Abstract
Symptomatic women with angina are more likely to have ischemia with no obstructive coronary arteries (INOCA) compared to men. In both men and women, the finding of INOCA is not benign and is associated with adverse cardiovascular events, including myocardial infarction, heart failure and angina hospitalizations. Women with INOCA have more angina and a lower quality of life compared to men, but they are often falsely reassured because of a lack of obstructive coronary artery disease (CAD) and a perception of low risk. Coronary microvascular dysfunction (CMD) is a key pathophysiologic contributor to INOCA, and non-invasive imaging methods are used to detect impaired microvascular flow. Coronary vasospasm is another mechanism of INOCA, and can co-exist with CMD, but usually requires invasive coronary function testing (CFT) with provocation testing for a definitive diagnosis. In addition to traditional heart disease risk factors, inflammatory, hormonal and psychological risk factors that impact microvascular tone are implicated in INOCA. Treatment of risk factors and use of anti-atherosclerotic and anti-anginal medications offer benefit. Increasing awareness and early referral to specialized centers that focus on INOCA management can improve patient-oriented outcomes. However, large, randomized treatment trials to investigate the impact on major adverse cardiovascular events (MACE) are needed. In this focused review, we discuss the prevalence, pathophysiology, presentation, diagnosis and treatment of INOCA.
摘要
与男性相比, 有症状的女性心绞痛更有可能出现无阻塞性冠状动脉缺血(INOCA)。在男性和女性中, INOCA的发现都不是良性的, 并与不良心血管事件相关, 包括心肌梗死、心力衰竭和心绞痛住院。与男性相比, 患有INOCA的女性患心绞痛的几率更高, 生活质量也较低, 但她们常常因为没有阻塞性冠状动脉疾病(CAD)而被错误地确定下来, 并被认为风险较低。冠状动脉微血管功能障碍(CMD)是一个关键的病理生理学因素, 并采用无创成像方法检测微血管血流受损。冠状动脉痉挛是INOCA的另一机制, 可与CMD共存, 但通常需要有创冠状动脉功能检测(CFT)和激发性检测才能确诊。除了传统的心脏病危险因素外, 影响微血管张力的炎症、激素和心理危险因素也与INOCA有关。治疗危险因素和使用抗动脉粥样硬化和抗心绞痛药物是有益的。提高意识和早期转诊到专注于INOCA管理的专科中心可以改善以患者为导向的结果。然而, 需要进行大型随机治疗试验来研究主要不良心血管事件(MACE)的影响。在这篇综述中, 我们讨论了INOCA的流行、病理生理、表现、诊断和治疗。
Introduction
Ischemic heart disease is a major contributor to death and disability in men and women, and it is estimated that more than 500,000 patients are diagnosed with angina annually [Citation1]. Patients with chest pain who are suspected of having myocardial ischemia often undergo cardiac stress testing to detect evidence of compromised blood flow to the heart due to significant coronary artery disease (CAD). If stress testing is abnormal, then coronary angiography is pursued to directly visualize epicardial coronary vessels and treat obstructive CAD. In addition to optimal medical therapy, obstructive CAD is treated with either percutaneous coronary stenting or coronary artery bypass grafting surgery. However, there is a substantial population of patients, particularly women, who have chest discomfort and abnormal stress testing, but are surprisingly found to have no obstructive CAD [Citation2,Citation3]. If there is no significant structural or valvular heart disease, then after the finding of no obstructive CAD they are often dismissed as having either a false positive stress test or non-cardiac chest pain. However, patients with ischemia with no obstructive coronary arteries (INOCA) are at risk of major adverse cardiovascular events (MACE) [Citation2,Citation4,Citation5]. Coronary vasomotor disorders such as coronary microvascular dysfunction (CMD) and coronary vasospasm are key pathophysiologic contributors to adverse outcomes in these patients. While INOCA occurs in both men and women, women are more likely to be impacted with recurrent angina presentations and have a lower quality of life with this challenging condition [Citation2,Citation4]. Despite comparable disability to those with obstructive CAD, patients with INOCA are less likely to be started on anti-anginal therapy or other cardiovascular therapies [Citation6]. In this focused review, we discuss the prevalence, pathophysiology, diagnosis and treatment of INOCA.
Nomenclature, epidemiology and prognosis
INOCA is diagnosed in the setting of chronic and recurrent anginal symptoms (usually several weeks to months) when there is evidence of ischemia on stress testing, but no obstructive CAD. Conventional stress testing includes exercise treadmill testing, stress echocardiography or nuclear stress testing (). Obstructive CAD is excluded, either by non-invasive coronary computed tomography angiography [Citation7] or by invasive left heart catheterization and coronary angiography. Obstructive CAD is typically defined as greater than 50% stenosis in at least one major epicardial coronary vessel or a hemodynamically significant stenosis determined by fractional flow reserve (<0.8) testing. Multiple studies now indicate that non-obstructive CAD is the predominant phenotype of ischemic heart disease, which may be partly due to preventive therapies such as statins [Citation8,Citation9]. Among those with ischemia, studies indicate that approximately two-thirds of women and one-third of men will not have any obstructive CAD [Citation2,Citation10]. One study found that more than two-thirds of almost 400,000 symptomatic patients with suspected CAD referred for elective angiography did not have any obstructive CAD [Citation11]. More recently, the term ‘ANOCA’ has been used to describe those with angina and no obstructive CAD, if ischemia is not confirmed on stress testing.
Table 1. Cardiac stress testing modalities and INOCA.
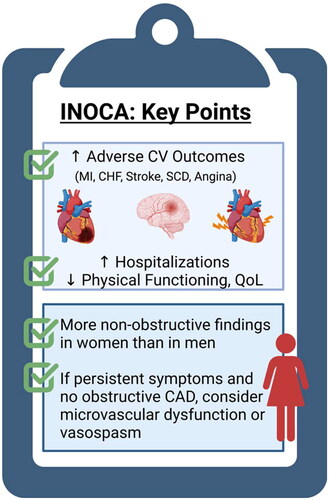
INOCA is associated with short-term and long-term increased mortality. A study in more than 30,000 patients undergoing elective coronary angiography found that those with coronary atherosclerosis (although non-obstructive) had increased mortality at 1 year compared to those without CAD [Citation10]. The Women’s Ischemia Syndrome Evaluation (WISE) study demonstrated a 13% risk of 10-year mortality in INOCA patients compared to 2.8% in asymptomatic controls [Citation5]. Despite not having obstructive atherosclerosis, patients can still have diffuse non-obstructive plaque as demonstrated by studies using intravascular ultrasound during coronary angiography [Citation12].
The pathophysiology of persistent chest pain in INOCA is incompletely understood, but abnormal coronary vascular reactivity (epicardial and microvascular) from endothelium-dependent or endothelium-independent mechanisms is evident in those who undergo advanced testing [Citation13,Citation14]. Endothelial dysfunction and related CMD is present in the majority of patients with INOCA [Citation15]. Furthermore, multiple studies have found that CMD is an important prognostic marker, and it is associated with two-fold to three-fold increased risk of MACE, including heart failure and myocardial infarction [Citation16,Citation17].
Myocardial infarction with no obstructive coronary arteries
Myocardial infarction with no obstructive coronary arteries (MINOCA) refers to those patients who present with acute coronary syndrome, with EKG changes and/or rise and fall of troponin elevation that indicate acute myocardial injury. While INOCA and MINOCA have some overlap in that they predominate in women, they are distinctly different in that INOCA is diagnosed in stable patients with episodes of angina, while MINOCA is diagnosed when there is acute myocardial infarction and other possible etiologies for troponin elevation are excluded (such as myocarditis, Takotsubo syndrome and non-ischemic cardiomyopathy) [Citation18]. Mechanisms of MINOCA include atherosclerotic plaque rupture/erosion, spontaneous coronary artery dissection, CMD, coronary vasospasm or thromboembolism [Citation19,Citation20]. Cardiac magnetic resonance imaging is an important modality in the investigation of MINOCA to diagnose myocarditis as an alternative explanation for clinical presentation [Citation20].
The relationship between INOCA and MINOCA is unclear; however, a patient with stable INOCA may develop a MINOCA event. Women are twice as likely as men to have MINOCA and comprise 50% of MINOCA patients. Large systematic reviews have shown that MINOCA patients tend to be younger women than patients with acute myocardial infarction with obstructive CAD. Prognosis of patients with MINOCA is concerning, with studies showing a 12-month all-cause mortality of 4.7% that is comparable to patients with acute myocardial infarction associated with single-vessel or double-vessel disease [Citation19,Citation21]. While a majority of MINOCA patients are relatively symptom free at 1 year, a subset continue to experience angina after their acute myocardial infarction event.
Symptoms and quality of life
INOCA patients present with substernal or left-sided chest pain (described as chest pain, discomfort, pressure or tightness); however, women are more likely to present with symptoms such as shortness of breath, jaw pain, nausea, profound weakness and fatigue [Citation22]. These ‘atypical’ symptoms are typical for women and therefore, per the new chest pain guidelines, describing cardiac symptoms as ‘atypical’ is no longer recommended [Citation23]. Just as limited blood flow due to obstructive CAD leads to angina, the inability of microvasculature to increase blood flow when demand is increased also leads to angina. Ischemic symptoms in this population can often go untreated because patients with non-obstructive CAD are less likely to be given a diagnosis of angina [Citation24]. For example, one survey done in those eventually diagnosed with INOCA found patients were, on average, seen by three cardiologists before being diagnosed and the time to diagnosis was 1–10 years after symptom onset. In the interim, 78% of patients were given non-cardiac diagnoses, and more than 30% were sent to a psychiatrist and/or prescribed antidepressants [Citation25]. High symptom burden in INOCA has psychosocial implications. For example, 80% of women report adverse effects on home and social life and 70% report reduced working hours or quitting their jobs [Citation25]. In the WISE study, anginal symptoms were more important determinants of quality of life than the degree of CAD severity [Citation5].
Women with INOCA also have a three-fold to four-fold increased risk of hospitalizations, which in turn contributes to high health-care costs [Citation26]. Women with angiographically normal coronaries were found to be four times more likely to be readmitted within 6 months for chest pain compared to men [Citation27,Citation28]. Women with persistent angina are predicted to have a lifetime cost of $750,000 for cardiovascular care [Citation29]. The estimated yearly economic impact of INOCA is ∼$21 billion dollars in the USA [Citation30]. These studies emphasize the burden INOCA can have in predominantly female patients and represent an area of opportunity to improve outcomes, symptoms and quality of life in this population.
Coronary vascular dysfunction in INOCA
Coronary microvascular dysfunction
In CMD there is impaired coronary blood flow either from structural or functional pathology in the microvasculature, leading to microvascular ischemia and angina. The coronary microcirculation includes epicardial pre-arterioles (less than 500 µm in diameter), intramyocardial arterioles and capillaries. Normally, an increase in myocardial oxygen demand is matched by an increase in coronary blood flow. However, in CMD, resistance in the arterioles can prevent an increase in myocardial blood supply and precipitate ischemia. Endothelial dysfunction is present in 80% of INOCA patients undergoing invasive testing [Citation31]. Normal endothelium regulates vascular tone by modulating vasodilatory factors (e.g. nitric oxide and prostacyclin) and vasoconstrictive factors (e.g. thromboxane and endothelin-1) [Citation32]. Patients are diagnosed with CMD when they have impaired vasodilatory responses or abnormal microvascular vasoconstriction. Increased resistance from structural effects on the vessels (e.g. arteriolar remodeling with perivascular fibrosis, abnormal perivascular fat, microvascular obstruction and capillary rarefaction) contributes to impaired microvascular flow [Citation33]. CMD is associated with endothelial dysfunction, which is considered a systemic process that impacts the microvasculature in multiple organ systems including the brain, eyes and kidneys [Citation34]. CMD may also be playing a role in patients who experience persistent angina despite percutaneous coronary intervention for treatment of obstructive CAD. INOCA patients have more diastolic dysfunction and fibrosis, and are at higher risk of developing heart failure with preserved ejection fraction compared to women in the general population [Citation5,Citation10,Citation35]. CMD is implicated in the pathophysiology leading to heart failure with preserved ejection fraction, a syndrome that is associated with cardiometabolic risk factors and also predominates in postmenopausal women.
Coronary vasospasm
Coronary artery spasm is a sudden hypercontraction of the microvascular or epicardial coronary vessels that causes near or total occlusion of the vessel and a reduction in blood flow. Triggers of coronary vasospasm are not completely understood, but risk factors such as smoking, hypertension and hyperlipidemia are associated with vasospasm. Two major mechanisms of vasospasm are sympathetically mediated vasoconstriction that can happen with mental stress, and predisposition to vasospasm due to upregulation of Rho-kinase. Rho-kinase is an enzyme that causes calcium-independent vascular smooth muscle contraction through myosin light chain phosphorylation [Citation36]. Inflammatory cytokines such as interleukin-1β (IL-1β) have been shown to increase expression of Rho-kinase in vascular smooth muscle cells and thus can precipitate spasm [Citation37]. Conversely, in in vivo models, estrogen inhibits Rho-kinase upregulation, suggesting spasm can be precipitated in low-estrogen states. In the Coronary Artery Spasm as a Frequent Cause for Acute Coronary Syndrome (CASPAR) study, approximately 50% of patients with acute coronary syndrome and no obstructive CAD were found to have coronary vasospasm on intracoronary acetylcholine (ACH) provocation testing [Citation38].
Risk factors and INOCA
Traditional risk factors
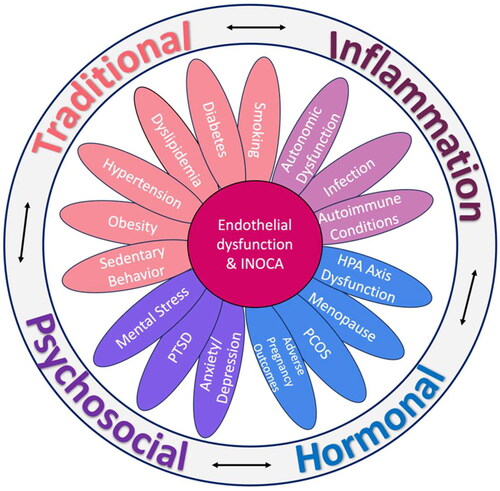
Traditional risk factors such as hypertension, diabetes mellitus, older age and smoking are associated with INOCA and CMD [Citation39,Citation40]. These risk factors are known to impair endothelial dysfunction and are also associated with inflammation and oxidative stress, which are key pathophysiologic drivers implicated in INOCA. Studies have shown lower coronary microvascular reserve and worse diastolic dysfunction in women with diabetes compared to men [Citation41,Citation42]. Obesity and metabolic syndrome are also associated with impaired microvascular function [Citation43]. In addition to traditional risk factors, there are inflammatory conditions, sex-specific factors and psychological factors that are associated with INOCA ().
Chronic inflammation
Women may be more susceptible to CMD due to factors such as inflammatory and auto-immune conditions, which contribute to endothelial dysfunction [Citation44,Citation45]. In patients with chronic rheumatologic disorders (systemic lupus erythematosus or rheumatoid arthritis) and no obstructive CAD or cardiac risk factors, impaired coronary flow reserve (CFR) was inversely related to disease duration [Citation46]. High-sensitivity C-reactive protein (hs-CRP) is a well-established marker of inflammation and has been linked to coronary endothelial dysfunction, and higher hs-CRP levels are associated with oxidative stress among adults without CAD, indicating that oxidative stress has pro-inflammatory effects [Citation47]. Higher interleukin-6 (IL-6) levels in INOCA predicted heart failure hospitalization and all-cause mortality in the WISE study [Citation48]. Soluble urokinase plasminogen activator receptor (suPAR) is a biomarker of inflammation and immune regulation that is released from various cell types including leukocytes and endothelial cells, and is an independent predictor of adverse outcomes [Citation49]. In patients with no obstructive CAD, the suPAR level was an independent predictor of CMD [Citation50]. More recently, in a large study of 1471 women, CMD was associated with biomarkers of inflammation (IL-6), hypertension (renin and adrenomedullin) and ventricular remodeling (BNP/NT-proBNP), independent of heart disease risk factors [Citation51].
Sex-specific risk factors
Sex-specific risk factors such as menopause [Citation52,Citation53], premature ovarian insufficiency [Citation54,Citation55], polycystic ovarian syndrome [Citation56] and adverse pregnancy outcomes (i.e. pre-eclampsia) are associated with endothelial dysfunction, and are heart disease risk factors in women [Citation57,Citation58]. Declining estrogen levels are associated with increased microvascular tone, and postmenopausal women make up the majority of patients with INOCA [Citation5]. There is an increased prevalence of vasospastic disorders postmenopause, given that estrogen has an inhibitory effect on Rho-kinase upregulation [Citation59]. Menopause is also associated with increases in heart disease risk factors such as hypertension and hyperlipidemia, which impact the vascular endothelium and accelerate vascular aging [Citation60]. Interestingly, there are younger women with INOCA who have exacerbations of their chest pain symptoms associated with the menstrual cycle [Citation61]. Although estrogen has positive vasodilatory effects, it is not typically used to treat INOCA or CMD as large randomized trials showing benefit are lacking.
Psychological risk factors and mental stress
Endothelial dysfunction and abnormal coronary vasoconstriction in response to laboratory-induced mental stress have been shown in several studies, and women may be particularly susceptible to adverse cardiac effects of mental stress [Citation62]. Mental stress can contribute to and exacerbate angina regardless of obstructive CAD. Vaccarino et al. found that mental stress-induced ischemia is more common in younger women (age ≤ 50 years) compared to younger men, but a sex difference was not present in those over the age of 50 years [Citation63]. Mental stress-induced endothelial dysfunction (measured by brachial artery flow-mediated dilation at 30 min post a mental stress task) was associated with worse cardiovascular outcomes, and this finding was more prominent in women compared to men [Citation64]. Presence of depression predicts angina frequency independently of CAD severity [Citation65]. In studies of symptomatic patients with no obstructive CAD, 34% were found to meet the criteria for having a panic disorder [Citation66], and those with high anxiety trait were found to be at risk of myocardial ischemia [Citation67]. Whether women with INOCA have more mental stress-related microvascular vasoreactivity compared to those women with obstructive CAD or compared to healthy women with no heart disease is under investigation (ClinicalTrials.gov NCT05401630).
Non-invasive stress testing
Exercise treadmill testing remains the first line in the evaluation of stable patients suspected of having myocardial ischemia, as it is widely available and prognostic. Positive exercise treadmill testing with no obstructive CAD on angiography can lead to a conclusion of ‘false-positive’ exercise treadmill testing; however, CMD should be considered as an etiology in such patients. If exercise stress is not an option due to orthopedic limitations, then pharmacologic stress testing using vasodilatory agents such as adenosine (ADO) or regadenoson is typically performed. CMD can be detected by positron emission tomography (PET), stress Doppler echocardiography or stress cardiac magnetic resonance imaging [Citation51,Citation68–70], and the test of choice depends on individual center expertise and insurance coverage (). The American College of Cardiology 2021 Chest pain guidelines recommend these non-invasive stress testing modalities to evaluate for ischemia in INOCA and to detect evidence of abnormal microvascular flow reserve [Citation23]. Cardiac PET is more widely available in the USA for CMD detection, and quantifies myocardial blood flow. PET provides the myocardial flow reserve, which is a ratio of blood flow during stress to rest, and an impaired reserve of less than 2.0 indicates CMD and predicts MACE regardless of CAD severity [Citation71,Citation72].
Invasive coronary function testing
Vasomotor disorders are a diagnostic challenge because invasive coronary function testing (CFT) is not routinely performed. Mechanisms of persistent and recurrent symptoms in INOCA are not well understood given that symptoms can be out of proportion to the ischemic burden detected on conventional stress testing. Indeed, conventional stress testing can often be normal in these patients, and only with invasive CFT is vasomotor dysfunction identified. The decision to pursue CFT depends on the availability of testing and when benefit outweighs the risk in an individual patient. A definitive diagnosis helps with prognostication and helps guide therapy to improve angina and quality of life outcomes [Citation73]. Therefore, an effort to identify the mechanism(s) of angina in patients with INOCA is crucial [Citation73]. In this section we briefly review the two distinct pathways that are tested in comprehensive CFT that contribute to angina in patients with INOCA (). For both pathways, wire-based techniques in the left anterior descending artery are commonly used, utilizing the Doppler wire (Volcano®) and the Thermodilution Radi X/Coroflow wire (Abbott Vascular®).
Figure 2. Endothelium-independent and endothelium-dependent angina mechanisms. IMR, Index of Microcirculatory Resistance. *Coronary flow reserve (CFR), an integrated measure of flow through both epicardial and microvascular vessels, but in the absence of epicardial disease it serves as a measure of microvascular function. Figure made using biorender.com. Image © Emory University, CC-by-SA.
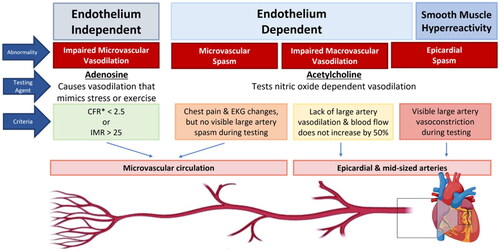
Endothelium-independent pathway
The endothelium-independent pathway uses ADO to create maximal hyperemia and assesses flow and resistance to flow in the microcirculation. ADO causes vasodilation of the microvasculature which mimics exercise and stress and can be used to obtain the CFR [Citation74]. The CFR is the ratio of coronary flow velocity during stress over coronary flow velocity during rest, and CFR < 2.5 in the absence of obstructive disease is used to diagnose CMD [Citation75]. Microvascular resistance can also be directly measured using the thermodilution technique, and an increased Index of Microcirculatory Resistance (IMR) > 25 is indicative of CMD [Citation76].
Endothelium-dependent pathway
The endothelium-dependent pathway can be tested using ACH, which normally causes vasodilation of the epicardial vessels. Vasoconstriction observed during administration of ACH is considered abnormal. ACH stimulates the healthy endothelium to release nitric oxide, which in turn mediates vascular smooth muscle cell relaxation via cyclic GMP. Low doses of ACH (1–50 μg) are used to evaluate endothelial dysfunction, whereas higher doses (100–200 μg) test for coronary vasospasm. In abnormal endothelial function, coronary blood flow does not increase by 50% with low doses of ACH [Citation77]. Severe endothelial dysfunction is defined as a decrease >20% in luminal diameter after intracoronary administration of low-dose ACH [Citation77]. If there is no visible epicardial spasm with higher doses of ACH but there is chest pain or EKG changes, then a diagnosis of microvascular spasm is made. Coronary vasospasm is defined as >90% narrowing of the vessel in response to ACH [Citation78]. Some clinical protocols accept spasm >75% during ACH as a diagnostic for epicardial spasm [Citation78]. Historically, ergonovine and methylergonovine were also utilized but due to higher rates of complications, including resistance to reversal of severe vasospasm and triggered electrical abnormalities, they are no longer widely used. The safety profile of ACH was evaluated in a recent meta-analysis showing very low risk <0.5% of major complications during spasm provocation [Citation13]. Patients with INOCA can have different and sometimes overlapping vascular pathways that are dysfunctional, which at the current time can only be tested comprehensively by CFT. The Coronary Microvascular Angina (CorMicA) trial demonstrated that determining the cause of INOCA followed by targeted treatment improves clinical outcomes [Citation73].
Treatment
In addition to addressing cardiac risk factors, treatment of INOCA focuses on the use of anti-atherosclerotic and anti-ischemic therapy to reduce adverse events, and the use of anti-anginal strategies to improve symptoms and quality of life (). To date there have been few large randomized controlled trials or large observational studies to determine the treatment options for patients with non-obstructive CAD. Large registry data suggest that half of the patients with stable INOCA are not treated with any cardiovascular medications [Citation6,Citation10,Citation79]. Treatment practices vary widely among cardiologists. For example, a recent survey of 130 cardiologists found 80% rarely to never ordered advanced imaging modalities to assess the CFR in patients with INOCA. More than 75% did not regularly prescribe anti-anginals, cardiac rehabilitation or antiplatelet agents for patients. Of those who did prescribe anti-anginals, approximately two-thirds reported prescribing beta-blockers or statins, but more than 70% never prescribed medications for afterload reduction such as an angiotensin converting enzyme inhibitor (ACE-I) or angiotensin receptor blocker (ARB) [Citation80].
Table 2. Therapy target and management strategies.
Previous studies have shown that treatment of cardiovascular risk factors can improve endothelial function and lower risk, and aggressive risk factor modification with lifestyle and medication changes is recommended in INOCA. For example, baseline statin therapy is associated with a reduction in mortality in those with non-obstructive disease compared to those with no CAD [Citation81]. All patients with INOCA should be screened for nicotine use and should be encouraged to quit with the help of support groups, medications and close follow-up to reduce the risk of recurrence [Citation82]. Cardiac rehabilitation improves risk factors, as well as cardiac morbidity and mortality [Citation83,Citation84]. Exercise training has benefits on cardiopulmonary function, vascular function, symptoms, psychological stress and quality of life. While chronic stable angina is a class I indication for cardiac rehabilitation (level of evidence B) [Citation85], this therapeutic strategy is underutilized, particularly in women [Citation84]. In a non-randomized study of 55 women with microvascular angina, cardiac rehabilitation improved functional capacity and myocardial perfusion [Citation86].
First-line treatment in INOCA revolves around reducing myocardial oxygen demand or increasing coronary blood supply with beta-blockers and calcium channel blockers. Although there are no formal guidelines on treatment, both medications have been shown to reduce anginal symptoms in patients with INOCA [Citation3,Citation87]. Calcium channel blockers, and short-acting and long-acting nitrates, are the first-line recommended treatment for vasospastic angina [Citation63,Citation88]. There are several agents that are available to manage CMD-related angina and ischemia, including beta-blockers and ranolazine [Citation89]. Several studies have found use of an ACE-I improves CFR and anginal symptoms [Citation90]. Patients often have multiple vascular pathways involved, and therefore treatment is individualized based on their response to therapy, hemodynamics (i.e. heart rate, blood pressure), side effects/intolerances and affordability. Trials such as the Beta Blocker and ACE-inhibitor/angiotensin Receptor Blocker Treatment in Myocardial Infarction with Non-obstructive Coronary Arteries (MINOCA-BAT) and the Women’s Ischemia Trial to Reduce Events in Non-obstructive CAD (WARRIOR) are currently underway to further illuminate the best treatment strategies [Citation91,Citation92].
Low-dose tricyclic antidepressants such as imipramine have also been shown to be helpful for patients with persistent chest pain where abnormal cardiac nociceptive abnormality is suspected [Citation93]. Often, these patients, who are more likely to be women, report chest pain with contrast injection in the catheterization laboratory during CFT and have high pain sensitivity. External counter pulsation is another treatment option that involves sessions where inflatable cuffs on lower extremities are used to augment diastolic flow and improve endothelial function [Citation94]. Implantation of a device in the coronary sinus (Neovasc Reducer™) is also being tested for treatment of CMD [Citation95].
Conclusions
INOCA is an important contributor to adverse cardiovascular outcomes and mortality in both men and women, but is under-recognized and undertreated. Women are more likely to present with INOCA compared to men and are more impacted by this condition with recurrent angina, low physical functioning and an impact on quality of life (). In addition to traditional heart disease risk factors, inflammatory, hormonal and psychological risk factors that impact microvascular tone are implicated in INOCA. A vast majority of INOCA patients have CMD, and non-invasive imaging methods combined with vasodilator test agents are used to detect CMD. Coronary vasospasm is another mechanism of INOCA, and can co-exist with CMD, but usually requires invasive CFT with provocation testing for a definitive diagnosis. Treatment of risk factors and use of anti-atherosclerotic and anti-anginal medications offer benefit. Referral to a specialist could lead to earlier diagnosis and treatment to improve quality of life in these patients. Large clinical trials are underway to investigate optimal medical therapy and impact on MACE in INOCA.
Potential conflict of interest
No potential conflict of interest was reported by the authors.
Acknowledgements
The authors acknowledge Esha Dave, MS for help with the figures for this manuscript. All figures were created using Biorender.com.
Additional information
Funding
References
- Benjamin EJ, Virani SS, Callaway CW, et al. Heart disease and stroke statistics-2018 update: a report from the American Heart Association. Circulation. 2018;137(12):e67–e492. doi: 10.1161/CIR.0000000000000558.
- Bairey Merz CN, Pepine CJ, Walsh MN, et al. Ischemia and No Obstructive Coronary Artery Disease (INOCA): developing evidence-based therapies and research agenda for the next decade. Circulation. 2017;135(11):1075–1092. doi: 10.1161/CIRCULATIONAHA.116.024534.
- Crea F, Camici PG, Bairey Merz CN. Coronary microvascular dysfunction: an update. Eur Heart J. 2014;35(17):1101–1111. doi: 10.1093/eurheartj/eht513.
- Shimokawa H, Suda A, Takahashi J, et al. Clinical characteristics and prognosis of patients with microvascular angina: an international and prospective cohort study by the Coronary Vasomotor Disorders International Study (COVADIS) Group. Eur Heart J. 2021;42(44):4592–4600. doi: 10.1093/eurheartj/ehab282.
- Gulati M, Cooper-DeHoff RM, McClure C, et al. Adverse cardiovascular outcomes in women with nonobstructive coronary artery disease: a report from the women’s ischemia syndrome evaluation study and the St James women take heart project. Arch Intern Med. 2009;169(9):843–850. doi: 10.1001/archinternmed.2009.50.
- Jespersen L, Hvelplund A, Abildstrøm SZ, et al. Stable angina pectoris with no obstructive coronary artery disease is associated with increased risks of major adverse cardiovascular events. Eur Heart J. 2012;33(6):734–744. doi: 10.1093/eurheartj/ehr331.
- Saraste A, Knuuti J. ESC 2019 guidelines for the diagnosis and management of chronic coronary syndromes: recommendations for cardiovascular imaging. Herz. 2020;45(5):409–420. doi: 10.1007/s00059-020-04935-x.
- Shaw LJ, Shaw RE, Merz CN, et al. Impact of ethnicity and gender differences on angiographic coronary artery disease prevalence and in-hospital mortality in the American College of Cardiology-National Cardiovascular Data Registry. Circulation. 2008;117(14):1787–1801. doi: 10.1161/CIRCULATIONAHA.107.726562.
- Merz NB, Johnson BD, Kelsey PSF, et al. Diagnostic, prognostic, and cost assessment of coronary artery disease in women. Am J Manag Care. 2001;7(10):959–965.
- Maddox TM, Stanislawski MA, Grunwald GK, et al. Nonobstructive coronary artery disease and risk of myocardial infarction. JAMA. 2014;312(17):1754–1763. doi: 10.1001/jama.2014.14681.
- Patel MR, Peterson ED, Dai D, et al. Low diagnostic yield of elective coronary angiography. N Engl J Med. 2010;362(10):886–895. doi: 10.1056/NEJMoa0907272.
- Khuddus MA, Pepine CJ, Handberg EM, et al. An intravascular ultrasound analysis in women experiencing chest pain in the absence of obstructive coronary artery disease: a substudy from the National Heart, Lung and Blood Institute-Sponsored Women’s Ischemia Syndrome Evaluation (WISE). J Interv Cardiol. 2010;23(6):511–519. doi: 10.1111/j.1540-8183.2010.00598.x.
- Takahashi T, Samuels BA, Li W, et al. Safety of provocative testing with intracoronary acetylcholine and implications for standard protocols. J Am Coll Cardiol. 2022;79(24):2367–2378. doi: 10.1016/j.jacc.2022.03.385.
- AlBadri A, Bairey Merz CN, Johnson BD, et al. Impact of abnormal coronary reactivity on long-term clinical outcomes in women. J Am Coll Cardiol. 2019;73(6):684–693. doi: 10.1016/j.jacc.2018.11.040.
- Sara JD, Widmer RJ, Matsuzawa Y, et al. Prevalence of coronary microvascular dysfunction among patients with chest pain and nonobstructive coronary artery disease. JACC Cardiovasc Interv. 2015;8(11):1445–1453. doi: 10.1016/j.jcin.2015.06.017.
- Gupta A, Taqueti VR, van de Hoef TP, et al. Integrated noninvasive physiological assessment of coronary circulatory function and impact on cardiovascular mortality in patients with stable coronary artery disease. Circulation. 2017;136(24):2325–2336. doi: 10.1161/CIRCULATIONAHA.117.029992.
- Ziadi MC, Dekemp RA, Williams KA, et al. Impaired myocardial flow reserve on rubidium-82 positron emission tomography imaging predicts adverse outcomes in patients assessed for myocardial ischemia. J Am Coll Cardiol. 2011;58(7):740–748. doi: 10.1016/j.jacc.2011.01.065.
- Pasupathy S, Tavella R, Beltrame JF. Myocardial Infarction with Nonobstructive Coronary Arteries (MINOCA): the past, present, and future management. Circulation. 2017;135(16):1490–1493. doi: 10.1161/CIRCULATIONAHA.117.027666.
- Agewall S, Beltrame JF, Reynolds HR, et al. ESC working group position paper on myocardial infarction with non-obstructive coronary arteries. Eur Heart J. 2017;38(3):143–153.
- Reynolds HR, Maehara A, Kwong RY, et al. Coronary optical coherence tomography and cardiac magnetic resonance imaging to determine underlying causes of myocardial infarction with nonobstructive coronary arteries in women. Circulation. 2021;144(12):e209–e210. doi: 10.1161/CIRCULATIONAHA.121.055516.
- Tamis-Holland JE, Jneid H, Reynolds HR, et al. Contemporary diagnosis and management of patients with myocardial infarction in the absence of obstructive coronary artery disease: a scientific statement from the American Heart Association. Circulation. 2019;139(18):e891–e908. doi: 10.1161/CIR.0000000000000670.
- Mieres JH, Heller GV, Hendel RC, et al. Signs and symptoms of suspected myocardial ischemia in women: results from the What is the Optimal Method for Ischemia Evaluation in WomeN? Trial. J Womens Health (Larchmt). 2011;20(9):1261–1268. doi: 10.1089/jwh.2010.2595.
- Gulati M, Levy PD, Mukherjee D, et al. 2021 AHA/ACC/ASE/CHEST/SAEM/SCCT/SCMR guideline for the evaluation and diagnosis of chest pain: executive summary: a report of the American College of Cardiology/American Heart Association Joint Committee on clinical practice guidelines. Circulation. 2021;144(22):e368–e454. doi: 10.1161/CIR.0000000000001030.
- Schumann CL, Mathew RC, Dean JL, et al. Functional and economic impact of INOCA and influence of coronary microvascular dysfunction. JACC Cardiovasc Imaging. 2021;14(7):1369–1379. doi: 10.1016/j.jcmg.2021.01.041.
- Gulati M, Khan N, George M, et al. Ischemia with no obstructive coronary artery disease (INOCA): a patient self-report quality of life survey from INOCA international. Int J Cardiol. 2023;371:28–39. doi: 10.1016/j.ijcard.2022.09.047.
- Aldiwani H, Zaya M, Suppogu N, et al. Angina hospitalization rates in women with signs and symptoms of ischemia but no obstructive coronary artery disease: a report from the WISE (Women’s Ischemia Syndrome Evaluation). J Am Heart Assoc. 2020;9(4):e013168.
- Humphries KH, Pu A, Gao M, et al. Angina with “normal” coronary arteries: sex differences in outcomes. Am Heart J. 2008;155(2):375–381. doi: 10.1016/j.ahj.2007.10.019.
- Kamlesh K. Microvascular coronary dysfunction in women: pathlophysiology, diagnosis, and management. Curr Probl Cardiol. 2011;36(8):27.
- Shaw LJ, Merz CN, Pepine CJ, et al. The economic burden of angina in women with suspected ischemic heart disease: results from the National Institutes of Health–National Heart, Lung, and Blood Institute–sponsored Women’s Ischemia Syndrome Evaluation. Circulation. 2006;114(9):894–904. doi: 10.1161/CIRCULATIONAHA.105.609990.
- Ferreira Vanessa M, Berry C. The health economics of ischemia with nonobstructive coronary arteries. ∗JACC Cardiovasc Imaging. 2021;14(7):1380–1383. doi: 10.1016/j.jcmg.2021.03.017.
- Feenstra RGT, Boerhout CKM, Woudstra J, et al. Presence of coronary endothelial dysfunction, coronary vasospasm, and adenosine-mediated vasodilatory disorders in patients with ischemia and nonobstructive coronary arteries. Circ Cardiovasc Interv. 2022;15(8):e012017. doi: 10.1161/CIRCINTERVENTIONS.122.012017.
- Godo S, Takahashi J, Yasuda S, et al. Endothelium in coronary macrovascular and microvascular diseases. J Cardiovasc Pharmacol. 2021;78(Suppl 6):S19–s29. doi: 10.1097/FJC.0000000000001089.
- Godo S, Suda A, Takahashi J, et al. Coronary microvascular dysfunction. Arterioscler Thromb Vasc Biol. 2021;41(5):1625–1637. doi: 10.1161/ATVBAHA.121.316025.
- Berry C, Sidik N, Pereira AC, et al. Small-vessel disease in the heart and brain: current knowledge, unmet therapeutic need, and future directions. J Am Heart Assoc. 2019;8(3):e011104.
- Nelson MD, Wei J, Bairey Merz CN. Coronary microvascular dysfunction and heart failure with preserved ejection fraction as female-pattern cardiovascular disease: the chicken or the egg? Eur Heart J. 2018;39(10):850–852. doi: 10.1093/eurheartj/ehx818.
- Masumoto A, Mohri M, Shimokawa H, et al. Suppression of coronary artery spasm by the rho-kinase inhibitor fasudil in patients with vasospastic angina. Circulation. 2002;105(13):1545–1547. Apr 2doi: 10.1161/hc1002.105938.
- Shimokawa H. 2014 Williams Harvey Lecture: importance of coronary vasomotion abnormalities-from bench to bedside. Eur Heart J. 2014;35(45):3180–3193. doi: 10.1093/eurheartj/ehu427.
- Ong P, Athanasiadis A, Borgulya G, et al. 3-Year follow-up of patients with coronary artery spasm as cause of acute coronary syndrome: the CASPAR (coronary artery spasm in patients with acute coronary syndrome) study follow-up. J Am Coll Cardiol. 2011;57(2):147–152. doi: 10.1016/j.jacc.2010.08.626.
- Mehta PK, Gaignard S, Schwartz A, et al. Traditional and emerging sex-specific risk factors for cardiovascular disease in women. Rev. Cardiovasc. Med. 2022;23(8):288. doi: 10.31083/j.rcm2308288.
- Waheed N, Elias-Smale S, Malas W, et al. Sex differences in non-obstructive coronary artery disease. Cardiovasc Res. 2020;116(4):829–840. doi: 10.1093/cvr/cvaa001.
- Haas AV, Rosner BA, Kwong RY, et al. Sex differences in coronary microvascular function in individuals with type 2 diabetes. Diabetes. 2019;68(3):631–636. Mardoi: 10.2337/db18-0650.
- Ohkuma T, Komorita Y, Peters SAE, et al. Diabetes as a risk factor for heart failure in women and men: a systematic review and meta-analysis of 47 cohorts including 12 million individuals. Diabetologia. 2019;62(9):1550–1560. Sepdoi: 10.1007/s00125-019-4926-x.
- Bajaj NS, Osborne MT, Gupta A, et al. Coronary microvascular dysfunction and cardiovascular risk in obese patients. J Am Coll Cardiol. 2018;72(7):707–717. doi: 10.1016/j.jacc.2018.05.049.
- Mehta PK, Levit RD, Wood MJ, et al. Chronic rheumatologic disorders and cardiovascular disease risk in women. Am Heart J Plus Cardiol Res Pract. 2023;27:100267. doi: 10.1016/j.ahjo.2023.100267.
- Chakrala T, Prakash R, Valdes C, et al. Circulating biomarkers in coronary microvascular dysfunction. J Am Heart Assoc. 2023;12(12):e029341.
- Recio-Mayoral A, Mason JC, Kaski JC, et al. Chronic inflammation and coronary microvascular dysfunction in patients without risk factors for coronary artery disease. Eur Heart J. 2009;30(15):1837–1843. doi: 10.1093/eurheartj/ehp205.
- Abramson JL, Hooper WC, Jones DP, et al. Association between novel oxidative stress markers and C-reactive protein among adults without clinical coronary heart disease. Atherosclerosis. 2005;178(1):115–121. doi: 10.1016/j.atherosclerosis.2004.08.007.
- AlBadri A, Lai K, Wei J, et al. Inflammatory biomarkers as predictors of heart failure in women without obstructive coronary artery disease: a report from the NHLBI-sponsored Women’s Ischemia Syndrome Evaluation (WISE). PLoS One. 2017;12(5):e0177684. doi: 10.1371/journal.pone.0177684.
- Eapen DJ, Manocha P, Ghasemzadeh N, et al. Soluble urokinase plasminogen activator receptor level is an independent predictor of the presence and severity of coronary artery disease and of future adverse events. J Am Heart Assoc. 2014;3(5):e001118.
- Mekonnen G, Corban MT, Hung OY, et al. Plasma soluble urokinase-type plasminogen activator receptor level is independently associated with coronary microvascular function in patients with non-obstructive coronary artery disease. Atherosclerosis. 2015;239(1):55–60. Mardoi: 10.1016/j.atherosclerosis.2014.12.025.
- Prescott E, Bove KB, Bechsgaard DF, et al. Biomarkers and coronary microvascular dysfunction in women with angina and no obstructive coronary artery disease. JACC: advances. 2023;2(2):100264. doi: 10.1016/j.jacadv.2023.100264.
- Moreau KL, Hildreth KL, Meditz AL, et al. Endothelial function is impaired across the stages of the menopause transition in healthy women. J Clin Endocrinol Metab. 2012;97(12):4692–4700. Decdoi: 10.1210/jc.2012-2244.
- Sickinghe AA, Korporaal SJA, den Ruijter HM, et al. Estrogen contributions to microvascular dysfunction evolving to heart failure with preserved ejection fraction. Front Endocrinol (Lausanne). 2019;10:442. doi: 10.3389/fendo.2019.00442.
- El Khoudary SR, Wildman RP, Matthews K, et al. Progression rates of carotid intima-media thickness and adventitial diameter during the menopausal transition. Menopause. 2013;20(1):8–14. doi: 10.1097/gme.0b013e3182611787.
- Stevenson JC, Collins P, Hamoda H, et al. Cardiometabolic health in premature ovarian insufficiency. Climacteric. 2021;24(5):474–480. doi: 10.1080/13697137.2021.1910232.
- Lakhani K, Leonard A, Seifalian AM, et al. Microvascular dysfunction in women with polycystic ovary syndrome. Hum Reprod. 2005;20(11):3219–3224. Novdoi: 10.1093/humrep/dei199.
- Cho L, Davis M, Elgendy I, et al. Summary of updated recommendations for primary prevention of cardiovascular disease in women: JACC state-of-the-Art review. J Am Coll Cardiol. 2020;75(20):2602–2618. doi: 10.1016/j.jacc.2020.03.060.
- Minissian MB, Mehta PK, Hayes SN, et al. Ischemic heart disease in young women: JACC review topic of the week. J Am Coll Cardiol. 2022;80(10):1014–1022. doi: 10.1016/j.jacc.2022.01.057.
- Chrissobolis S, Budzyn K, Marley PD, et al. Evidence that estrogen suppresses Rho-Kinase function in the cerebral circulation in vivo. Stroke. 2004;35(9):2200–2205. doi: 10.1161/01.STR.0000136951.85586.c8.
- El Khoudary SR, Aggarwal B, Beckie TM, et al. Menopause transition and cardiovascular disease risk: implications for timing of early prevention: a scientific statement from the American Heart Association. Circulation. 2020;142(25):e506–e532. doi: 10.1161/CIR.0000000000000912.
- Kawano H, Motoyama T, Ohgushi M, et al. Menstrual cyclic variation of myocardial ischemia in premenopausal women with variant angina. Ann Intern Med. 2001;135(11):977–981. doi: 10.7326/0003-4819-135-11-200112040-00009.
- Mehta PK, Sharma A, Bremner JD, et al. Mental stress-induced myocardial ischemia. Curr Cardiol Rep. 2022;24(12):2109–2120. doi: 10.1007/s11886-022-01821-2.
- Vaccarino V, Shah AJ, Rooks C, et al. Sex differences in mental stress-induced myocardial ischemia in young survivors of an acute myocardial infarction. Psychosom Med. 2014;76(3):171–180. doi: 10.1097/PSY.0000000000000045.
- Lima BB, Hammadah M, Kim JH, et al. Association of transient endothelial dysfunction induced by mental stress with major adverse cardiovascular events in men and women with coronary artery disease. JAMA Cardiol. 2019;4(10):988–996. doi: 10.1001/jamacardio.2019.3252.
- Hayek SS, Ko YA, Awad M, et al. Depression and chest pain in patients with coronary artery disease. Int J Cardiol. 2017;230:420–426. doi: 10.1016/j.ijcard.2016.12.091.
- Beitman BD, Mukerji V, Lamberti JW, et al. Panic disorder in patients with chest pain and angiographically normal coronary arteries. Am J Cardiol. 1989;63(18):1399–1403. doi: 10.1016/0002-9149(89)91056-4.
- Vermeltfoort IA, Raijmakers PG, Odekerken DA, et al. Association between anxiety disorder and the extent of ischemia observed in cardiac syndrome X. J Nucl Cardiol. 2009;16(3):405–410. doi: 10.1007/s12350-008-9032-2.
- Gould KL, Johnson NP, Bateman TM, et al. Anatomic versus physiologic assessment of coronary artery disease. Role of coronary flow reserve, fractional flow reserve, and positron emission tomography imaging in revascularization decision-making. J Am Coll Cardiol. 2013;62(18):1639–1653. doi: 10.1016/j.jacc.2013.07.076.
- Thomson LE, Wei J, Agarwal M, et al. Cardiac magnetic resonance myocardial perfusion reserve index is reduced in women with coronary microvascular dysfunction. A National Heart, Lung, and Blood Institute-sponsored study from the Women’s Ischemia Syndrome Evaluation. Circ Cardiovasc Imaging. 2015;8(4):e002481. doi: 10.1161/CIRCIMAGING.114.002481.
- Shufelt CL, Thomson LE, Goykhman P, et al. Cardiac magnetic resonance imaging myocardial perfusion reserve index assessment in women with microvascular coronary dysfunction and reference controls. Cardiovasc Diagn Ther. 2013;3(3):153–160.
- Murthy VL, Naya M, Foster CR, et al. Association between coronary vascular dysfunction and cardiac mortality in patients with and without diabetes mellitus. Circulation. 2012;126(15):1858–1868. Oct 9doi: 10.1161/CIRCULATIONAHA.112.120402.
- Taqueti VR, Hachamovitch R, Murthy VL, et al. Global coronary flow reserve is associated with adverse cardiovascular events independently of luminal angiographic severity and modifies the effect of early revascularization. Circulation. 2015;131(1):19–27. doi: 10.1161/CIRCULATIONAHA.114.011939.
- Ford Thomas J, Stanley B, Good R, et al. Stratified medical therapy using invasive coronary function testing in angina. J Am Coll Cardiol. 2018;72(23 Pt A):2841–2855. doi: 10.1016/j.jacc.2018.09.006.
- Camici PG, Crea F. Coronary microvascular dysfunction. N Engl J Med. 2007;356(8):830–840. doi: 10.1056/NEJMra061889.
- Demir OM, Boerhout CKM, de Waard GA, et al. Comparison of doppler flow velocity and thermodilution derived indexes of coronary physiology. JACC Cardiovasc Interv. 2022;15(10):1060–1070. doi: 10.1016/j.jcin.2022.03.015.
- Ng MK, Yeung AC, Fearon WF. Invasive assessment of the coronary microcirculation: superior reproducibility and less hemodynamic dependence of index of microcirculatory resistance compared with coronary flow reserve. Circulation. 2006;113(17):2054–2061. doi: 10.1161/CIRCULATIONAHA.105.603522.
- Suwaidi JA, Hamasaki S, Higano ST, et al. Long-term follow-up of patients with mild coronary artery disease and endothelial dysfunction. Circulation. 2000;101(9):948–954. doi: 10.1161/01.cir.101.9.948.
- Ong P, Athanasiadis A, Sechtem U. Patterns of coronary vasomotor responses to intracoronary acetylcholine provocation. Heart. 2013;99(17):1288–1295. doi: 10.1136/heartjnl-2012-302042.
- Johnston N, Schenck-Gustafsson K, Lagerqvist B. Are we using cardiovascular medications and coronary angiography appropriately in men and women with chest pain? Eur Heart J. 2011;32(11):1331–1336. doi: 10.1093/eurheartj/ehr009.
- Luu JM, Wei J, Shufelt CL, et al. Clinical practice variations in the management of ischemia with no obstructive coronary artery disease. J Am Heart Assoc. 2022;11(19):e022573.
- Chow BJW, Small G, Yam Y, et al. Prognostic and therapeutic implications of statin and aspirin therapy in individuals with nonobstructive coronary artery disease. Arterioscler Thromb Vasc Biol. 2015;35(4):981–989. doi: 10.1161/ATVBAHA.114.304351.
- Virani SS, Newby LK, Arnold SV, et al. 2023 AHA/ACC/ACCP/ASPC/NLA/PCNA guideline for the management of patients with chronic coronary disease: a report of the American Heart Association/American College of Cardiology Joint Committee on clinical practice guidelines. Circulation. 2023 Aug 29;148(9):e9-e119. doi: 10.1161/CIR.0000000000001168.
- Dibben G, Faulkner J, Oldridge N, et al. Exercise-based cardiac rehabilitation for coronary heart disease. Cochrane Database Syst Rev. 2021;11(11):CD001800.
- Sandesara PB, Lambert CT, Gordon NF, et al. Cardiac rehabilitation and risk reduction: time to “rebrand and reinvigorate”. J Am Coll Cardiol. 2015;65(4):389–395. doi: 10.1016/j.jacc.2014.10.059.
- Smith SCJr, Benjamin EJ, Bonow RO, et al. AHA/ACCF secondary prevention and risk reduction therapy for patients with coronary and other atherosclerotic vascular disease: 2011 update: a guideline from the American Heart Association and American College of Cardiology Foundation. Circulation. 2011;124(22):2458–2473. doi: 10.1161/CIR.0b013e318235eb4d.
- Szot W, Zając J, Kubinyi A, et al. The effects of cardiac rehabilitation on overall physical capacity and myocardial perfusion in women with microvascular angina. Kardiol Pol. 2016;74(5):431–438. doi: 10.5603/KP.a2015.0198.
- Turgeon RD, Pearson GJ, Graham MM. Pharmacologic treatment of patients with myocardial ischemia with no obstructive coronary artery disease. Am J Cardiol. 2018;121(7):888–895. doi: 10.1016/j.amjcard.2017.12.025.
- Takahashi J, Nihei T, Takagi Y, et al. Prognostic impact of chronic nitrate therapy in patients with vasospastic angina: multicentre registry study of the Japanese coronary spasm association. Eur Heart J. 2015;36(4):228–237. doi: 10.1093/eurheartj/ehu313.
- Bairey Merz CN, Pepine CJ, Shimokawa H, et al. Treatment of coronary microvascular dysfunction. Cardiovasc Res. 2020;116(4):856–870. doi: 10.1093/cvr/cvaa006.
- Pizzi C, Manfrini O, Fontana F, et al. Angiotensin-converting enzyme inhibitors and 3-hydroxy-3-methylglutaryl coenzyme a reductase in cardiac Syndrome X: role of superoxide dismutase activity. Circulation. 2004;109(1):53–58. doi: 10.1161/01.CIR.0000100722.34034.E4.
- Handberg EM, Merz CNB, Cooper-Dehoff RM, et al. Rationale and design of the Women’s Ischemia Trial to Reduce Events in Nonobstructive CAD (WARRIOR) trial. Am Heart J. 2021;237:90–103. doi: 10.1016/j.ahj.2021.03.011.
- Nordenskjold AM, Agewall S, Atar D, et al. Randomized evaluation of beta blocker and ACE-inhibitor/angiotensin receptor blocker treatment in patients with myocardial infarction with non-obstructive coronary arteries (MINOCA-BAT): rationale and design. Am Heart J. 2021;231:96–104. doi: 10.1016/j.ahj.2020.10.059.
- Cannon RO, 3rd, Quyyumi AA, Mincemoyer R, et al. Imipramine in patients with chest pain despite normal coronary angiograms. N Engl J Med. 1994;330(20):1411–1417. 19doi: 10.1056/NEJM199405193302003.
- Shechter M, Matetzky S, Feinberg MS, et al. External counterpulsation therapy improves endothelial function in patients with refractory angina pectoris. J Am Coll Cardiol. 2003;42(12):2090–2095. doi: 10.1016/j.jacc.2003.05.013.
- Gallone G, Baldetti L, Tzanis G, et al. Refractory angina: from pathophysiology to new therapeutic nonpharmacological technologies. JACC Cardiovasc Interv. 2020;13(1):1–19. doi: 10.1016/j.jcin.2019.08.055.