Abstract
Aim
We evaluated the cost-effectiveness of nivolumab in combination with ipilimumab (NIVO + IPI) versus platinum-doublet chemotherapy (PDC) for the first-line treatment of stage IV or recurrent non-small cell lung cancer (NSCLC) from a third-party payer perspective in the United States (US).
Methods
A partitioned survival model was developed using efficacy, safety, and utility inputs derived from Part 1 of the phase 3 CheckMate 227 trial (NCT02477826) with 37.7-month minimum follow-up for overall survival (OS). OS and progression-free (PF) survival were extrapolated over a 20-year time-horizon using parametric spline-based models selected based on goodness of fit and validated with data from external sources. Duration of treatment Kaplan–Meier curves were used for treatment cost calculations. US-specific costs (2021 dollars) for drug acquisition, administration, and monitoring; disease management (PF and progressed disease health states); end-of-life care; adverse events; and subsequent treatments were derived from publicly available sources. Time-to-death utilities were applied in the base case, whereas treatment-specific progression-based utilities were tested in a scenario analysis. Main outcomes included incremental cost per life-year gained (LYG) and quality-adjusted life-year (QALY). Model uncertainty was assessed through deterministic and probabilistic sensitivity analyses.
Results
NIVO + IPI resulted in 1.53 additional life-years, 1.33 additional QALYs, and $142 088 in additional costs compared with PDC. The incremental cost per LYG was $92 651, whereas incremental cost per QALY gained was $106 553. The application of treatment-specific progression-based utilities yielded an incremental cost per QALY gained of $117 076. Probabilistic sensitivity analysis revealed a 98% probability that NIVO + IPI was cost-effective versus PDC at a willingness-to-pay threshold of $150 000 per QALY.
Conclusions
NIVO + IPI was estimated to be cost-effective as a first-line treatment for stage IV or recurrent NSCLC in the US, with increased survival and higher cost compared with PDC.
Introduction
Lung cancer is the leading cause of cancer-related mortality, accounting for almost 25% of cancer deaths in the United States (US) and 18% worldwideCitation1,Citation2. In 2021, an estimated 235 760 people in the US were diagnosed with lung cancer, which was responsible for 131 880 deathsCitation2. Non-small cell lung cancer (NSCLC) accounts for 84% of all lung cancer casesCitation2. Advanced NSCLC patients treated with chemotherapy from September 2002 to October 2003 had a 5-year survival rate of 8% or lessCitation3,Citation4. Prior to recent advances, patients with advanced NSCLC had very poor prognoses; among US patients diagnosed between 2008 and 2014, the 5-year survival rate for newly diagnosed NSCLC was 22.7% and the survival rate was 5.5% for newly diagnosed NSCLC with distant metastasesCitation5. The introduction of immune checkpoint inhibitors (ICIs) to restore antitumor immunity, with the first approvals in advanced NSCLC in 2015, has substantially changed the landscape of treatment for NSCLC, with nivolumab in second-line treatment showing a 5-year survival rate of approximately 15%Citation6,Citation7.
Nivolumab (NIVO)Citation8 and ipilimumab (IPI)Citation9 are ICIs with distinct and complementary mechanisms of action, targeting programmed cell death receptor-1 and cytotoxic T-lymphocyte antigen-4, respectivelyCitation10. In combination, NIVO and IPI have been shown to confer a durable survival benefit in multiple tumor types and a benefit over chemotherapy in esophageal squamous cell carcinoma and unresectable pleural malignant mesotheliomaCitation11–16.
CheckMate 227 is a randomized, open-label, phase 3 clinical trial that includes adults with squamous and non-squamous stage IV or recurrent NSCLC who were previously untreated for advanced diseaseCitation17,Citation18. The design of CheckMate 227 has been described in detail elsewhereCitation17,Citation18. Briefly, patients with tumor programmed death ligand 1 (PD-L1) expression ≥1% (PD-L1 ≥ 1%; Part 1a) and PD-L1 expression <1% (PD-L1 < 1%; Part 1b) were enrolled (together, Part 1). Patients were randomly assigned (1:1:1) to receive NIVO (3 mg/kg every 2 weeks) plus IPI (1 mg/kg every 6 weeks), NIVO monotherapy (240 mg every 2 weeks), or histology-based platinum-doublet chemotherapy (PDC) every 3 weeks for up to 4 treatment cycles (Part 1a) or to receive NIVO + IPI, NIVO (360 mg every 3 weeks) plus PDC, or PDC alone (Part 1b). All patients were stratified by tumor histology (squamous vs non-squamous) in both study parts. Immunotherapy treatment continued until disease progression or unacceptable toxicity, for a maximum of 2 yearsCitation17,Citation18.
In the February 28, 2020 database lock (DBL) for CheckMate 227 after a minimum follow-up duration of 37.7 months (median, 43.1 months), median overall survival (OS) was improved with NIVO + IPI over PDC regardless of tumor PD-L1 expression, at 17.1 months versus 14.9 months in patients with PD-L1 ≥ 1% (hazard ratio for death [HR], 0.79; 95% confidence interval [CI], 0.67–0.93), and 17.2 months versus 12.2 months in those with PD-L1 < 1% (HR, 0.64; 95% CI, 0.51–0.81)Citation19. The proportion of patients with grade 3 or 4 treatment-related adverse events (AEs) was 33% with NIVO + IPI and 36% with PDC (minimum safety follow-up of >36.3 months)Citation19.
NIVO + IPI was approved by the US Food and Drug Administration (FDA) in May 2020 as a first-line treatment for adults with metastatic NSCLC expressing PD-L1 ≥ 1%, with no epidermal growth factor receptor (EGFR) or anaplastic lymphoma kinase (ALK) genomic tumor aberrationsCitation8,Citation9,Citation20. After the regulatory approval, cost-effectiveness analyses were undertaken from a third-party payer perspective in the US. The present study evaluated the cost-effectiveness of NIVO + IPI versus PDC for the first-line treatment of stage IV or recurrent NSCLC regardless of PD-L1 expressionCitation18,Citation19.
Methods
Model population
The modeled population was based on all of Part 1 of CheckMate 227 (NCT02477826), which comprised adults with squamous and non-squamous stage IV or recurrent NSCLC who were previously untreated for advanced diseaseCitation17,Citation18. Since the observed benefits of treatment with NIVO + IPI were independent of PD-L1 expression in CheckMate 227, the model population reflects the overall trial population (Part 1), thereby allowing preservation of the full trial sample size and a more robust analysis of the data.
CheckMate 227 was conducted in accordance with the Declaration of Helsinki and International Conference on Harmonisation Good Clinical Practice guidelines. The study protocol and protocol amendments were approved by institutional review boards/independent ethics committees at participating centers. Written informed consent was provided by all patients.
Model framework
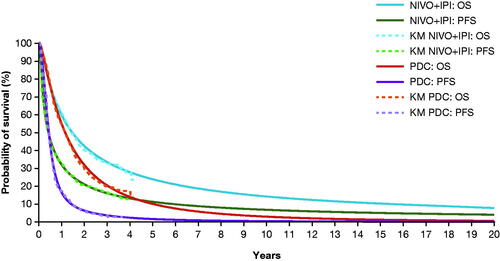
A cohort-based partitioned survival model was developed to evaluate the cost-effectiveness of NIVO + IPI compared with PDC. The model consisted of 3 mutually exclusive health states: progression free (PF), progressed disease (PD), and death. Duration of treatment (DoT) Kaplan–Meier (KM) curves were used to estimate treatment cost and DoT was defined as the time from the start dose date plus 1 day to the last dose date. Time spent in the PF and PD health states was estimated using fitted parametric and spline-based survival models for progression-free survival (PFS) and OS. Patients entered the model in the PF health state, where they could remain or transition into the PD or death health states. The patients in the PD state were based on the difference between patients who were alive (OS) and those remaining PF (PFS).
Costs and outcomes of treatments were calculated by combining occupancy in the PF and PD health states with the costs, disease management resource use, and measures of health effects associated with those states. The analysis adopted a third-party payer perspective in the US, including public (Medicare costs) and private (e.g. Kaiser Permanente) healthcare providers, and a 20-year lifetime horizon, consistent with published manuscripts on cost-effectiveness analysis of ICIs in NSCLCCitation21–26. Weekly model cycles were used for the first 28 weeks, followed by 4-week cycles. Half-cycle correction was applied. Costs and health outcomes were discounted at 3.0% annuallyCitation27. The main model outcomes included incremental cost per life-year gained (LYG) and incremental cost per quality-adjusted life-year (QALY) gained.
Survival analyses
OS, PFS, and DoT data were derived from Part 1 of CheckMate 227 for the NIVO + IPI and PDC treatment arms. Standard and spline-based curves were fitted to the OS and PFS data to smooth the stepped function of the KM curves and to extrapolate survival over the time horizon of the analysis. The survival analysis was conducted using the FlexSurv package in R.
Model fitting was conducted following National Institute for Health and Care Excellence (NICE) guidanceCitation21,Citation28. Statistical tests (e.g. Therneau and Grambsch correlation test) and log-cumulative hazard plots suggested that the proportional hazards assumption was violated between the treatment arms for both OS and PFS; therefore, survival models were fitted independently for each treatment arm.
The base-case survival models were selected based on goodness-of-fit statistics (Akaike information criterion and Bayesian information criterion), visual inspection, and validation of extrapolations with longer-term external data. To validate the OS extrapolations in the NIVO + IPI arm, a conditional survival curve was constructed using longer-term external data from other Bristol Myers Squibb-sponsored NSCLC studies and registry data ().
Figure 1. Constructed conditional survival curve for OS in the NIVO + IPI arm. Abbreviations. CM, CheckMate; IPI, ipilimumab; NIVO, nivolumab; OS, overall survival; PFS, progression-free survival; SEER, Surveillance, Epidemiology and End Results.
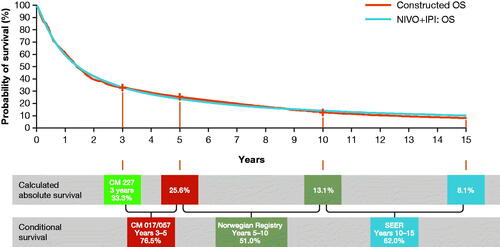
The conditional survival curve was constructed in a stepwise manner with each successive step adopting best available longer-term data from NSCLC patients with characteristics as close as possible to the trial population. OS for NIVO + IPI from 0 to year 3 reflects Part 1 of the CheckMate 227 trial, which had a minimum follow-up for OS of 37.7 months. Conditional survival estimates (defined as the percentage of patients alive in year X who will survive to year Y) from the CheckMate 017 and 057 trials of NIVO in pretreated NSCLC were used to predict OS for years 3–5Citation29. Conditional survival estimates for years 5–10 and for years 10–15 were obtained from Norwegian and Surveillance, Epidemiology, and End Results Program registry data.
The 5-, 10-, and 15-year OS estimates of 23.7%, 14.1%, and 10.1% derived from the constructed curve, shown in , can be compared with the 25.6%, 13.1%, and 8.1%, respectively, estimated extrapolating CheckMate 227 OS data using the 2 knots spline on odds.
The estimates of the constructed curve can be considered to produce conservative survival estimates for patients treated with NIVO + IPI for the first-line treatment of metastatic NSCLC given that it was derived using data from trials testing NIVO monotherapy in pretreated patients (CheckMate 017/057) and registries that to a large extent represent chemotherapy, since the data refer to a time before the advent of immuno-oncology therapy. Hence, the selected OS curve for NIVO + IPI was considered appropriate.
The use of parametric extrapolations was deemed unnecessary for DoT of NIVO + IPI and PDC due to the maturity of the data (there is a treatment stopping rule in the CheckMate 227 trial for NIVO + IPI at 24 months; as the trial has more than 24 months minimum follow-up, the data for this endpoint cover the required time). Therefore, KM curves were used directly for cost calculations in the base case, and the use of parametric extrapolations for DoT was tested in the scenario analysis. The base case survival models selected for the base case OS was the 2 knots spline on odds for the NIVO + IPI arm and 1 knot spline normal for the PDC arm (). For PFS, the 2 knots spline on odds for NIVO + IPI and 2 knots spline on hazard for PDC were selected. As noted, KM curves were used for DoT for each treatment arm ().
Safety data
As grade 3–5 AEs are likely to require active management and are therefore related to health care resource utilization and costs, only grade 3–5 treatment-related AEs experienced by ≥5% of patients receiving NIVO + IPI and PDC in Part 1 of CheckMate 227 were included in the analysis (Table S1).
Utilities and disutilities
The EQ-5D–3-level questionnaire results from CheckMate 227 Part 1 patient-level data were analyzed to derive the utility index using US weightsCitation30 with progression-based and time-to-death (TTD) approaches. A mixed model approach was used to account for repeat EQ-5D-3L measurements per patient within a health state. For both scenarios, two models (one without and one with treatment) were fitted. The variable(s) defining health states and treatment, if included, and the interaction between them were included in the model as fixed effects. Random intercept was used to account for repeated measurements within each patient. The health state utilities were estimated as least-square mean and standard errors and 95% confidence intervals. The addition of treatment was significant for progression-based but not for TTD models; therefore, treatment-specific progression-based utilities (Table S2) and overall TTD utilities (Table S3) were used in the economic analysis.
TTD utilities were used in the base case because they provided more granularity in capturing the deteriorating quality of life of patients with cancer as they approach death compared with progression-based utilities. They have been adopted in several previously published cost-effectiveness analyses of first-line treatments for metastatic NSCLC in the US settingCitation24,Citation25,Citation31,Citation32.
The disutilities related to AEs (Table S1) were derived from a review of previous health technology assessment submissions to NICE in advanced NSCLC and were applied as a one-off decrement in the first treatment cycle. When treatment-specific utilities were applied as a scenario analysis (see ), it was assumed that these would have indirectly accounted for any treatment-related AEs; therefore, disutilities due to AEs were excluded from the scenario to avoid potential double counting.
Table 1. Scenario analyses results for NIVO + IPI versus PDC.
Costs
US-specific unit costs for drug acquisition, administration, and monitoring; disease management (PF and PD health states); end-of-life care; management of AEs; and subsequent treatments were included in the model. Costs from older sources were inflated by the medical care consumer price index (CPI) to 2021 (US$) using the average index across all monthsCitation33. Disease-management costs were calculated to be $524 (PF health state) and $1 628 (PD health state) per 4 weeks (; Table S4).
Table 2. Model cost inputs.
An end-of-life cost of $12 303Citation34 was applied as a one-off fixed cost. The drug acquisition cost for NIVO was estimated at $6 271 per dose and $15 765 per dose for IPI (). For PDC, the per-dose cost of $5 203 () was calculated using a weighted average of the proportion of patients receiving each drug combination in Part 1 of CheckMate 227 (Table S5). Average patient weight and body surface area were used to calculate the mg per dose required for each treatment and the cost per dose for each treatment was calculated by assuming no vial sharing (i.e. if the full vial is not used, the remaining content was considered wastage). The option of including/excluding vial sharing was separate for each treatment included. In a scenario analysis, vial sharing was included for all treatments.
Drug administration costs () were calculated using Medicare costs based on time and number of infusions received at any administration for each treatment. Drug monitoring costs included office visits and laboratory tests (Table S4). The cost of AEs () was based on the cost of management of each AE and the proportion of patients incurring each AE for each treatment (Table S1). This was applied as a one-off cost in the first treatment cycle.
To calculate subsequent treatment costs following failure of first-line therapy, 38% of patients in the NIVO + IPI arm and 54% in the PDC arm were estimated to receive second-line systemic anticancer therapies as observed in Part 1 of CheckMate 227. Distribution, acquisition costs, and administration costs of subsequent treatments are shown in Table S6. The duration of second-line therapy was assumed to be 6.77 months with immuno-oncology therapies and 5.09 months with chemotherapy based on a previously conducted cost-effectiveness analysis of NIVO in pretreated NSCLCCitation35–37.
Sensitivity and scenario analyses
Deterministic and probabilistic sensitivity analyses were conducted to assess the impact of uncertainty in model input parameters on the results; these are described in Supplemental Methods, Sensitivity Analyses (Table S7 and S8). Scenario analyses were undertaken to investigate the impact of applying treatment-specific and overall progression-based utilities, as well as KM parametric extrapolation of DoT and more conservative approaches to OS extrapolation ().
Results
Cost-effectiveness analysis
In the base-case analysis, treatment with NIVO + IPI resulted in increased life-years, QALYs, and total costs compared with PDC (). The cost breakdown indicated that the higher total cost observed with NIVO + IPI compared with PDC ($262 689 versus $120 601) was due primarily to the difference in drug acquisition costs (). The incremental cost of NIVO + IPI versus PDC was $142 088 (). Incremental life-years for NIVO + IPI versus PDC were 1.53, and incremental QALYs were 1.33 (). The incremental cost per LYG was $92 651, and the incremental cost per QALY gained was $106 553.
Table 3. Base-case results for NIVO + IPI versus PDC.
Sensitivity analyses
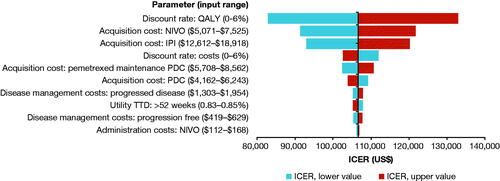
As expected, deterministic sensitivity analysis indicated that the discount rate applied to QALY, followed by the drug acquisition costs for NIVO + IPI () were the main driver of the results. The other most influential parameters affecting the results (>1% change in the incremental cost-effectiveness ratio) were discount rates applied to costs, drug acquisition cost for PDC, disease management costs for PD and PF health states, and utility weight for TTD >52 weeks (; Table S8).
Figure 3. Deterministic sensitivity analysis of NIVO + IPI versus PDC showing the 10 most impactful parameters on the model result. See Supplementary Material, Supplemental Methods, Sensitivity Analyses for details of the analysis. Abbreviations. ICER, incremental cost-effectiveness ratio; IPI, ipilimumab; NIVO, nivolumab; PDC, platinum-doublet chemotherapy; QALY, quality-adjusted life-year; TTD, time to death.
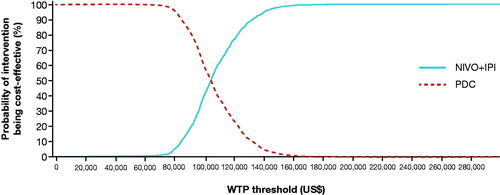
Probabilistic sensitivity analysis based on 1 000 iterations generated results consistent with the base case (incremental cost per QALY gained, $104 533 vs $106 553). The cost-effectiveness acceptability curve indicates that NIVO + IPI has a probability of 98% to be cost-effective at a willingness-to-pay (WTP) threshold of $150 000 per QALYCitation38 ().
Figure 4. Cost-effectiveness acceptability curve for NIVO + IPI and PDC. See Supplementary Material, Supplemental Methods, Sensitivity Analyses for details of the analysis. Abbreviations. IPI, ipilimumab; NIVO, nivolumab; PDC, platinum-doublet chemotherapy; WTP, willingness to pay.
Scenario analyses
All scenarios resulted in small changes versus base case (). The greatest magnitude of change was observed when applying treatment-specific progression-based utilities; this resulted in slightly lower QALYs in each treatment arm compared with the base case (NIVO + IPI, 2.73 vs 2.89; PDC, 1.52 vs 1.55) and incremental cost per QALY gained increased to $117 076 compared with the base case of $106 553.
Discussion
The present analysis estimates the cost-effectiveness of NIVO + IPI versus PDC as first-line treatment of adult patients with metastatic NSCLC with no EGFR or ALK genomic tumor aberrations in the US based on Part 1 of CheckMate 227. PDC was considered the most suitable comparator among the treatment evaluated in the CheckMate 227 as first-line NIVO monotherapy has not been approved by the US FDA for the treatment of adults with metastatic NSCLC. With distinct but complementary mechanisms of action, NIVO and IPI in combination have improved long-term survival versus PDC for patients with NSCLCCitation17,Citation18. The incremental cost per QALY gained for NIVO + IPI versus PDC was $106 553 using TTD utilities and $117 076 using progression-based, treatment-specific utilities.
TTD utilities were used in the base case because they provided a better statistical fit to the data and better captured the deteriorating quality of life compared with utilities based on progression. Scenario analyses conducted using progression-based utilities resulted in a modest (<10%) increase in the ICER.
Deterministic sensitivity analysis indicated that, other than drug acquisition costs, the variation of the discount rates applied to QALY and costs, the cost of disease management in PD and PF state, and the utility values applied to NSCLC patients before 52 weeks from death (TTD >52 weeks) had the highest impact on the results, whereas most parameters had an impact of less than 1%. The finding that the discount rates for costs and QALYs had the greatest impact on ICER was expected, because costs and benefits were distributed over a long-time horizon.
Probabilistic sensitivity analyses estimated that NIVO + IPI was 98% likely to be cost-effective versus PDC at a WTP threshold of $150 000 per QALY. In the absence of an explicit threshold in the US, Ubel et al. noted the cost-effectiveness threshold acceptable in 2003 approached $200 000 or more per QALYCitation39; adjusting to 2020 dollars, this threshold exceeds $300 000Citation33. More recent theoretical and empirical evidence has suggested that acceptable thresholds in metastatic cancer in the end-of-life context may be higherCitation40,Citation41. Compared to this body of evidence, the projected ICERs presented here are well within the range considered cost-effective in the US.
Assessment of three previously published studies evaluating the cost-effectiveness of NIVO + IPI for the first-line treatment of NSCLC revealed differences and weaknesses in model structure, assumptions, and inputs. These studies used published summary data from the CheckMate 227 trial for efficacy and safety rather than individual patient dataCitation42–44. Also, DoT was not available in the literature, and therefore PFS was used as a proxy; this would considerably overestimate the costs in this particular trial, as at 1 year, 31% of patients randomized to NIVO + IPI were PF but only 22% remained on treatment. All studies applied progression-based utilities from published literature. Of note, two of these previously published analyses used a shorter time horizon (10 years) than that used here, and thus may not have captured the predicted long-term benefits of NIVO + IPICitation42,Citation43. Outside of these studies involving ICIs including NIVO + IPI, authors have tended to support a horizon of 20 years or more in order to capture the long-term survival of ICIs in NSCLC and other tumorsCitation23–26.
Courtney et al.Citation42 reported an ICER of $401 700 with an incremental cost of $201 900 and an incremental QALY value of 0.50 (vs $139 197 and 1.53 QALYs in the present study). The shorter time horizon (10 vs 20 years) of the model and the use of PFS as to estimate treatment cost (overstating treatment duration in CheckMate 227, as noted above) are the likely important drivers of differences in results from the present study. Other aspects of the model structure and assumptions are also likely to contribute to differences in ICER estimates with the present study. Although the present study used a partitioned survival model and Courtney et al. used a Markov model, these two model types have been shown to yield similar ICER estimates in advanced NSCLCCitation45. Courtney et al. used four health states in their model – stable disease, stable disease during receipt of second-line treatment, disease progression, and death – but the health-state transitions and method of deriving the transition probabilities from the published CheckMate 227 endpoints were unclear. Extrapolation of OS and PFS beyond the trial’s follow-up was achieved by applying conditional survival from 42 months to both treatment armsCitation42. These survival data were derived from the SEER registry, and thus represent to a large extent the historical experience of patients treated with chemotherapy. However, in the present study, up to 5 years of available clinical trial data on NSCLC patients treated with ICIs were leveraged for validation of survival in the experimental arm, which may better reflect the experience of patients pretreated with NIVO + IPI in CheckMate 227; subsequently, conditional survival data from the SEER registry was used from 5 years onward to complete the validation curve. Finally, the current study was able to leverage utilities from the trial data, rather than those from the literature, based largely on the experience of patients treated with chemotherapy.
The analysis of Li et al. yielded an ICER of $180 307 with an incremental cost of $128 984 and an incremental QALY value of 0.715Citation43. The methodology applied in that study aligns with that in the present analysis in terms of both model structure and methodology used for survival extrapolations. However, Li et al. tested only standard parametric models and selected loglogistic analysis as a base-case, which likely influenced the results. The present analysis found that other parametric models not only provided a better statistical fit but also provided plausible long-term survival estimates. The extrapolated long-term survival estimates were validated by comparing them to those from the conditional survival curves constructed by utilizing external clinical trials with longer follow-up curves and real-world data sources provided a tool with which to select curves that provided realistic long-term survivals.
By contrast, Hu et al. only analyzed subgroups and found NIVO + IPI to be cost-effective for both the PD-L1 ≥ 1% and ≥50% populationsCitation44. In their three health-state model, Hu et al. selected Weibull distribution for the survival extrapolations, although their justification was not explained. In addition, the origin of the efficacy data for the PD-L1 ≥ 50% population that they used was unclear, as none of the cited studies reported a KM analysis for that subgroup.
Of note, this analysis included the overall population of Part 1 patients as the clinical benefits of NIVO + IPI have been consistently demonstrated regardless of PD-L1 expression in CheckMate 227Citation17,Citation19, whereas regulatory approval of NIVO + IPI for first-line treatment is limited to patients with NSCLC expressing PD-LI ≥1%, per the protocol-mandated primary endpoint of that study that led to this approvalCitation8,Citation9.
Strengths and limitations of the study
The partitioned survival model used in the present analysis is considered a valid approach, as demonstrated by Goeree et al.Citation45, who found that partitioned survival models and Markov models yield similar ICER estimates in advanced NSCLC. Using the partitioned survival approach has the advantage of being able to incorporate trial PFS and overall OS data directly, without separate estimation of transition probabilities. The reliance on individual patient data from the pivotal registrational trial CheckMate 227 directly comparing NIVO + IPI and PDC with a minimum follow-up of 3 years represents another strength of the study.
The extrapolations of OS and PFS beyond the trial were carried out in line with UK NICE Decision Support Unit guidelinesCitation21,Citation28. Long-term extrapolation of OS curves from clinical trials that do not extend for the expected survival length of patients is subject to uncertaintyCitation46. However, these extrapolations were validated against long-term data from other external sources, including randomized clinical trials in pretreated NSCLC patients and real-world data from cancer registries. A conditional survival curve was constructed to validate the long-term OS in the NIVO + IPI arm. The constructed OS curve can nonetheless be considered conservative, because the external sources involved clinical trials with pretreated patients and registry data that to a large extent pertain to chemotherapy-experienced patients treated prior to the advent of immuno-oncology, with SEER data specifically representing a US populationCitation47. That said, recently obtained 4-year survival data for CheckMate 227 demonstrate that the observed survival benefit with NIVO + IPI as a first-line therapy in these patients is maintainedCitation48. It should be noted that the efficacy of subsequent therapies was not directly modeled in our analysis.
The application of AE costs and disabilities in the first treatment cycle can be considered a conservative approach for two reasons. First, study week 1 has the maximum number of PF patients at risk of experiencing AEs, and second, the costs and outcomes (LYs and QALYs) accrued after 1 year on treatment would be discounted.
Our model was based on the all-randomized population from CheckMate 227 since the observed benefits of treatment with NIVO + IPI were independent of PD-L1 expression in the study. The absence of results from both PD-L1 ≥ 1% and PD-L1 < 1% populations is a limitation of the current analysis. Development of separate models for each of the PD-L1 expression groups remains a subject for future work.
A final limitation of the present analysis is that the resource use from the clinical trials may not represent that of real-world clinical practice. Like all clinical trials, CheckMate 227 was conducted in a strict clinical trial environment and may not represent patients seen in real-world settings. Moreover, disutilities for AEs were not directly reported in the CheckMate 227 trial and were therefore sourced from the published literature.
Conclusions
NIVO + IPI was associated with increased survival and higher costs compared with PDC in the first-line treatment of stage IV or recurrent NSCLC regardless of PD-L1 expression in the US. Estimated incremental cost-utility ratios for NIVO + IPI versus PDC were within a range considered acceptable in the US, particularly in the metastatic cancer settingCitation38–40.
Transparency
Declaration of funding
This study was funded by Bristol Myers Squibb. Parexel International was funded by Bristol Myers Squibb to conduct the analysis.
Declaration of financial/other interests
MB was an employee of Parexel International at the time of the analysis and is currently an employee of Sanofi. MAC, YY, NV, AL, SJL, and JRP are employees of Bristol Myers Squibb. PD was an employee of Parexel International at the time of the analysis and is currently employed as an independent consultant. ET is an employee of Parexel International. JK was an employee of Parexel International at the time of the analysis and is currently an employee of Daiichi-Sankyo Europe GmbH.
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Author contributions
All authors contributed to study conception and design, drafted the manuscript, and approved of the final version for submission. MB, ET, and JK performed the analysis.
Supplemental Material
Download MS Word (99.2 KB)Acknowledgements
Editorial support was provided by Parexel and was funded by Bristol Myers Squibb.
Data availability statement
The analysis reported in this study uses patient-level data from the CheckMate 227 trial. The results of the trial have been reported in a number of publicationsCitation18. The trial results supporting the findings of this analysis are presented graphically within the article. The survival analysis was implemented using the FlexSurv package in R.
References
- Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: Cancer J Clin. 2021;71(3):703–249.
- American Cancer Society. Lung cancer survival rates; 2021. [cited 2021 June 15] Available from: https://www.cancer.org/cancer/lung-cancer/detection-diagnosis-staging/survival-rates.html.
- Kaira K, Takahashi T, Murakami H, et al. Long-term survivors of more than 5 years in advanced non-small cell lung cancer. Lung Cancer. 2010;67(1):120–123.
- Lissoni P, Chilelli M, Villa S, et al. Five years survival in metastatic non-small cell lung cancer patients treated with chemotherapy alone or chemotherapy and melatonin: a randomized trial. J Pineal Res. 2003;35(1):12–15.
- Noone A, Howlader N, Krapcho M, et al. SEER Cancer Statistics Review (CSR) 1975-2015; 2018. [cited 2021 October 26] Available from: https://seer.cancer.gov/archive/csr/1975_2015/.
- Borghaei H, Gettinger S, Vokes EE, et al. Five-year outcomes from the randomized, phase III trials CheckMate 017 and 057: nivolumab versus docetaxel in previously treated Non-Small-Cell lung cancer. J Clin Oncol. 2021;39(7):723–733.
- Gettinger S, Horn L, Jackman D, et al. Five-year follow-up of nivolumab in previously treated advanced non-small-cell lung cancer: results from the CA209-003 study. J Clin Oncol. 2018;36(17):1675–1684.
- OPDIVO (nivolumab), [prescribing information]; 2021. Bristol Myers Squibb: Princeton, NJ.
- YERVOY (ipilimumab), [prescribing information]; 2020. Bristol Myers Squibb: Princeton, NJ.
- Mellman I, Coukos G, Dranoff G. Cancer immunotherapy comes of age. Nature. 2011;480(7378):480–489.
- Larkin J, Chiarion-Sileni V, Gonzalez R, et al. Five-year survival with combined nivolumab and ipilimumab in advanced melanoma. N Engl J Med. 2019;381(16):1535–1546.
- Tannir NM, Frontera OA, Hammers HJ, et al. Thirty-month follow-up of the phase III CheckMate 214 trial of first-line nivolumab + ipilimumab (N + I) or sunitinib (S) in patients (pts) with advanced renal cell carcinoma (aRCC). J Clin Oncol. 2019;37(7_suppl):547–547.
- Yau T, Kang Y-K, Kim T-Y, et al. Efficacy and safety of nivolumab plus ipilimumab in patients with advanced hepatocellular carcinoma previously treated with sorafenib: the CheckMate 040 randomized clinical trial. JAMA Oncol. 2020;6(11):e204564.
- Lenz H-J, Lonardi S, Zagonel V, et al. Nivolumab plus low-dose ipilimumab as first-line therapy in microsatellite instability-high/DNA mismatch repair deficient metastatic colorectal cancer: clinical update. J Clin Oncol. 2020;38(4_suppl):11–11.
- Baas P, Scherpereel A, Nowak AK, et al. First-line nivolumab plus ipilimumab in unresectable malignant pleural mesothelioma (CheckMate 743): a multicentre, randomised, open-label, phase 3 trial. Lancet. 2021;397(10272):375–386.
- Chau I, et al. Nivolumab (NIVO) plus ipilimumab (IPI) or NIVO plus chemotherapy (chemo) versus chemo as first-line (1L) treatment for advanced esophageal squamous cell carcinoma (ESCC): first results of the CheckMate 648 study. J Clin Oncol. 2021;39(18_suppl):LBA4001.
- Hellmann MD, Ciuleanu T-E, Pluzanski A, et al. Nivolumab plus ipilimumab in lung cancer with a high tumor mutational burden. N Engl J Med. 2018;378(22):2093–2104.
- Hellmann MD, Paz-Ares L, Bernabe Caro R, et al. Nivolumab plus ipilimumab in advanced non-small-cell lung cancer. N Engl J Med. 2019;381(21):2020–2031.
- Ramalingam SS, Ciuleanu TE, Pluzanski A, et al. Nivolumab + ipilimumab versus platinum-doublet chemotherapy as first-line treatment for advanced non-small cell lung cancer: three-year update from CheckMate 227 part 1. J Clin Oncol. 2020;38(15_suppl):9500–9500.
- US Food & Drug Administration, FDA approves nivolumab plus ipilimumab for first-line mNSCLC (PD-L1 tumor expression ≥1%). 2020.
- Latimer NR. NICE DSU technical support document 14: survival analysis for economic evaluations alongside clinical trials - extrapolation with patient-level data; 2011. [updated 2013 March; cited 2021 July 8]. Available from: https://www.sheffield.ac.uk/sites/default/files/2022-02/TSD14-Survival-analysis.updated-March-2013.v2.pdf.
- National Institute for Health and Care Excellence. NICE technology appraisal guidance [TA531]: pembrolizumab for untreated PD-L1-positive metastatic non-small-cell lung cancer; 2018. [updated 2021 February 13; cited 2021 June 15]; Available from: https://www.nice.org.uk/guidance/ta531/resources/pembrolizumab-for-untreated-pdl1positive-metastatic-nonsmallcell-lung-cancer-pdf-82606895901637.
- Chaudhary MA, Lubinga SJ, Smare C, et al. Cost-effectiveness of nivolumab in patients with NSCLC in the United States. Am J Manag Care. 2021;27(8):e254–e260.
- Huang M, Lou Y, Pellissier J, et al. Cost effectiveness of pembrolizumab vs. standard-of-care chemotherapy as first-line treatment for metastatic NSCLC that expresses high levels of PD-L1 in the United States. Pharmacoeconomics. 2017;35(8):831–844.
- Insinga RP, Vanness DJ, Feliciano JL, et al. Cost-effectiveness of pembrolizumab in combination with chemotherapy in the 1st line treatment of non-squamous NSCLC in the US. J Med Econ. 2018;21(12):1191–1205.
- Insinga RP, Vanness DJ, Feliciano JL, et al. Cost-effectiveness of pembrolizumab in combination with chemotherapy versus chemotherapy and pembrolizumab monotherapy in the first-line treatment of squamous non-small-cell lung cancer in the US. Curr Med Res Opin. 2019;35(7):1241–1256.
- Neumann PJ, Ganiats TG, Russell LB, et al. Cost effectiveness in health and medicine. Second ed. 2017. Oxford; New York: Oxford University Press: Oxford Scholarship Online.
- Rutherford MJ, Lambert PC, Sweeting MJ, et al. NICE DSU technical support document 21: flexible methods for survival analysis. Report by the Decision Support Unit; 2020. [cited 2021 July 8]. Available from: https://nicedsu.sites.sheffield.ac.uk/tsds/flexible-methods-for-survival-analysis-tsd.
- Gettinger S, Borghaei H, Brahmer J, et al. OA14.04 Five-year outcomes from the randomized, phase 3 trials CheckMate 017/057: nivolumab vs docetaxel in previously treated NSCLC. J Thorac Oncol. 2019;14(10):S244–S245.
- Shaw JW, Johnson JA, Coons SJ. US valuation of the EQ-5D health states: development and testing of the D1 valuation model. Med Care. 2005;43(3):203–220.
- Weng X, Luo S, Lin S, et al. Cost-utility analysis of pembrolizumab versus chemotherapy as first-line treatment for metastatic non-small cell lung cancer with different PD-L1 expression levels. Oncol Res. 2020;28(2):117–125.
- Criss SD, Mooradian MJ, Watson TR, et al. Cost-effectiveness of atezolizumab combination therapy for first-line treatment of metastatic nonsquamous non-small cell lung cancer in the United States. JAMA Netw Open. 2019;2(9):e1911952.
- US Bureau of Labor Statistics. Consumer price index; 2021. [updated 2021 March 17; cited 2021 June 15]. Available from: https://www.bls.gov/cpi/data.htm.
- Bremner KE, Krahn MD, Warren JL, et al. An international comparison of costs of end-of-life care for advanced lung cancer patients using health administrative data. Palliat Med. 2015;29(10):918–928.
- National Institute for Health and Care Excellence. Nivolumab for previously treated squamous non-small-cell lung cancer [TA483]; 2017. [updated 2018 May 28; cited 2021 May 15]. Available from: https://www.nice.org.uk/guidance/ta483.
- National Institute for Health and Care Excellence. Nivolumab for previously treated non-squamous non-small-cell lung cancer [TA484]; 2017. [updated 2018 May 28; cited 2021 May 15]. Available from: https://www.nice.org.uk/guidance/ta484.
- National Institute for Health and Care Excellence. NICE technology appraisal guidance [TA655]: nivolumab for advanced squamous non-small-cell lung cancer after chemotherapy; 2020. [updated 2021 June 15; cited 2021 June 15]. Available from: https://www.nice.org.uk/guidance/ta655.
- Marseille E, Larson B, Kazi DS, et al. Thresholds for the cost-effectiveness of interventions: alternative approaches. Bull World Health Organ. 2015;93(2):118–124.
- Ubel PA, Hirth RA, Chernew ME, et al. What is the price of life and why doesn't it increase at the rate of inflation? Arch Intern Med. 2003;163(14):1637–1641.
- Nadler E, Eckert B, Neumann PJ. Do oncologists believe new cancer drugs offer good value? Oncologist. 2006;11(2):90–95.
- Becker G, Murphy K, Philipson T. The value of life near its end and terminal care. National Bureau of Economic Research working paper No. 13333. August; 2007. [cited 2015 June 15]. Available from: https://www.nber.org/system/files/working_papers/w13333/w13333.pdf.
- Courtney PT, Yip AT, Cherry DR, et al. Cost-effectiveness of nivolumab-ipilimumab combination therapy for the treatment of advanced non-small cell lung cancer. JAMA Netw Open. 2021;4(5):e218787.
- Li J, Zhang T, Xu Y, et al. Cost-effectiveness analysis of nivolumab plus ipilimumab versus chemotherapy as first-line treatment in advanced NSCLC. Immunotherapy. 2020;12(14):1067–1075.
- Hu H, She L, Liao M, et al. Cost-effectiveness analysis of nivolumab plus ipilimumab vs. chemotherapy as first-line therapy in advanced non-small cell lung cancer. Front Oncol. 2020;10:1649.
- Goeree R, Villeneuve J, Goeree J, et al. Economic evaluation of nivolumab for the treatment of second-line advanced squamous NSCLC in Canada: a comparison of modeling approaches to estimate and extrapolate survival outcomes. J Med Econ. 2016;19(6):630–644.
- Gettinger SN, Horn L, Gandhi L, et al. Overall survival and long-term safety of nivolumab (anti-programmed death 1 antibody, BMS-936558, ONO-4538) in patients with previously treated advanced non-small-cell lung cancer. J Clin Oncol. 2015;33(18):2004–2012.
- National Cancer Institute. Cancer Query System: SEER survival statistics; 2021. [cited 2021 June 15]. Available from: https://seer.cancer.gov/canques/survival.html.
- Bristol Meyers Squibb. Four-year data from phase 3 CheckMate-227 trial show durable, long-term survival with Opdivo (nivolumab) plus Yervoy (ipilimumab) in patients with non-small cell lung cancer with PD-L1 expression ≥1%. 2021.
- Federal Reserve Economic Data. Consumer Price Index for All Urban Consumers: Medical Care in U.S. City Average; 2022. [cited 2022 February 28]. Available from: https://fred.stlouisfed.org/series/CPIMEDSL.
- Kluwer W. Medi-Span PriceRx database; 2021. [cited 2021 February 13]. Available from: https://pricerx.medispan.com.
- Centers for Medicare and Medicaid Services 2019 CMS clinical laboratory fee schedule; 2019.