency in 2018Citation12,Citation13.
Previous studies on cost-effectiveness analysis (CEA) of different seasonal influenza vaccines have been reported in the US and in European countries like Italy, Spain, and the UKCitation14–17. However, at the moment that the German health authorities are considering the reimbursement of QIVc, a CEA on the impact of QIVc in Germany is still lacking. As epidemiological data, vaccine coverage, and costs vary from country to country, CEA results from other European countries cannot be used for Germany and thus a German-specific model is necessary. Moreover, different vaccine effectiveness was used in the aforementioned CEA studies as uncertainties exist around the vaccine effectiveness. The study explores conservative scenarios of the vaccine effectiveness of QIVc. This study aims to evaluate cost-effectiveness, from the societal and payer perspectives, for the projected health and economic outcomes associated with the use of QIVc versus standard QIVe in individuals aged ≥9 years for preventing seasonal influenza in Germany.
Methods
Model framework
Transmission model
We used an extended version of the 4Flu model developed by Epimos GmbH and GlaxoSmithKline GmbH & Co. KGCitation18. The model simulates the concomitant and independent transmission of four different influenza viruses, i.e. A(H1N1), A(H3N2), B/Victoria, and B/Yamagata. Infected individuals pass through a latent period of 2 days and then become infectious for 2 days (adults) or 4 days (children)Citation18,Citation19. During this infectious period, they can infect any susceptible person within their contact network. Keeping 2019 as the base year, the model predicts the number of infected individuals per 100,000 persons by age, year, and risk group (normal and high risk) over a time horizon of 20 years. These outputs are used to feed the cost-effectiveness model (CEM). For a more detailed description of the simulation tool, see Supplementary Online Supporting Material.
Cost-effectiveness model
This CEM was developed in Microsoft Excel based on a previously published decision-tree model by Dolk et al.Citation20. The structure of the CEM shown in Supplementary Figure S2 represents the evolution of patients infected with influenza. The model considers that some of the infected patients will become symptomatic, while others will not develop clinical signs. A proportion of the symptomatic patients would seek medical attention while another proportion of patients with symptoms would be expected to develop complications like otitis, respiratory problems (bronchitis, pneumonia, upper respiratory tract infections), cardiac complications (myocarditis, myocardial infarction), neurological issues, or gastrointestinal bleeding. Each of these complications may be treated at home or in hospital. Hospitalized complications have a probability of being fatal. These complications were included as per Dolk et al.Citation20, along with the addition of myocardial infarction (MI) and stroke. The complications considered in the model are fully detailed in Supplementary Table S1. We have performed the necessary quality assurance steps to ensure the model produces predictable and accurate outputs. The quality assurance process involves an independent review of the model by a senior health economist.
Model perspective and patient populations
As mentioned, societal and payer perspectives were considered for this CEA. Following the German Standing Committee on Vaccination (STIKO) guidelines, the societal perspective was considered as the base caseCitation21, wherein the vaccination costs, GP visits, treatment for symptomatic influenza, complication costs, transportation costs, patient co-payments, absenteeism costs, and costs borne by child sickness benefit were included. In addition, costs associated with the loss of productivity for the caregiver due to influenza were included. Costs data from the payer perspective focused only on the vaccination costs, GP visits, treatment for symptomatic influenza, complication costs, and costs borne by child sickness benefit.
The simulations were performed on a cohort of 100,000 individuals and the outcome (i.e. number of infections) per treatment arm was analyzed in the CEM. Results were projected by age-distribution to the entire German population (82.5 million), as the clinical and health economic outcomes accumulated over seasons for a period of 20 years (time horizon of the 4Flu model).
The time horizon for the CEM was also 20 yearsCitation18. The costs and quality-adjusted life-years (QALYs) were discounted using a rate of 3% per annum after year one in compliance with the German guidelinesCitation22.
Vaccination with QIVe was compared to vaccination with QIVc in individuals aged ≥9 years and to QIVe in individuals under 9 years. A cut-off point of 9 years was used as QIVc is indicated for individuals aged ≥9 years per the label granted by the European Medicine Agency (EMA)Citation23.
Transmission model inputs
Vaccination for a given season primarily depends on the age and risk status of the individual. A re-vaccination factor of 4.25 was used in the model, meaning that previously vaccinated individuals are more than 4-times as likely to be vaccinated again as previously unvaccinated individuals. The re-vaccination factor was calculated from reported data on people who were vaccinated at least once in three subsequent seasons from 2004/5 to 2006/7 in GermanyCitation24. Vaccination coverage depends on the individuals’ age. Vaccine coverage in the 4Flu model is shown in Supplementary Table S2. The VE depends on the age of the individual and on the type of vaccine being used. Relative VE (i.e. rVE) describes the difference between QIVc and QIVe. It was assumed that, in years without egg-adaptation, the VE of QIVc against A(H3N2) was the same as that of QIVe. For years with egg-adaptation, it was assumed that the rVE of QIVc against A(H3N2) is 36.2% higher than that of QIVe (rVE: 36.2%; 95% confidence interval [CI]: 26.1–44.9)Citation25. For a more conservative scenario, the sensitivity analysis result of the same study was used, showing an rVE of 19.3% (95% CI: 9.5–28.0)Citation25. Absolute VE (i.e. aVE) per arm is based on the rVE and on previous findings reported by Belongia et al.Citation26, resulting in a mean VE (33%) of QIVe against A(H3N2) when averaged over all years (years with and without egg-adaptation).
The transmission model used different VE values depending on the base case assumption that egg-adaptation either occurs in 100% (Setting A) or in 55% (Setting B) of all years. The 55% assumption was derived from a study of Rajaram et al.Citation27 in which they reported that there was minimal or no antigenic similarity between type A(H3N2) circulating viruses and egg-adapted reference viruses in 16 of the 29 seasons analyzed. For the other influenza strains and lineages, the same VE was used for QIVc and QIVe in all yearsCitation28–30. The age-dependent VE against A(H3N2) with and without egg-adaptation for the base case analyses is shown in .
Table 1. Vaccine effectiveness for Settings A and B in the 4Flu model.
Cost-effectiveness model inputs
Clinical inputs
As reported by Carrat et al.Citation31, we assumed that 66.9% of the infected patients developed influenza symptoms. Age-specific probabilities of seeking medical attention by symptomatic cases are shown in Supplementary Table S3. The probability of receiving antivirals was 14.7% and 19.6% in normal and high-risk groups, respectivelyCitation20. Probabilities of most likely influenza attributable complications included in this model were taken from Dolk et al.Citation20. The model additionally includes MI and stroke, and the probabilities were based on published literatureCitation32–35. Emergency room (ER) visits could be required for patients with complications. These patients can be hospitalized or sent home. It was assumed that all patients that were hospitalized were admitted via ER. The percentage of patients being hospitalized and discharged from ER is shown in Supplementary Tables S4 and S5, respectively. Fatality rates with complications are shown in Supplementary Table S6.
Health-related quality-of-life inputs
The baseline utilities were taken from Dolk et al.Citation20 and are shown in Supplementary Table S7. Disutility associated with clinical influenza cases were obtained from averaging utility decrements during an influenza episode and were multiplied with the duration of influenza symptoms. Utility decrements were applied to symptomatic influenza, hospitalization related to complicated influenza, and for outpatient treatment for complicated influenza. The disutility and duration of symptomatic and complicated influenza were based on Dolk et al.Citation20, except for MI and strokeCitation35. Disutility due to symptomatic influenza was applied to both uncomplicated and complicated cases. To account for longer disease course and recovery time of hospitalized influenza patients compared to outpatient casesCitation36, disutilities for three extra ambulatory days were added to hospitalization duration. and depict the calculation of disutility values used in the model.
Table 2. Annualized disutility due to symptomatic influenza (calculated*).
Table 3. Disutility due to complications (calculated*).
Costs and resource inputs
All costs were updated to 2019, using an inflation rate of 1.74% and 1.85% for the years 2017 and 2018, respectivelyCitation37, and expressed in Euros (€). An estimated average price of €11.6 per dose was used for all QIVeCitation38. Vaccination cost for QIVc was €12.5 per dose. Other costs, taken from Dolk et al.Citation20, included vaccine administration, GP visits of symptomatic patients, antivirals, and complication treatment costs (except for MI and stroke in the ambulatory, ER and hospital settings, costs borne by child sickness benefit, transportation costs, and premature death costs).
The hospitalization costs, including patient co-payment, for MI and stroke, were derived from official German diagnosis-related groups (DRG) listings, weighted by the number of patients per disease severityCitation39. Ambulatory reimbursements and patient co-payments of MI and stroke management were sourced from the German uniform standard of evaluation (EBM, 2018), according to which outpatient services are billedCitation40.
Absenteeism costs were calculated based on the working days lost and the average net production per day, which was estimated with the labor costsCitation41 and the employment ratesCitation42. The working days lost were assumed to be the same as the disease duration in Dolk et al.Citation20. The working days lost for stroke (34 days) and MI (36 days) were sourced from the literatureCitation43. For individuals aged under 18 years, the working days lost were assumed to happen to their caregiver (only one parent was assumed to be affected).
Cost inputs are shown in Supplementary Table S8.
Cost-effectiveness analysis
Results are reported per 100,000 individuals and also projected to the German population, as explained earlier. The number of symptomatic cases, GP visits, complications, hospitalizations, and deaths were the reported clinical outcomes, whereas QALYs and incremental cost-effectiveness ratio (ICER) expressed as cost per QALY gained and net monetary benefit (NMB) were the considered economic outcomes. There is no official willingness-to-pay (WTP) threshold in Germany for CEA. In our analysis, a threshold of €30,000 per QALY gained was used in line with other published cost-effectiveness vaccines studies conducted earlier in GermanyCitation44.
Scenario and sensitivity analyses
As already explained in earlier sections, two base case analyses were conducted (Setting A and B). To assess the impact of uncertainty in 4Flu and cost-effective model parameters on the base case results, scenario and sensitivity analyses were conducted. From a payer perspective, a scenario in which rVE was set to 19.3% instead of 36.2% was consideredCitation25. Univariate deterministic sensitivity analyses (DSA) were conducted from the societal perspective only. Published lower and upper values, 95% CIs, or ±10% variations around base case were tested for epidemiological and health economic parameters (grouped and varied together per category level (e.g. costs of complication hospitalizations, ER discharges, sickness benefit, transportation, absenteeism and premature death, baseline utilities, and disutility). Second-order Monte Carlo probabilistic sensitivity analyses (PSAs) were performed. Beta distributions were used for baseline utilities, lognormal distributions were used for disutilities, and direct medical costs and normal distributions were used for indirect costs. As the CEM used epidemiological outcomes from the 4Flu model, PSAs of the 4Flu and the CEM models needed to be combined. For each iteration of the PSA (1,000 iterations in total), the CEM model used an average of 100 PSA random simulation outcomes of the transmission model as input, which is similar to the approach already deployed by Dolk et al.Citation20.
Results
Base case: Societal perspective
Setting A and B results
If 100% of the years are assumed to be egg-adapted years (Setting A), the use of QIVc instead of QIVe in subjects aged ≥9 years can annually prevent 4,054 symptomatic cases, 1,239 outpatient or GP visits, 419 complications, 51 hospitalizations, and two deaths in a cohort of 100,000 individuals (). QIVc was found to be a dominant strategy, with an annual gain of 39 QALYs and cost savings of €4,328,713; this resulted in a NMB of €5,512,075 (). If 55% of the years are assumed egg-adapted years (Setting B), the use of QIVc can annually prevent 2,092 symptomatic cases, 640 outpatient or GP visits, 216 complications, 26 hospitalizations, and one death (). QIVc was a dominant strategy in Setting B, with an annual gain of 21 QALYs per 100,000 individuals and cost savings of €2,080,721, thereby resulting in a NMB of €2,697,974 ().
Table 4. The annual clinical outcomes for Settings A and B in the cohort size of 100,000 individuals.
Table 5. The annual health economic outcomes for Settings A and B in the cohort size of 100,000 individuals.
The annual clinical and health economic outcomes for Settings A and B are depicted in and Citation5, respectively.
Extrapolated results to the entire German population
Results of the base cases (Settings A and B) were extrapolated to annual outcomes for the entire German population (82.5 million). For Setting A, QIVc can annually prevent 167,265 symptomatic cases, 51,114 outpatient or GP visits, 17,299 complications, 2,091 hospitalizations, and 103 deaths (Supplementary Table S9). This would amount to an annual QALY gain of 1,628 years and cost savings of €178,606,268. For Setting B, the annual QALY gain is 849 years and cost savings are €85,852,259. The NMBs were €227,432,752 and €111,320,638 for base case Settings A and B, respectively. QIVc was a dominant strategy with both base case settings.
Extrapolated clinical and health economic results for base case Settings A and B are summarized in Supplementary Tables S9 and S10, respectively.
Scenario and sensitivity analyses
Payer perspective
From a payer perspective with rVE of 36.2%, the ICERs per QALY were €2,285 and €8,984 for Settings A and B, respectively (). Similar ICER values were reported when Settings A and B results were extrapolated to the entire German population (82.5 million; ).
Table 6. Payer perspective scenario analysis annual health economic outcomes for Settings A (egg-adaptation occurs in 100% of all years) and B (egg-adaptation occurs in 55% of all years) in the cohort size of 100,000 individuals.
Table 7. Scenario analysis annual health economic outcomes for Settings A (egg-adaptation occurs in 100% of all years) and B (egg-adaptation occurs in 55% of all years) in the entire German population.
When a payer perspective was applied with an rVE of 19.3%, the ICERs were €8,199 per QALY and €22,845 per QALY for Settings A and B, respectively ().
Deterministic sensitivity analyses (DSAs)
Results of the DSAs are presented in . This figure highlights the ten parameters with the highest impact on the NMB of QIVc vs. QIVe for Settings A and B. For Setting A, the uncertainties around rVE (both 19.3% [9.5–28.0%] and 36.2% [26.1–44.9%]), aVE (33% [26–39%]) of QIVe, and the probabilities of being symptomatic were the major drivers of the NMB. For Setting B, rVE (19.3% [9.5–28.0%]) was the most important driver of the NMB, followed by the frequency of mismatched years (55% [35.69–73.55%]). Other parameters used in the model have a minor impact on the outcomes.
Figure 1. Deterministic sensitivity analysis: impact of the 10 key drivers on the NMB (Settings A [egg-adaptation occurs in 100% of all years] and B [egg-adaptation occurs in 55% of all years], societal perspective, 100,000 individuals). (a) Impact of the 10 key drivers on the NMB for Setting A. (b) Impact of the 10 key drivers on the NMB for Setting B. Abbreviations. aVE, absolute vaccine effectiveness; NMB, net monetary benefit; OSWA, one-way sensitivity analyses; rVE, relative vaccine effectiveness. Setting A: egg-adaptation occurs in 100% of all years. Setting B: egg-adaptation occurs in 55% of all years. % of death and % of hospitalizations are due to the following complications: bronchitis, pneumonia, unspecified upper tract respiratory infection, myocarditis, myocardial infarction, renal, central nervous system, stroke, otitis media, gastrointestinal bleedings where the base case values were changed with ±10%; disutilities due to influenza symptoms, baseline utilities, and disutilities due to influenza hospitalization changed with ±10%.
![Figure 1. Deterministic sensitivity analysis: impact of the 10 key drivers on the NMB (Settings A [egg-adaptation occurs in 100% of all years] and B [egg-adaptation occurs in 55% of all years], societal perspective, 100,000 individuals). (a) Impact of the 10 key drivers on the NMB for Setting A. (b) Impact of the 10 key drivers on the NMB for Setting B. Abbreviations. aVE, absolute vaccine effectiveness; NMB, net monetary benefit; OSWA, one-way sensitivity analyses; rVE, relative vaccine effectiveness. Setting A: egg-adaptation occurs in 100% of all years. Setting B: egg-adaptation occurs in 55% of all years. % of death and % of hospitalizations are due to the following complications: bronchitis, pneumonia, unspecified upper tract respiratory infection, myocarditis, myocardial infarction, renal, central nervous system, stroke, otitis media, gastrointestinal bleedings where the base case values were changed with ±10%; disutilities due to influenza symptoms, baseline utilities, and disutilities due to influenza hospitalization changed with ±10%.](/cms/asset/9415ea3e-865e-40de-afe4-badd83d4e842/ijme_a_1908000_f0001_c.jpg)
Probabilistic sensitivity analyses (PSAs)
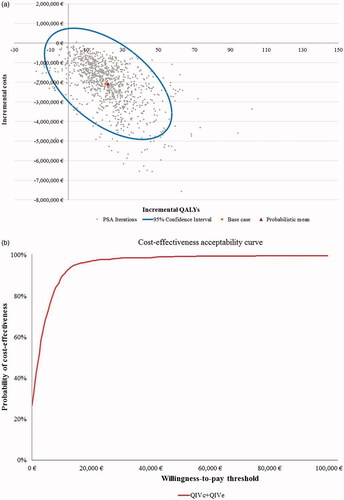
From a societal perspective, the cost-effectiveness acceptability curves (CEAC) showed that QIVc has a high likelihood of being a dominant vaccine strategy. PSA outcomes suggested that QIVc was cost-effective against QIVe in 100% and 99% of cases in Settings A and B, respectively ( and Citation3) at a WTP threshold of €30,000 per QALY. From the Payer perspective, the probability of QIVc being cost-effective was almost 100% and 85% of the analyses for Settings A and B, respectively, using the same WTP threshold (Supplementary Figure S3).
Figure 2. Scatter plot and cost-effectiveness acceptability curve for PSA with 1,000 iterations for Setting A (egg-adaptation occurs in 100% of years), societal perspective. (a) Scatter plot of PSA for Setting A. (b) Cost-effectiveness acceptability curve (CEAC) for Setting A. Abbreviations. CEAC, cost-effectiveness acceptability curve; PSA, probabilistic sensitivity analyses; QALYs, quality-adjusted life-years; QIVc, cell-based quadrivalent influenza vaccine; QIVe, egg-based quadrivalent influenza vaccine; €, Euros. Setting A: egg-adaptation occurs in 100% of all years.
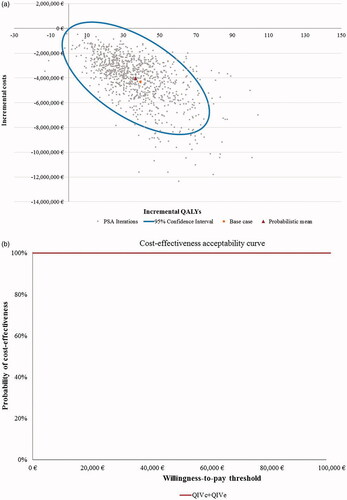
Figure 3. Scatter plot and cost-effectiveness acceptability curve for PSA with 1,000 iterations for Setting B (egg-adaptation occurs in 55% of years), societal perspective. (a) Scatter plot of PSA for Setting B. (b) Cost-effectiveness acceptability curve (CEAC) for Setting B. Abbreviations. CEAC, cost-effectiveness acceptability curve; PSA, probabilistic sensitivity analyses; QALYs, quality-adjusted life-years; QIVc, cell-based quadrivalent influenza vaccine; QIVe, egg-based quadrivalent influenza vaccine; €, Euros. Setting B: egg-adaptation occurs in 55% of all years.
Discussion
The QIV vaccines are commonly used seasonal influenza vaccines in Germany and most of them are produced using CVVs grown in eggs. However, lower VE has been reported with QIVe due to the adaptation of the vaccine antigen during the manufacturing process, that can create a mismatch with the selected influenza vaccine strain, particularly the A(H3N2) virusCitation8,Citation10. The production of vaccines using cell-derived influenza viruses circumvents viral mutations occurring in the vaccine antigen and can minimize viral antigenic dissimilarity with the vaccine virus strains. Potential increase in the VE has been reported with the use of cell-derived isolation and manufacturingCitation11. Flucelvax Tetra (QIVc) is currently approved for prophylaxis of influenza in adults and children aged ≥9 yearsCitation12.
In Germany, the previously mentioned CEA conducted by Dolk et al.Citation20 compared QIVe with egg-derived trivalent influenza vaccine (TIVe), and showed that QIVe was cost-effective (ICER: €14,461) from the payer perspective and a dominant vaccine strategy from the societal perspective. As QIVe is a common comparator to the analysis of Dolk et al. and our current analysis, we can compare the number of predicted cases and predicted costs (taking into account inflation of costs which is minor) between both analyses. In Dolk et al., annual costs of €1.3 million (€169 per symptomatic influenza case) were calculated from the payer perspective, while annual costs of €6.4 million (€794 per symptomatic influenza) from the societal perspective were accrued for 8,129 symptomatic influenza cases per 100,000 individuals, vaccinated with QIVe vaccine according the German vaccine coverageCitation20.
In the current analyses, we combined the 4Flu transmission model with a similar CEM. Over the time horizon of 20 years, €15.7 million from the payer perspective (Setting A) and €198.2 million from the societal perspective (Setting A) were accrued for 151,610 symptomatic influenza cases for a population of 100,000 individuals, vaccinated with QIVe vaccine according to the German vaccine coverage. This is equivalent to €103 and €1,307 per symptomatic influenza individual from the payer and societal perspective, respectively. These costs are in line with costs that were reported by Dolk et al.Citation20. However, there are certain differences in costs between both analyses due to differing assumptions on hospitalizations and deaths in the model. In our analysis, the probability to be hospitalized or to die is complication-specific and, where possible, age-dependent. In comparison, Dolk et al. assumed that hospitalization and mortality solely depend on age and not on the complication type.
Results from this study are in line with similar analyses conducted on the comparison of QIVc versus QIVe in the UK, Italy, Spain, and in the USCitation14–17. In all countries, the obtained ICERs for QIVc versus QIVe are far below the WTP threshold from the payer perspective, and QIVc is a dominant vaccination strategy from the societal perspective. Compared to our study, a different rVE was used in studies in the UK and Spain due to age restriction of QIVc in these two countries. In the analysis conducted in the UK population, a rVE (26.8%) was used since the use of QIVc is recommended only in those under 65 years in the UKCitation15,Citation45. Without these age restrictions, rVE in our German CEM was assumed to be 36.2% in the base case and 19.3% in the scenario analysis. In the UK-based study, it was assumed that 100% of the years are egg-adapted, and the resulting ICER was £598 from the payer perspectiveCitation15. In the analysis conducted in Spain, rVE was also assumed to be 26.8% due to a similar age restriction as that recommended in the UK. The reported ICER (QIVc vs. QIVe) was €12,852 from the payer perspective and QIVc was dominant from the societal perspectiveCitation17. Similar to our study, rVE was assumed to be 36.2% in the base case and 19.3% in scenario analyses for ItalyCitation14. The QIVc was cost-effective compared to QIVe from the payer perspective with ICER of €2,714 for the base case and €8,021 for a scenario analysis, and was reported as a dominant vaccine strategy in both the base case and scenario analysesCitation14. The study conducted in the US shows that using QIVc instead of QIVe can reduce annual outpatient visits and hospitalizations by three million and 84,185, respectively. Using QIVc can be a cost saving alternative vaccination strategy for the US Health Care SystemCitation16. Pro-rated to the German population size, using QIVc instead of QIVe results in a more modest reduction of outpatient visits and hospitalizations by 51,114 and 2,091 (for Setting A) and by 26,410 and 1,091 for Setting B, and in substantial (€170.8 million for Setting A and for €78.1 million for Setting B) savings from a societal perspective.
Note that there is also a study that does not show a significant better effectiveness of QIVcCitation46. In a case-control study, in patients aged < 65 years, statistically significant protection against influenza hospitalization of QIVc compared to QIVe was not observedCitation46. However, it should be noted that comorbidities were typically more present among recipients of QIVc than recipients of QIVe, especially among patients ages < 65 years in the study. Selection bias could occur if QIVc vaccinated patients, who had more comorbidities, were more likely to be hospitalized and tested for influenza than QIVe vaccinated individuals. Moreover, the population size of the QIVc group (75 patients < 65 years and 157 patients ≥ 65 years) is very small compared to the QIVe group (1,741 patients < 65 years and 3,498 patients ≥ 65 years). Furthermore, there may be other factors that affect VE, such as the immune response to prior influenza infection/vaccination and host characteristics, but that are not considered in the study.
This CEA has several strengths such as the flexibility to allow input per age. The model and analyses are based on a simple, transparent, and published model structure and the main source of inputs in our model came from one published model. The model also included stroke and MI as possible outcomes which were not included in the analyses of Dolk et al.Citation20 performed in a German population earlier. Our study was conducted from societal as well as payer perspectives. Therefore, study findings may provide support for reimbursement of QIVc in Germany. Uncertainty in model parameters was tested by performing a number of sensitivity analyses. The rVE and aVE of QIVe, probabilities of being symptomatic and the number of mismatched years were the main drivers of the NMB. All uncertainty testing demonstrated robustness of the model.
However, results from this study must be interpreted in light of a few limitations. The main limitation for this CEM was related to costs. Firstly, costs data used within CEM were mainly based on Dolk et al.Citation20 and were inflated to the current year. Hence, we assume that those cost sources are still valid in the current scenario. Secondly, absenteeism cost, which is a major cost item in this analysis, was estimated based on the assumption that the number of working days lost is the same as the duration of the disease. However, people may take fewer (or more) days off in reality and parents might not take time off to be with their children during sickness.
Furthermore, changes in medical management can impact medical resource use over time, which are not reflected in this CEM. Non-availability of absolute effectiveness levels for the different vaccines in matched and mismatched years can also be considered as a limitation for this study because the same relative effectiveness leads to different absolute differences depending on the baseline effectiveness value.
Conclusion
This study suggests that influenza vaccination with QIVc is cost-effective compared with QIVe in Germany. From a societal perspective, the introduction of QIVc can help in avoiding a significant number of influenza cases and complications while generating cost savings (dominant strategy).
Transparency
Declarations of funding
This work was funded by Seqirus Inc.
Declaration of financial/other interests
RC, LG, and ML are full-time employees of IQVIA. IQVIA received consulting fees from Seqirus for the development of the cost-effectiveness model. ME is a shareholder of Epimos GmbH, which has received funding from Seqirus to complete the work disclosed here as well as consultancy from other pharmaceutical companies. MS is a shareholder of ExploSYS GmbH, which has received funding from Epimos GmbH to complete the work disclosed here. SR and JFM-Q are employees of Seqirus and shareholders of CSL Limited.
JME peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Previous presentations
Results were presented as a poster at the Virtual European Ispor Conference 2020. Cai R, Eichner M, Schwehm M, Rajaram S, Mould-Quevedo J, Lamotte M. Cost-effectiveness of the CELL-based quadrivalent versus the standard egg-based quadrivalent influenza vaccine in Germany. Results of a dynamic transmission modeling approach. ISPOR Europe 2020 Virtual conference, Milano, November 16–19, Abstract ID# 105463.
Supplemental Material
Download MS Word (382 KB)Acknowledgements
Chandresh Kumar and Paranjoy Saharia are employees of IQVIA India, who have provided writing support for this manuscript.
References
- World Health Organization (WHO). Influenza (Seasonal) 2018. Available from: https://www.who.int/en/news-room/fact-sheets/detail/influenza-(seasonal).
- Robert Koch Institut (RKI). Report on the epidemiology of influenza in Germany - 2018/2019. Berlin, Germany; 2019.
- Sellers SA, Hagan RS, Hayden FG, et al. The hidden burden of influenza: a review of the extra-pulmonary complications of influenza infection. Influenza Other Respir Viruses. 2017;11(5):372–393.
- Centers for Disease Control and Prevention (CDC). People at high risk for flu complications. Available from: https://www.cdc.gov/flu/highrisk/index.htm.
- Robert Koch Institut (RKI). Recommendations of the Standing Committee on Vaccination (STIKO) at the Robert Koch Institute – 2017/2018. Berlin, Germany; 2018.
- Harding AT, Heaton NS. Efforts to improve the seasonal influenza vaccine. Vaccines (Basel). 2018;6(2):19.
- Rajaram S, Boikos C, Gelone DK, et al. Influenza vaccines: the potential benefits of cell-culture isolation and manufacturing. Ther Adv Vaccines Immunother. 2020;8:2515135520908121.
- Wu NC, Zost SJ, Thompson AJ, et al. A structural explanation for the low effectiveness of the seasonal influenza H3N2 vaccine. PLoS Pathog. 2017;13(10):e1006682.
- Zost SJ, Parkhouse K, Gumina ME, et al. Contemporary H3N2 influenza viruses have a glycosylation site that alters binding of antibodies elicited by egg-adapted vaccine strains. Proc Natl Acad Sci U S A. 2017;114(47):12578–12583.
- Skowronski DM, Janjua NZ, De Serres G, et al. Low 2012-13 influenza vaccine effectiveness associated with mutation in the egg-adapted H3N2 vaccine strain not antigenic drift in circulating viruses. PLoS One. 2014;9(3):e92153.
- Centers for Disease Control and Prevention (CDC). Cell-based flu vaccines. Available from: https://www.cdc.gov/flu/prevent/cell-based.htm.
- European Medicines Agency (EMA). Flucelvax Tetra, summary of product characteristics. Available from: https://www.ema.europa.eu/en/documents/product-information/flucelvax-tetra-epar-product-information_en.pdf.
- Seqirus A CSL company. First cell-based quadrivalent influenza vaccine approved for use in Europe. Available from: https://www.seqirus.com/news/first-cell-based-quadrivalent-influenza-vaccine-approved-in-europe.
- Calabro GE, Boccalini S, Del Riccio M, et al. Valutazione di health technology assessment (HTA) del vaccino antinfluenzale quadrivalente da coltura cellulare: FLUCELVAX TETR [Health technology assessment (HTA) evaluation of quadrivalent cell culture flu vaccine: FLUCELVAX TETR]. Ital J Public Health. 2019;8(5):113–143.
- Nguyen VH, Nasiri M, Mould-Quevedo J, et al. Cost-effectiveness of a cell culture-based quadrivalent influenza vaccine in the adult population of the United Kingdom. Presented at: 29th National Immunisation Conference for Health Care Workers, London, UK. 2018.
- Nguyen VH, Hilsky Y, Mould-Quevedo J. The epidemiological and economic impact of a cell-based quadrivalent influenza vaccine in an adult population in the US: a dynamic modelling approach. Research Square. 2020. DOI:https://doi.org/10.21203/rs.3.rs-27872/v1
- Ruiz-Aragón J, Gani R, Márquez S, et al. Estimated cost-effectiveness and burden of disease associated with quadrivalent cell-based and egg-based influenza vaccines in Spain. Hum Vaccin Immunother. 2020;16(9):2238–2244.
- Eichner M, Schwehm M, Hain J, et al. 4Flu – an individual based simulation tool to study the effects of quadrivalent vaccination on seasonal influenza in Germany. BMC Infect Dis. 2014;14:365.
- Haas W. Influenza: Prävention, Diagnostik, Therapie und öffentliche Gesundheit. Munich (Germany): Elsevier GmbH; 2009.
- Dolk C, Eichner M, Welte R, et al. Cost-utility of quadrivalent versus trivalent influenza vaccine in Germany, using an individual-based dynamic transmission model. Pharmacoeconomics. 2016;34(12):1299–1308. PubMed PMID: 27647004; PubMed Central PMCID: PMCPMC5110585 this study (GSK Study Identifier: HO-14-14994) and was involved in all stages of the study, including analysis of the data. GlaxoSmithKline Biologicals SA also covered all costs associated with the development and publication of this manuscript. Conflict of interest AA, RSO and RW are employees of the GSK group of companies. RSO, AA and RW declare restricted shares ownership in the GSK group of companies. ME is a shareholder of Epimos GmbH, which has received funding from the GSK group of companies to complete the work disclosed here as well as consultancy fees from AstraZeneca. MS is a shareholder of ExploSYS GmbH, which has received funding from Epimos GmbH to complete the work disclosed here. BPN’s, LAVB’s and IVV’s companies received consultancy fees from the GSK group of companies to complete the work disclosed here as well as for other work outside this study. MP received grants and honoraria from various pharmaceutical companies, inclusive of those developing, manufacturing and marketing influenza vaccines. CD declares no conflict of interest. eng.
- STIKO. 2016. Modelling methods for predicting epidemiological and health economic effects of vaccinations – Guidance for analyses to be presented to the German Standing Committee on Vaccination (STIKO) (last updated: 16 March 2016), Berlin.
- IQWiG. Institute for quality and efficiency in health care: general methods. Cologne, Germany;2015.
- European Medicines Agency (EMA). 2018. The Agency’s Committee for Medicinal Products for Human Use (CHMP): European public assessment report (EPAR). http://www.ema.europa.eu/ema/.
- Eichner M, Schwehm M, Eichner L, et al. Direct and indirect effects of influenza vaccination. BMC Infect Dis. 2017;17(1):308.
- Boikos C, Sylvester GC, Sampalis JS, et al. Effectiveness of the cell culture- and egg-derived, seasonal influenza vaccine during the 2017–2018 Northern Hemisphere Influenza Season. Atlanta (GA): National Adult and Influenza Immunization Summit; 2019.
- Belongia EA, Simpson MD, King JP, et al. Variable influenza vaccine effectiveness by subtype: a systematic review and meta-analysis of test-negative design studies. Lancet Infect Dis. 2016;16(8):942–951.
- Rajaram S, Suphaphiphat P, van Boxmeer J, et al. Retrospective assessment of the antigenic similarity of egg-propagated and cell culture-propagated reference influenza viruses as compared with circulating viruses across influenza seasons 2002–2003 to 2017–2018. IJERPH. 2020;17(15):5423.
- Jefferson T, Di Pietrantonj C, Al-Ansary LA, et al. Vaccines for preventing influenza in the elderly. Cochrane Database Syst Rev. 2018;2(2):CD004876.
- Jefferson T, Di Pietrantonj C, Rivetti A, et al. Vaccines for preventing influenza in healthy adults. Cochrane Database Syst Rev. 2010;2(7):CD001269.
- Jefferson T, Rivetti A, Di Pietrantonj C, et al. Vaccines for preventing influenza in healthy children. Cochrane Database Syst Rev. 2012;2012(8):CD004879.
- Carrat F, Vergu E, Ferguson NM, et al. Time lines of infection and disease in human influenza: a review of volunteer challenge studies. Am J Epidemiol. 2008;167(7):775–785.
- Boehme AK, Luna J, Kulick ER, et al. Influenza-like illness as a trigger for ischemic stroke. Ann Clin Transl Neurol. 2018;5(4):456–463.
- Kwong JC, Schwartz KL, Campitelli MA, et al. Acute myocardial infarction after laboratory-confirmed influenza infection. N Engl J Med. 2018;378(4):345–353.
- Palm F, Urbanek C, Rose S, et al. Stroke incidence and survival in Ludwigshafen am Rhein, Germany: the Ludwigshafen Stroke Study (LuSSt). Stroke. 2010;41(9):1865–1870.
- Ara R, Brazier JE. Using health state utility values from the general population to approximate baselines in decision analytic models when condition-specific data are not available. Value Health. 2011;14(4):539–545.
- Mauskopf JA, Cates SC, Griffin AD, et al. Cost effectiveness of Zanamivir for the treatment of influenza in a high risk population in Australia. Pharmacoeconomics. 2000;17(6):611–620.
- Inflation rates. Inflation rates in Germany 2018. Available from: https://www.inflation.eu/inflation-rates/germany/historic-inflation/cpi-inflation-germany-2017.aspx.
- Robert Koch Institut (RKI). Influenza surveillance: vaccinnation coverage per region in Germany from the year 2009 onwards. Berlin (Germany): Robert Koch Institute; 2018.
- G-DRG-Browser. G-DRG-Browser 2018 2018. Available from: http://www.g-drg.de.
- EBM. Uniform rating scale: the uniform standard of evaluation (EBM) is the remuneration system of contract medical or contract psychotherapeutic care in Germany 2018. Available from: https://www.bundesgesundheitsministerium.de/service/begriffe-von-a-z/e/einheitlicher-bewertungsmassstab-ebm.html.
- EUROSTAT. Labour costs. https://ec.europa.eu/eurostat/web/labour-market/labour-costs. 2019a.
- EUROSTAT. Employment and unemployment. https://ec.europa.eu/eurostat/web/lfs/data/database. 2019b.
- Kotseva K, Gerlier L, Sidelnikov E, et al. Patient and caregiver productivity loss and indirect costs associated with cardiovascular events in Europe. Eur J Prev Cardiol. 2019;26(11):1150–1157.
- Stahmeyer JT, Rossol S, Liersch S, et al. Cost-effectiveness of treating hepatitis C with sofosbuvir/ledipasvir in Germany. PloS One. 2017;12(1):e0169401.
- JCVI PHE (UK). Joint Committee on Vaccination and Immunisation: Advice on influenza vaccines for 2020/21. London (UK): Department of Health & Social Care, Government of the United Kingdom; 2019.
- Bruxvoort KJ, Luo Y, Ackerson B, et al. Comparison of vaccine effectiveness against influenza hospitalization of cell-based and egg-based influenza vaccines, 2017–2018. Vaccine. 2019;37(39):5807–5811.
