?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Context: Pancreatic α-amylase and α-glucosidase inhibitors serve as important strategies in the management of blood glucose. Even though Syzygium cumini (L.) Skeels (Myrtaceae) (SC) is used extensively to treat diabetes; scientific evidence on antidiabetic effects of SC leaves is scarce.
Objective: SC leaf extract was investigated for α-amylase inhibitory effect and continued with isolation and identification of α-amylase inhibitors.
Materials and methods: Bioassay-guided fractionation was conducted using in vitro α-amylase inhibitory assay (with 20–1000 μg/mL test material) to isolate the inhibitory compounds from ethyl acetate extract of SC leaves. Structures of the isolated inhibitory compounds were elucidated using 1H NMR and 13C NMR spectroscopic analysis and direct TLC and HPLC comparison with authentic samples. Study period was from October 2013 to October 2015.
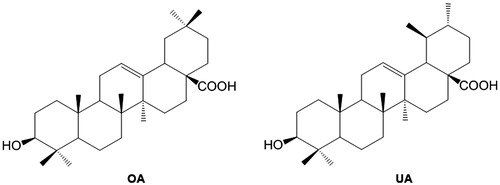
Results: An active fraction obtained with chromatographic separation of the extract inhibited porcine pancreatic α-amylase with an IC50 of 39.9 μg/mL. Furthermore, it showed a strong inhibition on α-glucosidase with an IC50 of 28.2 μg/mL. The active fraction was determined to be a 3:1 mixture of ursolic acid and oleanolic acid. Pure ursolic acid and oleanolic acid showed IC50 values of 6.7 and 57.4 μg/mL, respectively, against α-amylase and 3.1 and 44.1 μg/mL respectively, against α-glucosidase.
Discussion and conclusions: The present study revealed strong α-amylase and α-glucosidase inhibitory effects of ursolic acid and oleanolic acid isolated from SC leaves for the first time validating the use of SC leaves in antidiabetic therapy.
Keywords:
Introduction
Diabetes mellitus is a global health concern with 415 million affected people in 2015. Approximately 75% of diabetic patients live in low and middle income countries and most affected people are in the productive years of their lives (International Diabetes Federation Citation2015). The disease causes premature illness and death due to complications, mainly through the increased risk of cardiovascular diseases. Diabetes has also become a leading cause of blindness, non-traumatic amputation and renal failure. These complications account for much of the social and financial burden of diabetes (World Health Organization and International Diabetes Federation Citation2004). In 2015, five million people died from the complications associated with this disease (IDF Citation2015).
Primary target of management of diabetes is to lower the blood glucose concentration, as hyperglycaemia is the clinical hallmark of diabetes (Sheard et al. Citation2004). Mechanisms reducing blood glucose concentration include lowering of the postprandial blood glucose elevation. Pancreatic α-amylase catalyzes the initial step of starch hydrolysis by breaking the α-1,4-glycosidic link yielding a mixture of maltose and a number of small oligosaccharides (Sales et al. Citation2012). α-Glucosidases present in the intestinal brush border release glucose by hydrolyzing the maltose and oligosaccharides (Kumar et al. Citation2011). Inhibitors of pancreatic α-amylase and α-glucosidase are known to serve as important strategies in the management of blood glucose concentration in diabetic patients as they cause a reduction in the postprandial increase in serum glucose concentration (Alagesan et al. Citation2012a).
Despite the availability of antidiabetic drugs, diabetes and the related complications continue to be a major medical problem. Many of these oral antidiabetic agents have a number of serious adverse effects, thus managing diabetes without side effects is still a challenge. Therefore, the search for more effective and safer drugs continues as an important area of investigation. Currently, there is more interest in plant-based remedies as an affordable and safer mode of therapy in controlling diabetes mellitus. Studies of traditional remedies used around the world have identified more than 1200 species of plants with hypoglycaemic activity (Grover et al. Citation2002) as a hidden wealth of potentially useful natural products for diabetes control. Although many of these remedies are known to be effective for thousands of years, only few have been scientifically validated and investigated for the bioactive compounds despite of the recommendations of World Health Organization (WHO) for further investigation. Natural products with antidiabetic potential isolated from medicinal plants were reported as saponins, flavonoids, alkaloids, anthraquinones, terpenoids, coumarins, phenolics and some polysaccharides (Qi et al. Citation2010). Only a few studies have been reported on the isolation of α-amylase inhibitors from plants (Ali et al. Citation2006; Karthic et al. Citation2008).
Syzygium cumini (L.) Skeels (Myrtaceae) (SC) is a large evergreen tropical tree, widely distributed from historical times in the South Asian countries such as Sri Lanka, India, Bangladesh, Nepal and Pakistan (Ayyanar & Subash-Babu Citation2012). SC (local name: Madan) is also known as Eugenia jambalaya Lam., Myrtus cumini L., Syzygium jambolanum DC. and Eugenia cumini (L.) Druce (Ayyanar & Subash-Babu Citation2012). Widespread use of almost all the parts of SC in traditional medicine reflects its pharmacological value against many diseases. SC is one of the medicinal plants that are recognized as most effective in the treatment of diabetes (Jung et al. Citation2006). There are some studies validating the effectiveness of the bark and seed of SC (see discussion section). However, only limited data are available on the SC leaves even though it is a plant source widely available throughout the year.
The objective of this study was to isolate α-amylase inhibitors from SC leaves using bioassay-guided fractionation and to identify the active compounds.
Materials and methods
General materials
1H and 13C NMR spectra were recorded with a Bruker DRX-500 spectrometer (500 and 125 MHz for 1H and 13C) in CDCl3 containing a drop of CD3OD. 1H chemical shifts are expressed in reference to the internal standard tetramethylsilane (δ 0.00), while 13C chemical shifts are referenced to CDCl3 solvent (δ 77.0). Column chromatography was carried out on silica gel [Merck Art 7734 (70–230 mesh) and 9385 (230–400 mesh)]. Thin-layer chromatography (TLC) and preparative TLC (PTLC) were conducted on aluminium sheets pre-coated with silica gel (Merck 1.05554.0007, 60F254). HPLC was performed on a Shimadzu LC-6A chromatograph with a UV detector. Ursolic acid and oleanolic acid hydrate were purchased from Tokyo Chemical Industry Co., Ltd. and the latter was used for the assay without further drying. Porcine pancreatic α-amylase, p-nitrophenyl α-d-glucopyranoside, α-glucosidase, acarbose and other general reagents were from Sigma.
Plant material
Syzygium cumini leaves were collected from Jaffna District, Sri Lanka in October 2013 and March 2014. Isolation was conducted with the latter. Plant was authenticated by Dr A.M.A.S. Attanayake, Deputy Director, National Herbarium, Royal Botanical Gardens and the voucher specimen (HKIP-JKP-BIO-2013-01) was deposited at the Royal Botanical Gardens, Peradeniya, Sri Lanka. Duration of the study was from October 2013 to October 2015.
Extraction of SC leaves
SC leaves were cleaned and dried under shade for one week. Dried leaves were ground to a powder using a grinder. The leaf powder (1.00 kg) was sequentially extracted with hexane, ethyl acetate, methanol and water (each solvent 2 L × 3). Respective solvents were filtered and evaporated using a rotary evaporator at 45–50 °C to give 25, 47.5, 172 and 95 g of dry extracts. Another batch of the leaf powder (278 g) was directly extracted with ethyl acetate in a similar manner to give 25 g of dry extract. The dried extracts were stored in a refrigerator until use for the inhibitory assays. Parts of the dried extracts were re-suspended in deionized water to the required concentrations for the assays. DMSO was used to solubilize the extracts, if necessary.
Chromatographic fractionation of ethyl acetate extract
Ethyl acetate dry extract (25 g) obtained directly from SC leaf powder was subjected to column chromatography over silica gel (70–230 mesh) with gradient elutions of hexane:ethyl acetate followed by ethyl acetate:methanol. Fractions were collected at uniform intervals and the progress of separation was monitored by TLC. Compounds were visualized by employing a UV lamp (254 and 360 nm) and using p-anisaldehyde reagent. Column fractions were combined based on the TLC pattern and the solvents were evaporated using a rotary evaporator. Combined fractions were analyzed for α-amylase inhibitory activity, which indicated the fraction eluted from 1:1 to 0:1 hexane:ethyl acetate was found to be most active. The active fraction was chromatographed again over silica gel (70–230 mesh) with gradient elutions of hexane:dichloromethane followed by dichloromethane:methanol. Fraction collected from 1:3 hexane:dichloromethane to 19:1 dichloromethane:methanol was found to be most active. This active fraction was further chromatographed over silica gel (230–400 mesh) with gradient elutions of hexane:dichloromethane followed by dichloromethane-methanol. The fraction eluted with dichloromethane:methanol (19:1 to 17:3) was further purified by PTLC (developed with 3% methanol:chloroform in a chamber saturated with NH3) to give the active fraction (28 mg) as a white solid.
Structure elucidation
Active compound was identified by detailed analysis of 1H NMR and 13C NMR spectroscopic data and the direct comparison with TLC.
Measurement of α-amylase inhibitory effects
Percentage α-amylase inhibitions of the test samples were determined according to the published method (Geethalakshmi et al. Citation2010). Briefly, porcine pancreatic α-amylase was dissolved in ice-cold distilled water (5 unit/mL solution). Potato starch (1% w/v) in 20 mM phosphate buffer (pH 6.9) containing 6.7 mM sodium chloride was used as the substrate solution. Plant extract or the fraction (40 μL) was mixed with 80 μL of 20 mM phosphate-buffered saline (pH 6.9) and with 40 μL α-amylase. Eppendorf tubes with the reaction mixtures were pre-incubated for 15 min at 37 °C and then 1% potato starch (40 μL) was added to the tubes. Final concentrations used for the extract or the fraction included 20–1000 μg/mL. Final concentrations used for ursolic acid and oleanolic acid were 2–50 μg/mL and 20–80 μg/mL, respectively. Control was carried out in the absence of plant extract or standard inhibitor. Test blanks were conducted in the presence of plant extracts without α-amylase. A blank reaction was carried out with 40 μL of the respective solvent replacing the plant extract. Acarbose (Sigma) was used as the standard inhibitor. Reaction mixtures were incubated for 3 min at 37 °C. Dinitrosalicylic acid colour reagent (96 mM 3,5-dinitrosalicylic acid, 5.31 M sodium potassium tartarate in 2 M NaOH) was added (100 μL) to all the tubes and kept immediately in a water bath at 85 °C for 15 min. Distilled water (900 μL) was added to each tube and the absorbance was measured at 540 nm.
Measurement of α-glucosidase inhibitory effects
Inhibitory effects of the fraction isolated from PTLC (pure fraction), UA and OA against α-glucosidase were determined using the published method (Elya et al. Citation2012). Briefly, 200 μL of 67 mM sodium phosphate buffer (pH 6.8) and 120 μL of 10 mM p-nitrophenyl α-D-glucopyranoside (Sigma) were added to the Eppendorf tubes. Isolated pure fraction (40 μL) containing final concentration of 10–60 μg/mL was added to the test and test blank. Final concentrations used for ursolic acid and oleanolic acid were 2–32 μg/mL and 20–80 μg/mL, respectively. Mixtures were pre-incubated for 15 min at 37 °C. Thereafter, 40 μL of α-glucosidase (0.1 U) from Saccharomyces cerevisiae (Sigma) was added to the tests and control. Reaction mixtures were incubated for 15 min at 37 °C and terminated by adding 200 mM sodium carbonate (800 μL). The hydrolysis of α-d-glucopyranoside to p-nitrophenol was measured at 405 nm.
Calculation of percentage inhibition of enzyme activities and IC50
Percentage inhibition was calculated using the following formula (Olaokun et al. Citation2013).
The concentration of the extract or the fraction or the compound that inhibits 50% of the enzyme activity (IC50) was calculated using a series of suitable concentrations. IC50 values were determined by plotting per cent inhibition (Y axis) versus log10 extract concentration (X axis) and calculated by logarithmic regression analysis from the mean inhibitory values (Sudha et al. Citation2011).
Statistical analysis
Enzyme inhibitory assays were carried out on three separate occasions. Each measurement was taken in triplicates. Data are expressed as mean ± standard deviation. Statistical analysis was performed using ANOVA to compare the means of different fractions. Values of p < 0.05 were considered as significantly different.
Results
Each extract of dried leaves of the S. cumini was subjected to porcine pancreatic α-amylase inhibitory bioassay. Methanol and water extracts showed the highest α-amylase inhibitory activity (98.3 ± 2.3 and 98.6 ± 1.6%, respectively, with 1 mg/mL extracts). Ethyl acetate extract obtained directly from SC leaf powder showed 72.6 ± 3.75% inhibitory activity while no inhibitory activity was observed with hexane fraction (). Significantly higher inhibitory effects (p < 0.0005) on amylase were recovered from methanol and water extracts of SC leaves compared to that of ethyl acetate extract according to the effects observed with 200 μg/mL extracts (). Preliminary attempts to obtain active substances from methanol and water was not successful due to the very complex nature of these extracts, and the purification of these extracts remains as the subject of further study. Thus, in the present study the ethyl acetate extract obtained directly from SC leaf powder was subjected to bioassay-guided fractionation. The fraction eluted between 5 and 15% methanol to dichloromethane at the final step of silica gel column chromatography was found to be active against α-amylase, showing 97% inhibition at 200 μg/mL (). TLC analysis of the fraction indicated that the fraction consists of UV-absorbing and UV-non absorbing compounds (). The mixture was successfully separated by PTLC developed with 3% methanol:chloroform in a TLC chamber saturated with NH3 () and the UV-nonabsorbing compound was isolated. Isolated fraction showed a single peak with HPLC. The pure fraction isolated showed 99.6% inhibition on α-amylase at 100 μg. Inhibition % demonstrated with different concentrations (20–100 μg/mL) of pure fraction on amylase are shown in . There was a significant enhancement of the inhibitory effects on amylase with the progress of purification (p < 0.0005) with a decrease in IC50 from 704 to 39.9 μg/mL (). The bioassay-guided fractionation of the ethyl acetate extract indicated that the UV-nonabsorbing pure fraction was responsible for the α-amylase inhibitory activity. In addition to the expected inhibition on amylase, pure fraction has also showed a strong inhibition on α-glucosidase with an IC50 of 28.2 μg/mL. Inhibition % demonstrated with different concentrations of pure fraction (10–60 mg/mL) on α-glucosidase are shown in and the inhibition demonstrated was significantly higher than the inhibitory effects on α-amylase (p < 0.01). These findings suggest two modes for the possible hypoglycaemic effects of the pure fraction isolated from SC leaves.
Figure 1. TLC of the active fractions before and after final purification. (A) Prior to the final purification, (B) After the final purification with PTLC Solvent mixture: 5% MeOH: Chloroform.
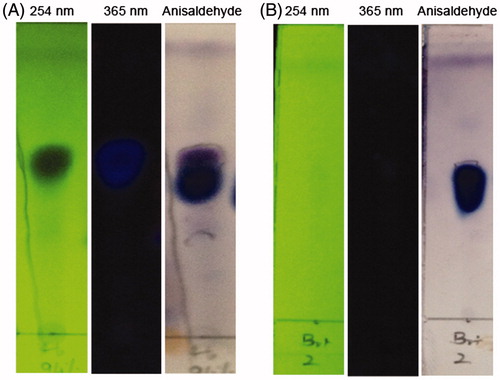
Table 1. Porcine pancreatic α-amylase inhibitory activity of SC fractions.
Table 2. Percentage inhibition of α-amylase and α-glucosidase by pure fraction.
Active compound was identified by detailed analysis of 1H NMR and 13C NMR spectroscopic data and the direct comparison with TLC. The 1H NMR spectrum (recorded in CDCl3 containing a drop of CD3OD) of the active fraction showed signals of an oxymethine proton at δ 3.21 (dd, J = 10.9, 4.7 Hz) assignable to 3-proton of typical pentacyclic triterpenes and olefinic protons at δ 5.25 and 5.28 (brt each, J = 3.0 Hz) in a ratio of 3:1, characteristic of H-12 of ursolic acid and oleanolic acid, respectively. In addition, proton signals assignable to H-18 of ursolic acid and oleanolic acid were discerned at δ 2.19 (d, J = 11.4 Hz) δ 2.83 (dd, J = 13.2, 3.6 Hz) in a 3:1 ratio (Gohari et al. Citation2009; Kolak et al. Citation2009). The 13C NMR spectrum exhibited signals for an oxymethine carbon at δ 78.9 assignable to C-3 of pentacyclic triterpenes, olefinic carbons at 125.6 and 138.1, and C-28 carboxyl carbon at δ 170.5 for ursolic acid (Gohari et al. Citation2009; Kolak et al. Citation2009). TLC comparison (solvent: hexane:ethyl acetate 2:1) of the sample with authentic ursolic acid and oleanolic acid indicated the identity of the material. Finally, the sample was analyzed by reversed-phase HPLC in comparison with authentic acids (column, GL Sciences Inc. Inertsil ODS-3 (4.6 mm (i.d.) × 150 mm); solvent, methanol:water 10:1 containing 0.1% trifluoroacetic acid; flow rate 0.5 mL/min; detected at 215 nm). The sample exhibited two peaks at 16.5 and 17.2 min in a 3:1 ratio, which corresponded to the retention times of authentic ursolic acid (UA) and oleanolic acid (OA), respectively. Structures of UA and OA are shown in . According to up to date literature, there has been no previous reports on identification of amylase inhibitors from SC leaf. When the activity of commercially available UA and hydrated form of OA was investigated, UA showed significantly higher inhibitory effects on both α-amylase and α-glucosidase compared to that of OA (p < 0.0005). UA and OA showed IC50 of 6.7 and 57.4 μg/mL, respectively, against α-amylase and 3.1 and 44.1 μg/mL respectively, against α-glucosidase. Inhibition % with different concentrations of UA (2–50 μg/mL) and OA (20–80 μg/mL) on α-amylase and α-glucosidase are shown in and , respectively. Inhibitory effects of UA and OA on amylase and glucosidase isolated from various other sources have been shown previously although the IC50 values were varying from report to report (Komaki et al. Citation2003; Ali et al. Citation2006; Tiwari et al. Citation2010).
Table 3. Percentage inhibition of α-amylase and α-glucosidase by ursolic acid.
Table 4. Percentage inhibition of α-amylase and α-glucosidase by oleanolic acid.
Discussion
Bark, fruits, seeds and leaves of S. cumini are frequently used to treat diabetes in many parts of the world including Sri Lanka for centuries (Schoenfelder et al. Citation2010; Ediriweera & Ratnasooriya Citation2009). However, scientific data are lacking on the antidiabetic effects of SC leaves. Therefore, SC leaf extracts were investigated for α-amylase inhibitory effects and bioassay-guided fractionation was carried out in search of α-amylase inhibitors. Fractionation of the ethyl acetate extract afforded a UV-nonabsorbing fraction with a strong α-amylase inhibitory activity (IC50 39.9 μg/mL). Even though the current study was not aimed to identify α-glucosidase inhibitors, the isolated fraction showed a strong inhibition on α-glucosidase too with an IC50 of 28.2 μg/mL. The active fraction was determined to be a 3:1 mixture of ursolic acid (UA) and oleanolic acid (OA).
Several studies have revealed the antidiabetic effects of SC bark and seeds in streptozotocin or alloxan induced diabetic rats. SC bark was shown to improve the oral glucose tolerance, elevate plasma insulin and stimulate pancreatic β-cell regeneration when an aqueous extract was given (Schossler et al. Citation2004; Saravanan & Leelavinothan Citation2006; Saravanan & Pari Citation2008). Oral administration of ethyl acetate and methanol extracts of SC seeds has reduced blood glucose concentration in diabetic rats (Kumar et al. Citation2008). SC seed extract also has shown inhibitory effects against α-amylase (Karthic et al. Citation2008) and α-glucosidase (Shinde et al. Citation2008).
There are contradictory findings with regard to the effectiveness of SC leaves as an antidiabetic agent. Essential oil from SC leaves inhibited α-amylase in vitro (Nishandhini et al. Citation2014). Chloroform extract was also found to inhibit α-amylase in vitro while no inhibition was revealed with methanol and water extracts (Bhat et al. Citation2011). In contrast to this observation, α-amylase inhibitory activities detected in methanol and water extracts in the present study were significantly high (p < 0.0005). Findings of some other investigations too suggest antidiabetic effects of methanol, ethanol and water extracts. When the extracts of SC leaves, root, seeds and bark were administered to alloxan-induced diabetic rats the maximum antidiabetic efficacies were observed with methanol and aqueous extracts of leaves (Deb et al. Citation2013). Ethanol extract of SC leaves was found to cause a decrease in the blood glucose, triglycerides and cholesterol concentrations when given to alloxan-induced diabetic rats (Schoenfelder et al. Citation2010). In another study, ethanol extract of SC leaves exhibited α-glucosidase inhibitory activity with an IC50 of 17.4 μg/mL (Saraswaty Citation2012). However, some investigations did not reveal antidiabetic effects of SC leaves. For instance, no antihyperglycaemic effects were observed upon administration of tea prepared from SC leaves to streptozotocin-induced diabetic rats for 2 weeks (Teixeira et al. Citation2000) and to patients with type 2 diabetes for 4 weeks (Teixeira et al. Citation2006). Similar negative outcome was reported when butanol fraction of leaves was given to diabetic mice (Oliveira et al. Citation2005).
UA and OA are two isomeric pentacyclic triterpenoids, which are found in plants (Xu et al. Citation2012). Triterpenoids are reported to be endowed with many pharmacological effects including antidiabetic effects (Castellano et al. Citation2013). Multiple beneficial actions of UA and OA against diabetes are documented. These effects include preservation of pancreatic β-cells, improvement of insulin response (Castellano et al. Citation2013), decrease in glucose 6-phosphatase activity, increase in glucokinase activity, glucose transporter 2 mRNA levels and liver glycogen (Lee et al. Citation2014), stimulation of glucose uptake (He et al. Citation2014), normalization of polyol pathway (Lee et al. Citation2014) and superoxide anion scavenging activity, chelating effect, reducing power and inhibitory effects on glycation (Yin & Chan Citation2007). Therapeutic effectiveness with no apparent side effects also has been reported with OA (Castellano et al. Citation2013).
According to up to date literature, only one study has previously reported the identification of amylase inhibitors from SC. Betulinic acid and 3,5,7,4′-tetrahydroxyflavanone isolated from aqueous extract of SC seeds were found to be strong inhibitors of α-amylase (Karthic et al. Citation2008). Even though UA was previously isolated from chloroform extract (Boonruad & Chansuwanich Citation2012) and dichloromethane extracts (Ragasa et al. Citation2014) of SC leaves its antidiabetic effects have not been reported. One study has documented identification of an α-glucosidase inhibitor (a flavonoid apigenin 7-O-glucoside) from SC seeds (Alagesan et al. Citation2012b).
The present study revealed the inhibitory activities of UA and OA isolated from SC leaves on α-amylase and α-glucosidase. A 2:1 mixture of OA and UA isolated from the hexane extract of Phyllanthus amarus Schum & Th. has exhibited a potent amylase inhibitory activity with IC50 of 2 μg/mL (Ali et al. Citation2006). In another study, OA was found to inhibit α-amylase with an IC50 of 100 μg/mL (Komaki et al. Citation2003). OA has been isolated from chloroform fraction of Allium sativum L. leaves (IC50 83.6 μg/mL) (Meshram & Khamkar Citation2014). OA and UA isolated from the dichloromethane and methanol extracts of Callistemon lanceolatus (Sm.) at 50 μg/kg also showed α-amylase inhibition (62.5 and 55.0%, respectively) (Kumar et al. Citation2013). Furthermore, α-glucosidase inhibitory effects were found in OA isolated from fruits of Sonneratia caseolaris L. (IC50 15.0 μM) (Tiwari et al. Citation2010), OA and UA isolated from the dichloromethane and methanol extracts of Callistemon lanceolatus (84.2, 68.0%, respectively) at 50 μg/kg (Kumar et al. Citation2013), UA and OA isolated from ethyl acetate extract of Osmanthus fragrans Lour. (IC50 3.4 and 3.3 μg/mL, respectively) (Kang et al. Citation2012) and UA and OA isolated from flowers of Punica granatum L. (IC50 39.0 and 35.0 μM, respectively) (Salah El Dine et al. Citation2014). In the present study UA and OA were recovered together during purification. It is reported that the separation of UA and OA is difficult as their chromatographic properties are very similar (Xu et al. Citation2012).
Findings of the present study provide evidence for the effects of SC leaves in bringing down postprandial blood glucose concentrations. Reported antidiabetic effects of UA and OA isolated from other plants (Yin & Chan Citation2007; Castellano et al. Citation2013; He et al. Citation2014; Lee et al. Citation2014) suggest that there may be number of other antidiabetic effects too in UA and OA isolated from SC.
Conclusions
This study demonstrated strong α-amylase inhibitory effects in ethyl acetate, methanol and water fractions of SC leaves. Two α-amylase inhibitors were identified through bioassay-guided fractionation of the ethyl acetate fraction. Final active fraction exhibited a porcine α-amylase inhibitory activity with an IC50 of 39.9 μg/mL and the content of the fraction was determined as a mixture of UA and OA (3:1) in a detail NMR analysis. The present study reports strong α-amylase and α-glucosidase inhibitory effects of UA and OA isolated from the SC leaves and validates the use of SC leaves in antidiabetic therapy. Furthermore, this study suggested the presence of other active compounds in the methanol and water fractions. As the fact indicates SC leaves are plausible for diabetic treatment, further studies to determine active compounds from methanol and water fractions are in progress.
Disclosure statement
The authors report no declarations of interest.
References
- Alagesan K, Raghupathi PK, Sankarnarayanan S. 2012a. Amylase inhibitors: potential source of anti-diabetic drug discovery from medicinal plants. Int J Pharm Life Sci. 3:1407–1412.
- Alagesan K, Thennarasu P, Kumar V, Sankarnarayanan S, Balsamy T. 2012b. Identification of α-glucosidase inhibitors from Psidium guajava leaves and Syzygium cumini Linn. seeds. Int J Pharm Sci Res. 3:316–322.
- Ali H, Houghton PJ, Soumyanath A. 2006 . alpha-Amylase inhibitory activity of some Malaysian plants used to treat diabetes; with particular reference to Phyllanthus amarus. J Ethnopharmacol. 107:449–455.
- Ayyanar M, Subash-Babu P. 2012. Syzygium cumini (L.) Skeels: a review of its phytochemical constituents and traditional uses. Asian Pac J Trop Biomed. 2:240–246.
- Bhat M, Zinjarde SS, Bhargava SY, Kumar AR, Joshi BN. 2011. Antidiabetic Indian plants: a good source of potent amylase inhibitors. Evid Based Complement Alternat Med. 2011:1–6.
- Boonruad T, Chansuwanich N. 2012. Chemical constituents of antibacterial active fraction from Syzygium cumini. (L.) Skeels leaves extract. Bull Dep Med Sci. 42:109–118.
- Castellano JM, Guinda A, Delgado T, Rada M, Cayuela JA. 2013. Biochemical basis of the antidiabetic activity of oleanolic acid and related pentacyclic triterpenes. Diabetes. 62:1791–1799.
- Deb L, Bhattacharjee C, Shetty R, Dutta A. 2013. Evaluation of anti-diabetic potential of the Syzygium cumini (Linn) Skeels by reverse pharmacological approaches. Bull Pharm Res. 3:135–145.
- Ediriweera ERHSS, Ratnasooriya WD. 2009. A review on herbs used in treatment of diabetes mellitus by Sri Lankan ayurvedic and traditional physicians. Ayu. 30:373–391.
- Elya B, Basah K, Munim A, Yuliastuti W, Bangun A, Septiana EK. 2012. Screening of α-glucosidase inhibitory activity from some plants of Apocynaceae, Clusiaceae, Euphorbiaceae, and Rubiaceae. J Biomed Biotechnol. 2012:1–6.
- Geethalakshmi R, Sarada DVL, Marimuthu P, Ramasamy K. 2010. α-Amylase inhibitory activity of Trianthema decandra L. Int J Biochem Biotechnol. 6:369–376.
- Gohari A, Saeidnia S, Hadjiakhoondi A, Abdoullahi M, Nezafati M. 2009. Isolation and quantificative analysis of oleanolic acid from Satureja mutica. Fisch. & C.A. Mey. J Med Plants. 5:65–69.
- Grover JK, Yadav S, Vitas V. 2002 . Medicinal plants of India with anti-diabetic potential. J Ethnopharmacol. 81:81–100.
- He Y, Li W, Li Y, Zhang S, Wang Y, Sun C. 2014. Ursolic acid increases glucose uptake through the PI3K signaling pathway in adipocytes. PLoS One. 9:1–8.
- International Diabetes Federation (IDF). 2015. IDF diabetes atlas [Internet]. 7th ed. Brussels, Belgium: International Diabetes Federation. Available from: http://www.diabetesatlas.org/.
- Jung M, Park M, Lee CH, Kang Y, Kang ES, Kim SK. 2006. Antidiabetic agents from medicinal plants. Curr Med Chem. 13:1203–1218.
- Kang W, Song Y, Gu X. 2012. α-glucosidase inhibitory in vitro and antidiabetic activity in vivo of Osmanthus fragrans. J Med Plants Res. 6:2850–2856.
- Karthic K, Kirthiram KS, Sadasivam S, Thayumanavan B, Palvannan T. 2008 . Identification of alpha amylase inhibitors from Syzygium cumini Linn seeds . Indian J Exp Biol. 46:677–680.
- Kolak U, Hacibekiroglu I, Ozturk M, Ozgokce F, Topcu G, Ulubelen A. 2009. Antioxidant anticholinesterase constituents of Salvia poculata. Turkish J Chem. 33:813–823.
- Komaki E, Yamaguchi S, Maru I, Kinoshita M, Kakehi K, Ohta Y, Tsukada Y. 2003. Identification of anti-alpha-amylase components from olive leaf extracts. Food Sci Technol Res. 9:35–39.
- Kumar A, Ilavarasan R, Jayachandran T, Deecaraman M, Aravindan P, Padmanabhan N, Krishan MRV. 2008. Anti-diabetic activity of Syzygium cumini and its isolated compound against streptozotocin-induced diabetic rats. J Med Plants Res. 2:246–249.
- Kumar S, Kumar V, Prakash O, Ali M. 2013. Enzymes inhibition and antidiabetic effect of isolated constituents from Callistemon lanceoalatus. J Nat Prod. 3:252–259.
- Kumar S, Narwal S, Kumar V, Prakash O. 2011. α-Glucosidase inhibitors from plants: a natural approach to treat diabetes. Pharmacogn Rev. 5:19–29.
- Lee J, Lee HI, Seo KI, Cho HW, Kim MJ, Park EM, Lee MK. 2014. Effects of ursolic acid on glucose metabolism, the polyol pathway and dyslipidemia in non-obese type 2 diabetic mice. Indian J Exp Biol. 52:683–691.
- Meshram GA, Khamkar SS. 2014. Effect of oleanolic acid isolated from garlic leaves on carbohydrate metabolizing enzymes, in vitro. Int J Pharm Sci Res. 5:988–991.
- Nishandhini S, Sudha V, Mallavarapu GR, Murugan R. 2014. Chemical compositions, α-amylase inhibitory and antioxidant activities of the essential oils from unripe fruit pulp and leaves. Int J Pharm Pharm Sci. 7:511–514.
- Olaokun OO, McGaw LJ, Eloff JN, Naidoo V. 2013. Evaluation of the inhibition of carbohydrate hydrolysing enzymes, antioxidant activity and polyphenolic content of extracts of ten African Ficus species (Moraceae) used traditionally to treat diabetes. BMC Complement Altern Med. 13:94–103.
- Oliveira ACP, Endringer DC, Amorim LAS, Brandão MGL, Coelho MM. 2005. Effect of the extracts and fractions of Baccharis trimera and Syzygium cumini on glycaemia of diabetic and non-diabetic mice. J Ethnopharmacol. 102:465–469.
- Qi LW, Liu EH, Chu C, Peng YB, Cai HX, Li P. 2010. Anti-diabetic agents from natural products-an update from 2004 to 2009. Curr Top Med Chem. 10:434–457.
- Ragasa CY, Torres OB, Shen CC, Lachica MKEG, Sulit AB, Chua DBDL, Ancheta ADM, Ismail CJB, Bernaldez FTE, Raga DD. 2014. Triterpenes from the leaves of Syzygium polycephalum, S. cumini and S. samarangense. Chem Nat Compd. 50:942–944.
- Salah El Dine R, Ma Q, Kandil ZA, El-Halawany AM. 2014. Triterpenes as uncompetitive inhibitors of α-glucosidase from flowers of Punica granatum L. Nat Prod Res. 28:2191–2194.
- Sales PM, Souza PM, Simeoni LA, Magalhães PO, Silveira D. 2012. α-Amylase inhibitors: a review of raw material and isolated compounds from plant source. J Pharm Pharm Sci. 15:141–183.
- Saraswaty V. 2012. Alpha glucosidase inhibitory activity from Syzigium sp. J Teknol Indon. 33:33–37.
- Saravanan G, Leelavinothan P. 2006. Effects of Syzygium cumini bark on blood glucose, plasma insulin and C-peptide in streptozotocin induced diabetic rats. Int J Endocrinol Metab. 4:93–105.
- Saravanan G, Pari L. 2008. Hypoglycaemic and antihyperglycaemic effect of Syzygium cumini bark in streptozotocin-induced diabetic rats. J Pharm Toxic. 3:1–10.
- Schoenfelder T, Warmlin CZ, Manfredini MS, Pavei LL, Réus JV, Tristão TC, Fernandes MS, Costa-Campos L. 2010. Hypoglycemic and hypolipidemic effect of leaves from Syzygium cumini (L.) Skeels, Myrtaceae in diabetic rats. Rev Bras Farmacogn. 20:222–227.
- Schossler DRC, Mazzanti CM, Luz SCAD, Filappi A, Prestes D, Silveira AFD, Cecim M. 2004. Syzygium cumini and the regeneration of insulin positive cells from the pancreatic duct. Braz J Vet Res Anim Sci. 41:236–239.
- Sheard NF, Clark NG, Brand-Miller JC, Franz MJ, Pi-Sunyer FX, Mayer-Davis E, Kulkarni K, Geil P. 2004 . Dietary carbohydrate (amount and type) in the prevention and management of diabetes: a statement by the american diabetes association. Diabetes Care. 27:2266–2271.
- Shinde J, Taldone T, Barletta M, Kunaparaju N, Hu B, Kumar S, Placido J, Zito SW. 2008. α-Glucosidase inhibitory activity of Syzygium cumini (Linn.) Skeels seed kernel in vitro and in Goto–Kakizaki (GK) rats. Carbohydr Res. 343:1278–1281.
- Sudha P, Zinjarde SS, Bhargava SY, Kumar AR. 2011. Potent α-amylase inhibitory activity of Indian Ayurvedic medicinal plants. BMC Complement Altern Med. 11:5–10.
- Teixeira CC, Fuchs FD, Weinert LS, Esteves J. 2006. The efficacy of folk medicines in the management of type 2 diabetes mellitus: results of a randomized controlled trial of Syzygium cumini (L.) Skeels. J Clin Pharm Ther. 31:1–5.
- Teixeira CC, Rava CA, Silva PM, Melchior R, Argenta R, Anselmi F, Almeida CRC, Fuchs FD. 2000. Absence of antihyperglycemic effect of jambolan in experimental and clinical models. J Ethnopharmacol. 71:343–347.
- Tiwari AK, Viswanadh V, Gowri PM, Ali AZ, Radhakrishnan SV, Agawane SB, Madhusudana K, Rao JM. 2010. Oleanolic acid & alpha-glucosidase inhibitory and antihyperglycemic active compound from the fruits of Sonneratia caseolaris. Open Access J Med Aromat Plants. 1:19–23.
- World Health Organization and International Diabetes Federation (WHO & IDF). 2004. Diabetes action now. An initiative of the World Health Organization and International Federation of Diabetes [Internet]. Available from: http://apps.who.int/iris/bitstream/10665/42934/1/924159151X.pdf.
- Xu XH, Su Q, Zang ZH. 2012. Simultaneous determination of oleanolic acid and ursolic acid by RP-HPLC in the leaves of Eriobotrya japonica Lindl. J Pharm Biomed Anal. 2:238–240.
- Yin MC, Chan KC. 2007. Nonenzymatic antioxidative and antiglycative effects of oleanolic acid and ursolic acid. J Agric Food Chem. 55:7177–7181.