 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Purpose
The aim of this study was to design and fabricate a three-dimensional (3D) printed artificial ovary.
Methods
We first compared the printability of gelatin-methacryloyl (GelMA), alginate and GelMA–alginate bioinks, of which GelMA was selected for further investigation. The swelling properties, degradation kinetics and shape fidelity of GelMA scaffolds were characterized by equilibrium swelling/lyophilization, collagenase processing and micro-computed tomography evaluation. Commercial ovarian tumor cell lines (COV434, KGN, ID8) and primary culture ovarian somatic cells were utilized to perform cell-laden 3D printing, and the results were evaluated by live/dead assays and TUNEL detection. Murine ovarian follicles were seeded in the ovarian scaffold and their diameters were recorded every day. Finally, in vitro maturation was performed, and the ovulated oocytes were collected and observed.
Results
Our results indicated that GelMA was suitable for 3D printing fabrication. Its scaffolds performed well in terms of hygroscopicity, degradation kinetics and shape fidelity. The viability of ovarian somatic cells was lower than that of commercial cell lines, suggesting that extrusion-based 3D culture fabrication is not suitable for primary ovarian cells. Nevertheless, the GelMA-based 3D printing system provided an appropriate microenvironment for ovarian follicles, which successfully grew and ovulated in the scaffolds. Metaphase II oocytes were also observed after in vitro maturation.
Conclusions
The GelMA-based 3D printing culture system is a viable alternative option for follicular growth, development and transfer. Accordingly, it shows promise for clinical application in the treatment of female endocrine and reproductive conditions.
明胶-甲基丙烯酰基生物墨水挤出式3D生物打印人工卵巢 摘要
目的:本研究的目的是设计和制造一个3D打印的人工卵巢。
方法:我们首先比较了明胶-甲基丙烯酰基 (GelMA)、藻酸盐和 GelMA-藻酸盐生物墨水的可印刷性, 并选取GelMA 进行进一步研究。 GelMA支架的溶胀和降解性能、形状保真度通过溶胀/冻干平衡、胶原酶降解和微型计算机断层扫描评估来表征。利用商业化的卵巢肿瘤细胞系(COV434、KGN、ID8)和原代培养卵巢体细胞进行载细胞3D打印, 并通过活性检测和 TUNEL 检测评估结果。将鼠卵巢卵泡接种在卵巢支架中并每天记录其直径。最后进行体外成熟, 收集并观察排卵的卵母细胞。
结果:我们的结果表明GelMA适合用于3D打印。它的支架在吸湿性、降解动力学和形状保真度方面表现良好。卵巢体细胞的活性低于商业细胞系, 这表明挤出式3D 培养不适合原代卵巢细胞。尽管如此, 基于 GelMA的3D打印系统为卵巢卵泡提供了合适的微环境, 卵泡在支架中成功生长和排卵。在体外成熟后也观察到MII期卵母细胞。
结论:基于GelMA的3D打印培养系统是卵泡生长、发育和转移的可行替代选择。因此基于GelMA的3D打印培养系统在治疗女性内分泌和生殖疾病方面有很好的临床应用前景。
Introduction
The ovary is the endocrine organ of the female reproductive system and controls follicular development and sex steroid secretion [Citation1]. The onset of puberty, establishment of the menstrual cycle and menopausal conditions are all associated with the state of the ovary [Citation2–4]. However, pathologies such as malignant tumor, primary ovarian diseases and autoimmune diseases can disrupt hormone secretion and follicular development [Citation5–8]. Furthermore, iatrogenic conditions due to chemotherapy, radiotherapy and oophorectomy can also damage ovarian function to a degree, resulting in premature menstruation or amenorrhea, as well as decreased fertility [Citation5]. Disorders of the ovary are also responsible for many mental/psychological issues and organ dysfunction, such as osteoporosis, cognitive decline and cardiovascular disease. As a means to treat these conditions, bioengineering artificial ovaries that mimic natural ovaries are of potentially far-reaching significance. Bioengineered ovaries are designed to integrate into the hypothalamic–pituitary–ovary axis and provide optimal microenvironments for follicular growth [Citation9].
An artificial ovary typically comprises a supporting bioscaffold and follicles at different developmental stages. Conventional methods for the fabrication of artificial ovaries involve encapsulating ovarian follicles in plasma clots, synthetic hydrogels or natural polymers, such as collagen, fibrin and alginate [Citation10–13]. Microfluidic chips have also been created for in vitro culture of ovarian follicles [Citation14–16]. However, they are limited to small-scale production and are very unlike real ovaries. In fact, in the majority of studies, artificial ovaries are replaced with ovarian follicles, which are the functional units of the ovary. Overall, accurately mimicking the complex cellular compartmentalization, dynamic extracellular matrix and mechanical properties of a real ovary in an engineered ovary is extremely challenging.
The emergence of three-dimensional (3D) printing technology has provided researchers with numerous strategies for creating prosthetic organs or tissues that are biocompatible, degradable and functional [Citation17]. In recent years, 3D printing has demonstrated its potential in regenerative medicine, being used to create patient-specific beating hearts, distensile airways and central nervous system scaffolds [Citation18–20]. With the advancement of 3D printing technology and cell biology, more versatile organs can be created using novel bioinks or in vitro methods. However, its application to female reproductive therapy is currently limited [Citation21–23]. To date, 3D printed ovaries have only been attempted by Laronda et al. in 2017, who reported 3D fabrication of gonad tissues that partially restored ovarian function (i.e. hormone secretion and egg production) [Citation21]. Accordingly, there is still much work to be done if such systems are to advance from experimental exploration to clinical application. Investigating 3D printing systems for bioengineering reproductive tissues is of paramount importance for in vitro culture of follicles, ovary tissue transplantation and menopausal hormonal therapy.
In this study, we developed a 3D printed ovary using gelatin-methacryloyl (GelMA), a type of bioink that shows excellent biocompatibility and mechanical properties, with exogenous follicles deposited in the scaffolds. The seeded ovarian follicles gradually develop and finally produce mature oocytes. We also tested the efficacy of ovarian somatic-cell-laden 3D bioprinting technology. This is the first study in which GelMA hydrogel is used to construct an artificial 3D ovary. We have demonstrated that the 3D printed scaffold creates an appropriate microenvironment for follicular development, which is an important step toward realizing the clinical application of this technology to female reproductive disorders and provides a novel strategy for in vitro follicle growth.
Materials and methods
Materials
The 3D bioprinter (SUNP BIOMAKER 2); freeze-dried bioink powders, including GelMA (SP-BI-G01-2), alginate (SP-BI-GA01-2) and GelMA–alginate (SP-BI-G02-2); the cross-linking agent (SP-BI-C01-1); and lithium phenyl-2,4,6-trimethylbenzoylphosphinate (LAP) (SP-BI-C02-1) were provided by SunP Biotech (Beijing, China). Red fluorescence GelMA (EFL-GM RF-60) was purchased from Suzhou Intelligent Manufacturing Research Institute (Suzhou, China). Collagenase agents of type I (G5026), type II (G5027) and type IV (G5029), as well as DNase I (G5043) and trypan blue (GP1101), were purchased from Servicebio (Wuhan, China). Insulin transferrin selenium (ITS) (41400-045) and α-minimum essential medium (α-MEM) (12492013) were purchased from ThermoFisher (Runcorn, Cheshire, UK). Human chorionic gonadotropin (hCG), McCoy’s 5A powder (M4892) and Leibovitz’s 15 medium (L1518) were purchased from Sigma-Aldrich (St. Louis, MO, USA). Calcein-AM/propidium iodide Double Stain Kits (40747ES76) were purchased from YEASEN (Shanghai, China). In situ cell death detection kits (C1090) were purchased from Beyotime (Shanghai, China). Three-week-old and 4-week-old female C57BL/6J mice were purchased from the Hubei Center for Disease Control and Prevention Animals Center.
Bioink preparation
The bioinks were prepared at a concentration of 10% (w/v) according to the manufacturer’s instructions. Briefly, the bioink powders were dissolved in McCoy’s 5A medium, sterilized at 70 °C for 30 min three times with intervals of 30 min and filtered through 0.22-μm filters (Millex-GP; Millipore, Bedford, Massachusetts, USA). The sterilized solutions were stored at 4 °C in 1.5-ml Eppendorf tubes and incubated at 37 °C before use.
Isolation and culturing of ovarian somatic cells
The 4-week-old female C57BL/6J mice were euthanized by cervical dislocation and their ovaries were removed by dissection in preincubated Leibovitz’s 15 medium supplemented with 100 μg/ml streptomycin and 100 IU/ml penicillin. Ovarian somatic cells are mainly composed of theca-interstitial cells (TICs), granulosa cells (GCs) and mesenchymal cells. Ovaries were punctured with a needle to release the GCs into the Leibovitz’s 15 medium. Residual tissues were reserved for TIC and mesenchymal cell isolation. They were incubated for 60 min at 37 °C in 200 μl per ovary of collagenase–DNase solution containing 4 mg/ml collagenase IV, 10 mg/ml DNase I and 10 mg/ml bovine serum albumin (BSA) in McCoy’s 5A medium. During this time, the ovarian tissues were agitated using a pipette at least 10 times at intervals of 10 min. Then, the cell suspension was centrifuged at 1000 rpm for 5 min. Cells from passage one were fixed with 4% paraformaldehyde (w/v) for immunofluorescence staining. Cells from passage two were trypsinized and prepared for cell-laden bioprinting.
3D printing and cell-laden bioprinting
For extrusion-based printing, a bioink solution alone or one loaded with ovarian somatic cells (2.0 × 106 cells/ml) was prepared in a 5-ml sterile Luer lock syringe. In the mixed solution, the volume ratio of GelMA to cell suspension to LAP was 5:4:1 according to the manufacturer’s instructions. Before printing, the initial temperature of the extruder and bed were set at 19–23 °C and 10 °C, respectively. To obtain high-precision struts, the solution was printed at a speed of 8 mm/s and an extrusion speed of 4.5 mm/s. The line height and distance were set to 200 μm and 550 μm, respectively. Constructs were printed in standard coated six-well plates cross-linked with blue light (405 nm) using the recommended manufacturer settings of 5 mW/cm2 for 10 s at 3 cm. When alginate hydrogel was used, it was incubated with the cross-linking agents for 2 min and rinsed with saline water two times. Constructs were subsequently cut into equal parts with a 3-mm biopsy punch and immersed in 1–2 ml medium to avoid evaporation.
Swelling testing
To measure the equilibrium swellings of the hydrogels, the GelMA bioink was cross-linked into a square-grid shape (thickness = 1.2 mm, diameter = 8 mm) in a 3-mm Petri dish. The gel cubes were immersed in 2 ml medium at 37 °C for 48 h to reach equilibrium swelling. After removal from the medium, the gel cubes were gently touched with blotting paper to remove excess liquid and weighed (recorded as Ww). The samples were subsequently lyophilized and weighed again to determine the dry weight of each cube (recorded as Wd). The equilibrium water content (%) was calculated according to the following equation:
In vitro degradation in collagenase
Separate type I, type II and type IV collagenase solutions were prepared at a concentration of 0.5 mg/ml. The initial appearances of the samples were recorded photographically immediately after printing. Then, the samples were incubated in the different collagenase solutions at 37 °C and photographed at predetermined time points (20, 40 and 80 min). The degree of degradation (%) was defined as the residual volume of the structure divided by its initial volume.
Micro-computed tomography evaluation
The micro-architectural details of the fabricated scaffolds were observed using micro-computed tomography apparatus (SkyScan 1276; Bruker Co., Bremen, Germany). SkyScan1276 software was used to acquire appropriate images with an image pixel size of 9 μm and a depth of 16 bits. The reconstruction process was performed with NRecon software.
Culturing and in vitro ovulation of follicles
The 3-week-old female C57BL/6J mice were euthanized by cervical dislocation. Ovarian tissues were separated from surrounding tissues using two 25-gauge needles. After mechanical dissection, follicles having diameters of 80–180 μm, an intact basal membrane, a central and spherical oocyte, and surrounding GCs were selected for seeding onto pre-cut 3D printed scaffolds. Follicles were allowed to sediment for 30 min and follicle growth medium were added. The follicle growth medium consisted of α-MEM supplemented with 10% fetal bovine serum (FBS), 100 μg/ml streptomycin, 100 IU/ml penicillin and ITS (insulin 10 μg/ml, transferrin 5.5 μg/ml, selenium 6.7 ng/ml). Photographs of each follicle were taken every day. The medium was changed every 2 days.
In vitro maturation was performed on 6–8 days of follicle culturing. Follicles were incubated in maturation medium for 14–16 h at 37 °C under a 5% CO2 atmosphere. The maturation medium consisted of α-MEM medium with 10% FBS, 1.5 IU/ml hCG, 10 ng/ml epidermal growth factor and 10 mIU/ml recombinant follicle stimulating hormone.
Live/dead cell assays
All constructs were rinsed in buffer solution and incubated for 15 min in a mixture containing 0.5 μM calcein-AM and 0.5 μM propidium iodide at room temperature. After incubation, the constructs were rinsed again and imaged with an inverted fluorescence microscope. Cell viability was quantified using ImageJ software according to the following equation:
Immunofluorescence staining
The structures were removed from the medium and fixed with 4% paraformaldehyde (w/v) for 30 min at room temperature and their cell membranes were permeabilized with 1% Triton X-100 (v/v) for 10 min. For blocking, samples were incubated in 1% BSA with 0.5% Triton X-100 at 37 °C for 1 h. A solution of primary antibody against proliferating cell nuclear antigen (PCNA) (1:200) was prepared in 0.1% BSA. Hydrogels were incubated with the primary antibody overnight at 4 °C. The next day, samples were washed with phosphate buffered saline (PBS) three times for 5 min and incubated with secondary antibodies (Alexa Fluor 594 donkey anti-rabbit or Alexa Fluor 488 donkey anti-rabbit) at 37 °C for 1 h. Hydrogels were then rinsed three times for 5 min and cellular nuclei were stained with 4′,6-diamidino-2-phenylindole (DAPI) (1:2000) for 10 min at room temperature. Two-dimensional (2D) adhered cell immunofluorescence staining was modified from the aforementioned method with primary antibodies against CYP11A1, CYP17A1, CYP19A1 and α-smooth muscle actin (α-SMA).
Cell viability by trypan blue assay
The primary cells were trypsinized and centrifuged for 5 min at 800 rpm. The supernatant was then discarded and the cell pellet resuspended in 1 ml PBS. The cells were exposed to 0.2% trypan blue reagent for 3 min at room temperature and counted under an optical microscope. Viable cells were not stained by the trypan blue dye, and the bright cells were counted as living cells. The percent of viable cells was quantified according to the following equation:
TUNEL staining
According to the manufacturer’s instructions, each scaffold was incubated with terminal deoxynucleotidyl transferase dUTP nick end-labeling (TUNEL) reaction mixture (enzyme solution:label solution = 1:5) at 37 °C for 1 h. The red fluorescence of the apoptotic cells against the blue background was observed using a microscope and the images were acquired with cellSens Dimension software.
Statistical analysis
GraphPad Prism (version 8.3.0) was used for statistical analysis. Two-way analysis of variance with Tukey’s post hoc test or Student’s t-test was used depending on the number of comparisons. Data are given as mean ± standard deviation (SD) and p ≤ 0.05 was reported as statistically significant.
Results
Printability and comparison of hydrogel bioinks
The use of bioink hydrogels has recently flourished in the bioprinting field. When first used for constructing a specific tissue, a bioink should be evaluated in terms of its bioprintability, affordability, mechanical strength, swelling ability, degradation behavior and structural integrity [Citation24]. In this study, we evaluated a natural polymer (alginate), a chemically modified natural polymer (GelMA) and their combination (GelMA–alginate) in a 3D printing system. The printability was directly verified by the shape and integrity of gel fibers printed using the bioinks at the same concentration (10%) (Supplementary Figure S1). In the case of alginate, the scaffolds were found to be fragile and unstable, degenerating significantly in vitro within several days. GelMA–alginate formed well-structured scaffolds. However, the grid was too opaque to observe the follicles clearly. Moreover, we had to utilize LAP, low temperature and calcium ion cross-linking agents to solidify the GelMA–alginate bioink, which is more complicated and expensive than using a single-component bioink. In contrast, GelMA formed regular, smooth and clear grids. They could also be clipped using tweezers, so the scaffolds could be used for further processing (data not shown) [Citation25]. Accordingly, alginate and GelMA–alginate were eliminated from our investigation and GelMA was used in further experiments.
Characterization of 3D printed scaffolds
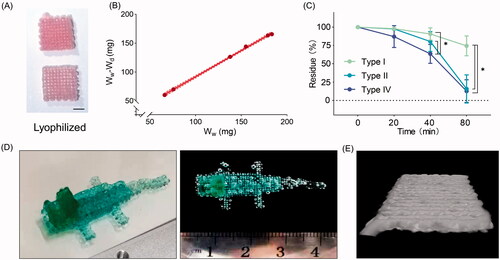
To further validate the performance of GelMA, the biological properties of its printed scaffold were evaluated. Using lyophilization, the water in the scaffolds was removed in the form of water vapor, leaving a solid structure (). The swelling behavior of GelMA is presented in , and the equilibrium water content is 91.6%. Suitable degradation kinetics is necessary for cell growth in vivo, as structures that degrade too rapidly cannot maintain the cell microenvironment, while long-term existence of the grafts in vivo may cause chronic fibrosis and immune responses. Our results showed that collagenase accelerates the degradation of the 3D scaffolds (). Collagenases of type II and type IV degrade the structure within 80 min. However, nearly 75% remains after collagenase type I treatment. To test the printability of the bioink, we fabricated a complex structure in the shape of a crocodile (). Morphology observation revealed that the grids are continuous and form regular corners (), confirming that the printed scaffolds possess high shape fidelity.
Figure 1. Biological properties of the scaffolds. (A) Image of lyophilized gelatin-methacryloyl (GelMA) scaffolds. Scale bar = 400 μm. (B) Characterization of equilibrium water content (n = 6). (C) In vitro degradation kinetics of three-dimensional (3D) printed structures using different collagenase solutions (n = 3). *Adjusted p < 0.05. (D) Photographs of a printed scaffold in the shape of a crocodile. (E) Representative micro-computed tomography 3D reconstruction of GelMA structures. Wd, dry weight; Ww, wet weight.
Cell-laden bioprinting for artificial ovaries
Cell-laden bioprinting has been demonstrated to maintain the viability and morphology of cells [Citation26–28]. Therefore, we first used commercial ovarian tumor cell lines, including COV434, KGN and ID8, to perform cell-laden bioprinting. During the process, the cell-laden bioink was extruded from a low-temperature nozzle and formed the grids according to our pre-set shape (Supplementary Figure S2(A)). We observed more live than dead cells through live/dead staining (Supplementary Figure S2(B)). The same phenomenon was observed after culturing COV434 for 5 days, and we observed a two-fold increase in cell numbers (Supplementary Figure S2(C)). These results indicate that extrusion-based cell-laden 3D bioprinting is feasible for ovarian tumor cell lines and that the GelMA hydrogel is non-toxic to the cells, exhibiting very good biocompatibility.
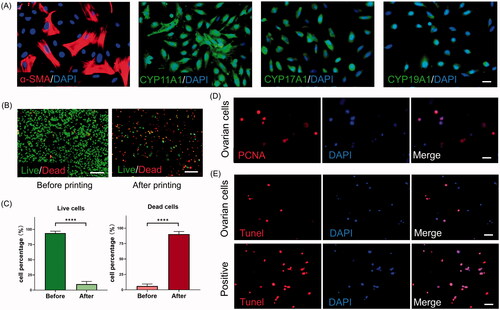
Next, we intended to load primary culture ovarian somatic cells in GelMA. It was expected that the growth factors secreted by somatic cells would benefit follicle survival through the paracrine (or other) mechanism. The isolated murine ovarian somatic cells were a group of mixed cells. Immunofluorescence analysis of the cells showed that they express the representative GC marker CYP19A1 and the TIC marker CYP17A1 () [Citation9]. Both GCs and TICs express CYP11A1, which encodes the enzyme P450scc. The marker α-SMA is mainly detected in mesenchymal cells. We found that the 2D culture prepared using GelMA maintained primary cells well, with little cell death being observed (). However, when fabricated by the extrusion-based cell-laden bioprinting method, most cells (90%) died (). A massive loss of live cells (p < 0.0001) and a dramatic increase in dead cells (p < 0.0001) were observed through the cell viability assay (). Even if the remaining cells were positively PCNA stained, they would not continue to proliferate during the 5-day culturing (). Furthermore, trypan blue staining revealed no dead cells after trypsinization (data not shown). This result indicates that the extrusion force used in this study causes irreversible damage to cells and even cellular death. To reveal the mechanism of this extensive cell death, we performed a TUNEL assay immediately after bioprinting. The results revealed the activation of the apoptosis signaling pathway in the primary cells (). We thus speculate that the extrusion damage is specific to ovarian somatic cells and does not affect ovarian tumor cell lines. Therefore, cell-laden bioprinting appear to be unsuitable for primary cells.
Figure 2. Functional investigation of cell-laden printing by an extrusion-based method. (A) Immunofluorescence staining against α-smooth muscle actin (α-SMA), CYP11A1, CYP17A1 and CYP19A1 in a two-dimensional (2D) culture system. Scale bar = 25 μm. (B) Calcein-AM/propidium iodide staining and (C) quantification of the cell viability before and after printing. ****p < 0.0001. Scale bars = 500 μm. (D) Immunofluorescence staining against proliferating cell nuclear antigen (PCNA) for cells included in scaffolds. Scale bar = 25 μm. (E) Apoptotic ovarian somatic cells in the scaffolds were detected by terminal deoxynucleotidyl transferase dUTP nick end-labeling (TUNEL) staining. DAPI, 4′,6-diamidino-2-phenylindole. Scale bar = 25 μm.
Growth and maturation of ovarian follicles
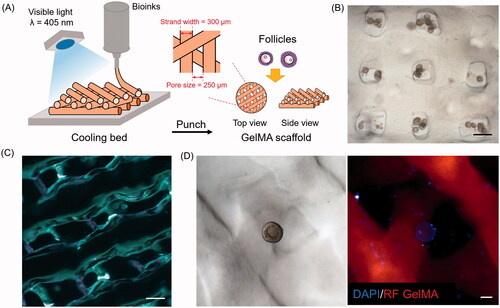
We subsequently deposited exogenous follicles onto the scaffolds without using cell-laden bioprinting. shows the workflow chart for constructing 3D ovaries using GelMA bioink. We obtained round-shaped scaffolds using a 3-mm biopsy punch machine. First, we tested the design of 90° scaffolds (i.e. those with a 90° angle between the adjacent upper and lower layers) for follicle culture (). However, most follicles fell into the pores between the grids, thus adhering to the bottom of the cell plates in a 2D culture. The same situation even occurred after repeatedly shaking the cell plate. After culturing for several days, these follicles lose their shape, and the GCs spread across the surface of the plate. Therefore, we decreased the angle from 90° to 60° (). In this way, the follicles were able to distribute randomly, as shown in . Under a fluorescence microscope, ovarian cells stained blue could be observed (). After culturing for 7 days in vitro, 84% of the follicles were observed to be alive; that is, five-fold more than were observed to be dead (p < 0.0001). These results clearly demonstrate that culturing murine follicles in a GelMA hydrogel fabricated with a moderate angle 60° supports follicular survival.
Figure 3. Deposition of ovarian follicles in optimized scaffolds. (A) Schematic representation of three-dimensional (3D) printing of gelatin-methacryloyl (GelMA) scaffolds. (B) Photograph of follicles in 90° scaffolds. Scale bar = 500 μm. (C) Optical microscopy images of GelMA 60° scaffolds. Scale bar = 500 μm. (D) Follicles attached in 60° scaffolds under bright white light and fluorescence microscope. Scale bar = 50 μm. DAPI, 4′,6-diamidino-2-phenylindole; RF, red fluorescence.
We also compared the growth ability of two-layer secondary follicles (100–130 μm) and multi-layer secondary follicles (130–180 μm) [Citation29]. We found that the diameters of the smaller follicles do not significantly change during the 5-day culturing (). Conversely, multi-layer secondary follicles were observed to have significantly expanded by the fifth day (p < 0.05) (). Interestingly, we observed a slight (but not significant) decrease in diameter at day 2 for both groups.
Figure 4. Development of follicles within scaffolds. Diameter changes of (A) small follicles and (B) large follicles over 5-day culture. ns, no significance; *p < 0.05; **p < 0.01. Histogram of (C) oocyte diameter and (D) diameter ratio of follicles versus oocytes. (E) Morphological observation of follicle growth and ovulation. Scale bar = 50 μm. (F) Representative images of oocytes of different stages. Scale bar = 50 μm. GV, germinal vesicle; GVBD, germinal vesicle breakdown; MII, metaphase II.
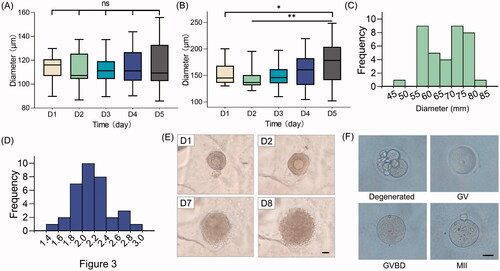
Maturation is critical for follicular function. Accordingly, we retrospectively analyzed the parameters of the spontaneously ovulated follicles on the day before ovulation. The diameters of these oocytes ranged from 55 to 75 μm (). The ratio of follicle diameter to oocyte diameter was found to be 2.0 (). These parameters can help us predict the timing of spontaneous ovulation, allowing us to perform in vitro maturation ahead of their spontaneous behavior. The follicles successfully released oocytes after in vitro maturation (). The ovulated oocytes float on the medium. Oocytes at all developmental stages, including degenerated, germinal vesicle, germinal vesicle breakdown and metaphase II, were observed in our study (). Oocytes at the metaphase II stage have completed the first meiotic cell division and are ready for fertilization.
Discussion
3D bioprinting technology has been extensively investigated in recent years. In terms of the female reproductive system, Laronda et al. successfully fabricated artificial ovaries that led to live birth in mice [Citation21]. However, 3D bioprinting requires significant development before it can be clinically applied [Citation23]. In this study, we developed 3D printed ovaries using GelMA bioinks and demonstrated that they successfully support follicular activities, including growth and maturation. We also compared the effects of cell-laden printing by an extrusion-based method on commercial tumor cell lines and ovarian somatic cells. We propose that 3D bioprinting with additional follicle deposition can be used to construct artificial ovaries.
We compared the effect of GelMA with that of alginate and GelMA–alginate hydrogels. We chose alginate in 3D printing because it has long been used to construct 3D follicle culture systems since around 2004 [Citation30]. The system is extensively used and accepted by other groups around the world, yielding promising results [Citation31,Citation32]. GelMA is produced with a methacrylation modification from gelatin, which has already been used to construct 3D printed artificial ovaries [Citation21]. It can be easily photo-cross-linked using ultraviolet or visible light [Citation33], and has been tested for regeneration of various tissues [Citation28,Citation34,Citation35]. However, studies on the effect of GelMA in female reproductive tissues have not been reported. Our results suggest that GelMA performs well in terms of swelling behavior and stability. In terms of biodegradability, type I and type II collagenase were widely used in degradation experiments [Citation36–39]. As GelMA is a chemically modified gelatin B and collagenase type IV is known to cleave gelatin B [Citation40], we also added a group using collagen type IV treatment. The three types of collagenase help us understand the degradation behavior of the scaffolds more comprehensively. Our crocodile model and micro-computed tomography evaluation confirmed the good fidelity of the 3D printing system. Furthermore, the resolution of the printed architecture is also influenced by the type of bioink used, nozzle diameter and deposition speed.
Extrusion-based cell-laden bioprinting has been widely used with commercially available cell lines and stem cells for 3D culture and drug screening [Citation22,Citation27,Citation41,Citation42]. We expected that ovarian somatic cells embedded in the hydrogel could produce sex hormones and nutrients to better maintain the survival of neighboring follicles. However, we found that the viability of ovarian somatic cells under such conditions is extremely low, which is quite different from the results of previous studies [Citation18,Citation43]. Since trypan blue staining of the cells revealed few dead ones after trypsin digestion, we speculate that it might be attributed to the low-temperature manipulation and shear stress occurring during the printing process [Citation44]. These external stimuli inevitably cause cell damage through apoptosis signaling, according to our results. Thus, we concluded that primary cells from murine ovaries are not suitable for extrusion-based printing. To address this problem, photopolymerization-based bioprinting techniques, such as digital-light processing [Citation45], show better performance in maintaining the cell viability when manufacturing artificial ovaries (data not shown).
We found that two-layer secondary follicles did not grow in our scaffolds during culturing. This seems to be unsuitable for in vitro culture of immature follicles. In fact, the same is true of traditional in vitro follicle cultures, as early-stage follicles are not well understood and are difficult to culture [Citation46,Citation47]. However, it is possible for them to continue development following in vivo transplantation [Citation48]. Multi-layer secondary follicles are more likely to grow after a short adaptive phase. This reveals that the efficiency of 3D printed scaffolds also depends on the size and developmental stage of the follicles [Citation49,Citation50]. Furthermore, all of the follicles underwent a slight, but not statistically significant, decrease in diameter. We speculate that this is because, during the first several days, the follicles adhere and spread along the strands, restricting follicular expansion.
Much work needs be done to confirm the efficacy of 3D bioprinting in the reproductive medicine field, such as further animal experiments, more complex printed architecture and comparison with conventional in vitro follicle culturing. The 3D printing system is sophisticated and requires expensive equipment, which limits its clinical application. An artificial ovary should enable both restoration of fertility and resumption of endocrine function. In terms of seeding cells, most studies used adult somatic cells. Cell-based hormone replacement constructs, containing GCs and theca cells, could function for as long as 90 days [Citation9]. Adding mesenchymal stem cells or progenitor cells of GCs or TICs to the artificial ovary construction may further prolong function. Stem cells might be advantageous for ovarian bioengineering since they support GCs and steroid secretion and have follicle-rejuvenating effects when transplanted into the ovary [Citation51–53].
Conclusion
In this study, we designed a 3D bioprinted ovary using a GelMA bioink and demonstrated that it can support follicle morphology, growth and maturation in vitro. Furthermore, we performed cell-laden 3D printing to construct artificial ovaries using commercial ovarian tumor cell lines and ovarian somatic cells for the first time, revealing extensive primary cell death within the grids. As more and more low-cost 3D bioprinters, operation systems and multifunctional bioinks become commercially available, more novel 3D bioprinting technologies will benefit female reproductive diseases treatment. Nevertheless, further studies with different types of bioprosthetic artificial ovaries containing human ovarian follicles are necessary for clinical application in the future.
Potential conflict of interest
No potential conflict of interest was reported by the authors.
Supplemental Material
Download TIFF Image (2.7 MB)Supplemental Material
Download TIFF Image (3.4 MB)Additional information
Funding
References
- Holesh JE, Bass AN, Lord M. Physiology, Ovulation. In: StatPearls. StatPearls Publishing Copyright © 2020. Treasure Island (FL): StatPearls Publishing LLC; 2020.
- Louwers YV, Laven JSE. Characteristics of polycystic ovary syndrome throughout life. Ther Adv Reprod Health. 2020;14:2633494120911038.
- Silber S, Pineda J, Lenahan K, et al. Fresh and cryopreserved ovary transplantation and resting follicle recruitment. Reprod Biomed Online. 2015;30(6):643–650.
- Li L, Wang Z. Ovarian aging and osteoporosis. Adv Exp Med Biol. 2018;1086:199–215.
- Balachandren N, Davies M. Fertility, ovarian reserve and cancer. Maturitas. 2017;105:64–68.
- Donnez J, García-Solares J, Dolmans MM. Ovarian endometriosis and fertility preservation: a challenge in 2018. Minerva Ginecol. 2018;70(4) :408–414.
- Laven JS. Primary ovarian insufficiency. Semin Reprod Med. 2016;34(4):230–234.
- Vanni VS, De Lorenzo R, Privitera L, et al. Safety of fertility treatments in women with systemic autoimmune diseases (SADs). Expert Opin Drug Saf. 2019;18(9):841–852.
- Sittadjody S, Saul JM, McQuilling JP, et al. In vivo transplantation of 3D encapsulated ovarian constructs in rats corrects abnormalities of ovarian failure. Nat Commun. 2017;8(1):1858.
- Rios PD, Kniazeva E, Lee HC, et al. Retrievable hydrogels for ovarian follicle transplantation and oocyte collection. Biotechnol Bioeng. 2018;115(8):2075–2086.
- Dolmans M-M, Yuan WY, Camboni A, et al. Development of antral follicles after xenografting of isolated small human preantral follicles. Reprod Biomed Online. 2008;16(5):705–711.
- Chiti MC, Dolmans M-M, Mortiaux L, et al. A novel fibrin-based artificial ovary prototype resembling human ovarian tissue in terms of architecture and rigidity. J Assist Reprod Genet. 2018;35(1):41–48.
- Kim EJ, Yang C, Lee J, et al. The new biocompatible material for mouse ovarian follicle development in three-dimensional in vitro culture systems. Theriogenology. 2020;144:33–40.
- Aziz A, Fu M, Deng J, et al. A microfluidic device for culturing an encapsulated ovarian follicle. Micromachines (Basel). 2017;8(11):335.
- He X. Microfluidic encapsulation of ovarian follicles for 3D culture. Ann Biomed Eng. 2017;45(7):1676–1684.
- Nawroth J, Rogal J, Weiss M, et al. Organ-on-a-chip systems for women’s health applications. Adv Healthc Mater. 2018;7(2):1700550.
- Shiju TM, Carlos de Oliveira R, Wilson SE. 3D in vitro corneal models: a review of current technologies. Exp Eye Res. 2020;200:108213.
- Lee A, Hudson AR, Shiwarski DJ, et al. 3D bioprinting of collagen to rebuild components of the human heart. Science. 2019;365(6452):482–487.
- Grigoryan B, Paulsen SJ, Corbett DC, et al. Multivascular networks and functional intravascular topologies within biocompatible hydrogels. Science. 2019;364(6439):458–464.
- Koffler J, Zhu W, Qu X, et al. Biomimetic 3D-printed scaffolds for spinal cord injury repair. Nat Med. 2019;25(2):263–269.
- Laronda MM, Rutz AL, Xiao S, et al. A bioprosthetic ovary created using 3D printed microporous scaffolds restores ovarian function in sterilized mice. Nat Commun. 2017;8:15261.
- Paul K, Darzi S, McPhee G, et al. 3D bioprinted endometrial stem cells on melt electrospun poly ε-caprolactone mesh for pelvic floor application promote anti-inflammatory responses in mice. Acta Biomater. 2019;97:162–176.
- Gargus ES, Rogers HB, McKinnon KE, et al. Engineered reproductive tissues. Nat Biomed Eng. 2020;4(4):381–393.
- Hospodiuk M, Dey M, Sosnoski D, et al. The bioink: a comprehensive review on bioprintable materials. Biotechnol Adv. 2017;35(2):217–239.
- Jakus AE, Laronda MM, Rashedi AS, et al. “Tissue Papers” from organ-specific decellularized extracellular matrices. Adv Funct Mater. 2017;27(3):1700992.
- Zhang J, Wehrle E, Vetsch JR, et al. Alginate dependent changes of physical properties in 3D bioprinted cell-laden porous scaffolds affect cell viability and cell morphology. Biomed Mater. 2019;14(6):065009.
- Jang J, Park H-J, Kim S-W, et al. 3D printed complex tissue construct using stem cell-laden decellularized extracellular matrix bioinks for cardiac repair. Biomaterials. 2017;112:264–274.
- Luo C, Xie R, Zhang J, et al. Low-temperature three-dimensional printing of tissue cartilage engineered with gelatin methacrylamide. Tissue Eng Part C Methods. 2020;26(6):306–316.
- Kreeger PK, Fernandes NN, Woodruff TK, et al. Regulation of mouse follicle development by follicle-stimulating hormone in a three-dimensional in vitro culture system is dependent on follicle stage and dose. Biol Reprod. 2005;73(5):942–950.
- Heise M, Koepsel R, Russell AJ, et al. Calcium alginate microencapsulation of ovarian follicles impacts FSH delivery and follicle morphology. Reprod Biol Endocrinol. 2005;3(1):47.
- Vanacker J, Amorim CA. Alginate: a versatile biomaterial to encapsulate isolated ovarian follicles. Ann Biomed Eng. 2017;45(7):1633–1649.
- Brito IR, Lima IMT, Xu M, et al. Three-dimensional systems for in vitro follicular culture: overview of alginate-based matrices. Reprod Fertil Dev. 2014;26(7):915–930.
- Yue K, Trujillo-de Santiago G, Alvarez MM, et al. Synthesis, properties, and biomedical applications of gelatin methacryloyl (GelMA) hydrogels. Biomaterials. 2015;73:254–271.
- Zhang X, Li J, Ye P, et al. Coculture of mesenchymal stem cells and endothelial cells enhances host tissue integration and epidermis maturation through AKT activation in gelatin methacryloyl hydrogel-based skin model. Acta Biomater. 2017;59:317–326.
- Kilic Bektas C, Hasirci V. Cell loaded 3D bioprinted GelMA hydrogels for corneal stroma engineering. Biomater Sci. 2019;8(1):438–449.
- Pepelanova I, Kruppa K, Scheper T, et al. Gelatin-methacryloyl (GelMA) hydrogels with defined degree of functionalization as a versatile toolkit for 3D cell culture and extrusion bioprinting. Bioengineering (Basel), 2018;5(3):55.
- Bae L, Shirahama H, Kim MH, et al. Colloidal templating of highly ordered gelatin methacryloyl-based hydrogel platforms for three-dimensional tissue analogues. NPG Asia Mater. 2017;9:e412.
- Xiao W, He J, Nichol JW, et al. Synthesis and characterization of photocrosslinkable gelatin and silk fibroin interpenetrating polymer network hydrogels. Acta Biomater. 2011;7(6):2384–2393.
- Xiao W, Li J, Qu X, et al. Cell-laden interpenetrating network hydrogels formed from methacrylated gelatin and silk fibroin via a combination of sonication and photocrosslinking approaches. Mater Sci Eng C Mater Biol Appl. 2019;99:57–67.
- Chaudhary AK, Chaudhary S, Ghosh K, et al. Secretion and expression of matrix metalloproteinase-2 and 9 from bone marrow mononuclear cells in myelodysplastic syndrome and acute myeloid leukemia. Asian Pac J Cancer Prev. 2016;17(3):1519–1529.
- Zhang J, Wehrle E, Adamek P, et al. Optimization of mechanical stiffness and cell density of 3D bioprinted cell-laden scaffolds improves extracellular matrix mineralization and cellular organization for bone tissue engineering. Acta Biomater. 2020;114:307–322.
- Kim BS, Gao G, Kim JY, et al. 3D cell printing of perfusable vascularized human skin equivalent composed of epidermis, dermis, and hypodermis for better structural recapitulation of native skin. Adv Healthc Mater. 2019;8(7):e1801019.
- Tytgat L, Van Damme L, Ortega Arevalo MDP, et al. Extrusion-based 3D printing of photo-crosslinkable gelatin and κ-carrageenan hydrogel blends for adipose tissue regeneration. Int J Biol Macromol. 2019;140:929–938.
- Mota C, Camarero-Espinosa S, Baker MB, et al. Bioprinting: from tissue and organ development to in vitro models. Chem Rev. 2020;120:10547–10607.
- Schmidleithner C, Malferrari S, Palgrave R, et al. Application of high resolution DLP stereolithography for fabrication of tricalcium phosphate scaffolds for bone regeneration. Biomed Mater. 2019;14(4):045018.
- Tagler D, Makanji Y, Tu T, et al. Promoting extracellular matrix remodeling via ascorbic acid enhances the survival of primary ovarian follicles encapsulated in alginate hydrogels. Biotechnol Bioeng. 2014;111(7):1417–1429.
- Jin SY, Lei L, Shikanov A, et al. A novel two-step strategy for in vitro culture of early-stage ovarian follicles in the mouse. Fertil Steril. 2010;93(8):2633–2639.
- Kniazeva E, Hardy AN, Boukaidi SA, et al. Primordial follicle transplantation within designer biomaterial grafts produce live births in a mouse infertility model. Sci Rep. 2016;5(1):17709.
- Xu M, West E, Shea LD, et al. Identification of a stage-specific permissive in vitro culture environment for follicle growth and oocyte development. Biol Reprod. 2006;75(6):916–923.
- Woodruff TK. Lessons from bioengineering the ovarian follicle: a personal perspective. Reproduction. 2019;158(6):F113–f126.
- Hummitzsch K, Anderson RA, Wilhelm D, et al. Stem cells, progenitor cells, and lineage decisions in the ovary. Endocr Rev. 2015;36(1):65–91.
- Herraiz S, Romeu M, Buigues A, et al. Autologous stem cell ovarian transplantation to increase reproductive potential in patients who are poor responders. Fertil Steril. 2018;110(3):496–505.e1.
- Lai D, Wang F, Yao X, et al. Human endometrial mesenchymal stem cells restore ovarian function through improving the renewal of germline stem cells in a mouse model of premature ovarian failure. J Transl Med. 2015;13(1):155.
