Abstract
This paper describes and explores how translational research models, embedded in institutions and standards, interact with the epistemic and material practices of cell biologists of ageing, a field re-energized by emergent technoscientific promises that hinge on the possibility of eliminating or manipulating senescent cells to tackle age-related diseases. Drawing on a 3-year long lab ethnography, the paper suggests that knowledge making in cell biology of ageing relies on two different epistemic and material cultures, to then argue that these cultures combine in four different types of experimental systems, only one of which can properly be seen as pertaining to translation as usually conceived. The paper further analyses how cell biologists articulate the linear temporality of translational research with the unfolding experimental chains where, by shifting between types of experimental system, cell biologists are able to generatively reconfigure their epistemic objects, and the consequences of this fragile arrangements for the field.
Wednesdays AM Lab Meeting Roundtable: Peter asked to go first as he had just received the reviews on the collaborative grant the Tread Lab and his own group had submitted a few weeks ago to a UK major research charity. He had prepared a presentation, recalling the aims of the original proposal, the reviewers’ main points and objections, and his proposed response to the reviewers. He then summarised what he saw as the main issue coming from the reviews: that the proposal was focused on understanding fundamental mechanisms of senescence in cardio-vascular disease, which, in his view, were still poorly understood, while the reviewers were asking for a “translational” focus.
“It is difficult”, Peter sighed. There was general, if silent, agreement around the table about this conclusion. He added more context to their predicament: that the research charity had recently appointed a new director, who had publicly committed to funding more “high impact, translational” grants, with clear, direct benefit for the patient.
Oscar, the Tread Lab PI, condemned the director’s wide-ranging aim of funding translational research, because, he said, it is inadequate for situations where the “basic biology” is not well understood. He argued further that the strength of their proposal was to explore not only important processes in cardio-vascular illness but to also use specific disease mechanisms as a model to understand senescent responses more widely. He added that understanding the biological functions of senescence was a key problem in contemporary biology.
“I don’t know how much [the director] cares about the biology”, Peter replied. Oscar smiled, conceding the point. (FN_ TreadLab 10/11/17_b)
This extract from my field-notes succinctly describes a tension between “biology” and “translational research,” which I have observed many times during my fieldwork in the Tread Lab. The Tread Lab’s main focus was on exploring the role of mitochondria and telomeres in cell senescence but was increasingly involved in researching how these processes impacted on age-associated diseases in collaborations with other labs such as Peter’s, whose work concerned specifically cell-based therapies in cardio-vascular disease. As the extract recounts, the continued collaboration between the labs hinged on obtaining funds from a medical research charity which prioritized “high impact, translational” grants. In the meeting, they agreed that, pragmatically, in order to get the project funded, it was necessary to emphasize the possible, more immediate benefits the research might yield for patients, and to downplay the distal, longer term, wider implications of their experiments for the biology of senescence. This meant not only that the relationship between the labs would have to be recast during the project, Peter’s assuming a more prominent role, but also that resources would have to shift from more exploratory experimental work to the focused development of clinical applications.
In this paper, my point of departure is that the predicament deployed at the Tread Lab’s Wednesday morning roundtable meeting in 2017 partakes in an outspreading transformation of biology of ageing where “translational research” models increasingly shape scientific practices in that field. This transformation, it is argued, is necessary to bring to bear new developments in the field of cell biology of ageing that revive the possibility of extending human “health span” (e.g. Deursen Citation2019; Campisi et al. Citation2019). Such new developments concern the identification and evaluation of senolytic drugs. These, according to James Kirkland and colleagues “are agents that selectively induce apoptosis [cell death] of senescent cells” (Kirkland et al. Citation2017, 2297). Cell senescence, in turn, is defined as a state of cell arrest – i.e. irreversible loss of division potential in somatic cells – that arises as a response to diverse stimuli such as development, wounds, DNA damage or oncogene activation (e.g. Herranz and Gil Citation2018). Senescent cells are believed to accumulate in many tissues with ageing and at sites of pathology in multiple chronic diseases, with a variety of deleterious effects (Deursen Citation2014), making their elimination an ideal strategy to manage age-associated diseases such as cardiovascular disease, arthritis or Alzheimer's.
Publicized in reports in newspapers like the Guardian (Fleming Citation2019), New York Times (Bakalar Citation2018), or magazines like the Wire in the last few years, these new developments have led to a regulatory reframing of the premises of biomedical research of ageing. In this, for example, the FDA has shifted its requirement that clinical trials be tied to some recognized clinical or nosological entity – a disease – to allow an evaluation of the effectiveness of metformin – a drug already approved for the treatment of diabetes – on the risk of onset of a variety of age-associated illness (Hayden Citation2015). The hope is that this reframing will facilitate the implementation of translational research models in ageing research, whose work had been hindered by their own reluctance to configure ageing as a disease (e.g. Miller Citation2002)
Translational research models are often portrayed in the form of a pipeline, guiding techniques and applications across the laboratory towards clinical practice or the market, resulting in the implementation of a technique or therapy with clear, measurable patient benefit, currently exemplified by the MRC Translational Pathway (See ). Another model, embodied in the US National Institute of Health translational framework (2004–2014), is that of the roadmap, marking specific pathways back and forth between forms of research and clinical practice. One key, common feature of these models is the emphasis on the forward direction of knowledge making and technological development, transferring “basic” scientific knowledge from “bench to bedside” by testing its validity in clinical practice, moving technologies from the laboratory to clinic or market. These models occasionally depict the journey of molecules, techniques and applications as one that is not without setbacks, revisions or even failure, making it all the more important that potential technologies are not diverted from the path of translation, if we are to maximize the return, i.e. the benefit in health and functionality yielded from the financial and human capital investment in research.
Figure 1. Translational pathway. MRC translational funding program [https://mrc.ukri.org/funding/science-areas/translation/about-our-translational-research/].
![Figure 1. Translational pathway. MRC translational funding program [https://mrc.ukri.org/funding/science-areas/translation/about-our-translational-research/].](/cms/asset/c62fbb9f-74df-4885-ae2c-f410c2151e37/cngs_a_1825932_f0001_oc.jpg)
In this paper, my concern is not whether these models are valid representations of innovation in the biomedical sciences (e.g. Vos Citation1991), but instead to understand how, as formalized representations, stabilized in institutions and material forms, they interact, shape and interfere with the epistemic and material practices of cell biologists of ageing. In this regard, the paper combines two main strands of social science research on translational research: work on the role of technological expectations in structuring translational networks and practices (e.g. Gardner and Webster Citation2017; Addison Citation2017; Brosnan and Michael Citation2014; Mittra Citation2013), and those problematizing and reconceptualizing translation processes in biomedicine (e.g. Keating and Cambrosio Citation2013; Lewis, Hughes, and Atkinson Citation2014).
Drawing on ethnographic fieldwork, I first describe how knowledge making in cell biology of ageing relies on two different epistemic and material cultures – two different ways of making senescence: visualization and quantification. I argue that the focus of cell biologists’ work on “mechanisms,” “biomarkers” or “biomedical translation” is related to how uncertainty is distributed across cell biology’s two machineries of knowledge production. This analysis suggests thus that the ability to work on translational applications is a contingent, difficult to achieve and rare outcome of the alignment between two epistemic cultures on which cell biology of ageing relies. In the following section (Experiments and their futures), I explore how biologist of ageing build unfolding experimental chains that, by shifting between types of experimental system, generatively reconfigure their epistemic objects. I argue that the institutionally embedded, spacialized temporality of translational pathways models – the way in which it deploys linear enactments of the future – is in tension with this complex, open choreography of practice through which cell biologists pursue their inquiries. Finally, I describe how cell biologists attempt to nest the latter form of practice within translational, biomedical projects, and the reasons why these arrangements are inherently fragile.
Methodology
This paper draws on data collected through ethnographic fieldwork. The fieldwork followed the procedures and orientation of laboratory studies, an approach to the study of science established by Latour, Lynch and Knorr Cetina, amongst others, between the late 1970s and the 1990s. The underlying assumption of laboratory studies was that the establishment of scientific facts and theories was a practical achievement, best understood through close observation and analysis of the routine working order of setting up, conducting and interpreting experiments. More recently, the assumptions of laboratory studies about the central place of the laboratory in the scientific enterprise have been criticized, because the approach reinforces, rather than challenges, this position, favoring particular locations within complex technoscientific networks, and their associated ways of knowing (Doing Citation2008; Ziewitz and Lynch, Citation2018). Taking these into account, the study focused on how scientists navigated and composed diverse ways of knowing and modes of coordination to enact their everyday experimental practices.
The fieldwork took place between February 2015 and December 2018 in a laboratory focusing on the role of telomeres and mitochondria in ageing and senescence. The Tread Lab has two PIs. Usually, depending on grant money, there are 2 or 3 postdocs, about 5 PhDs, and the cyclical presence of MA and undergraduate project students. It is part of a wider research center, with offices for the PIs and two shared, bigger offices for PhDs and PDs, and one main lab with 8 benches. The lab shares facilities such as tissues culture rooms, freezers and microscopes, and technicians with three other labs focused on other aspects of ageing working in the same research center. The Tread Lab collaborates with a variety of other laboratories with similar or complementary interest in the UK, US and Continental Europe, exchanging experimental materials, techniques and expertise, regularly hosting visiting researchers.
During fieldwork, I regularly attended the lab’s weekly meeting where progress, analysis and experimental results were discussed. I shadowed all of the lab’s post-docs and PhDs in their goings about the lab, taking photographs. I also conducted unstructured interviews with most members of the lab, asking questions about biology of ageing, and the procedures and techniques they were using. These were recorded in the fieldwork diary. In addition, I participated in social events organized by the lab’s members, such as Christmas parties, which did not get recorded in field-notes.
In ethnography, data collection and analysis are closely interwoven. In this, ethnographers “search simultaneously for an explanation that will fit all the evidence and for a definition of the problem that, without ‘cooking’ or hiding data, makes relevant only the evidence that fits the explanation” (Katz Citation2002, 485), guiding further data collection and analysis. This process was strengthened by obtaining respondent validation at various stages of the fieldwork, through presentation and discussion, with lab members, of the emerging analysis/explanation as it was developing.
The study was approved by the Durham University Department of Sociology Ethics Committee. All names used in the paper are pseudonyms.
Visualization and quantification in cell biology of ageing
During the early period of fieldwork, analysis of my notes identified an interesting pattern: that, while preparing and conducting experiments, and discussing data, lab members constantly shifted between – what I then provisionally called – two repertoires. One of those was concerned with “looking” and “seeing,” or with the production and interpretation of images; the other was about “counting,” measuring, and calculating data obtained in experiments. This pattern was most evident in the main forms of evidence used in the papers produced by the lab, which combined, on the one hand, pictures of exemplary cells under the (fluorescence) microscope, and on the other, plots, charts and calculations statistical of differences between batches of cells in relation to specific measurable indicators of the presence of a protein or some other biological entity (See ). As the fieldwork developed, this provisional hypothesis became key to understand experimental practices within the lab.
There is nothing new or atypical about cell biologists concern with visualization or quantification. Indeed, it has been accepted for many years, within Science and Technology Studies, that visualization and quantification are two of the main procedures used by scientists to represent and stabilize the entities they create in laboratories (Lynch and Woolgar Citation1990; Coopmans et al. Citation2014; Porter Citation1995; Martin and Lynch Citation2009). While it is recognized that scientists might visualize to count or visualize calculations in graphs, most research has tended to focus on one repertoire or the other. In the Tread Lab, however, the relationship between the two repertoires was of principal importance. This was because the lab members had a particular investment in demonstrating the validity of a biomarker which identified a mechanistic driver of cellular senescence, rather than simply tracking by-products of the process of cell arrest such as inflammation. Further, their proposed method for identifying what they often categorized as a “signature of senescence” was at odds with the established understanding in the field that telomere shortening was the key driver of senescence. It was the view of lab members, and specially of Oscar, the Lab’s PI, based on a numbers of critical peer reviews and some paper rejections, that to persuade others of the strength of their method and explanation, it was necessary to produce clear, easy to interpret images of the proposed biomarker backed by robust quantifications. Thus, the lab members’ work and routine discussions often topicalized the features of these repertoires and their possible relationships (e.g. Lynch Citation1991).
For lab members, the practical question was not to choose one repertoire, or “machinery of knowledge production” (Knorr-Cetina Citation1997) over another, but how to arrange or compose their articulation. While the emphasis was often on ensuring the overall alignment or coordination of these cultures to be able to generate a robust object, in practice, there was diversity in the ways in which epistemic cultures related to each other within cell biology of ageing. Understanding this multiplicity requires focusing on the experimental nature of cell biology: lab members’ main focus was on setting up, running and interpreting experiments, drawing on local materials resources, standards, institutional conditions, etc. As Rheinberger (Citation1997) argues, these “experimental systems” are primarily situations where the relationship between what is known and what is not known is negotiated. Knowledge making in the experimental biological sciences relies on a careful arrangement between what he calls “technical objects” (known, established, certain), and “epistemic things” (unknown or uncertain), which are the object of inquiry. Composing the relationship between knowledge machineries relies on how uncertainty is differently distributed between them, within an experimental system.
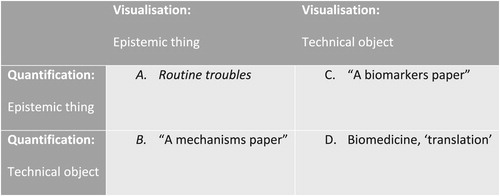
Selecting which aspects are “black boxed” and which ones are open to investigation is not a choice exercised by lab members alone but an action deployed by the experimental system as an “agencement” (Caliskan and Callon Citation2010, 9). In this, epistemic machineries are key because they make accountable and actionable not only what is known (or not) but also how it might become knowable. That is to say, epistemic cultures enable not only the identification of stable, robust objects, but also indicate how uncertainties might be explored. Experimental systems in cell biology of ageing are, then, ordered by the way in which uncertainty is distributed across the epistemic cultures of visualization and quantification. From this analysis, it is possible to identify four ideal types of experimental system ().
On the top left (Cell A), we have what could be labeled “routine troubles” because it refers to frequently occurring situations where both known markers and the identification of recognizable visual features are challenged by experiment. Because of the Thread Lab’s focus on seeking alignment between visualization and quantification, these situations gained special relevance in the working order of the lab. Where, for example, the visual differences between batches of cells “looked larger than the quantification,” the question of whether the visualization was misleading or the wrong measurement procedures used would habitually arise. These were results that, as Oscar put it in a lab session, “cannot be taken seriously” and set forth a variety of inquiries. In such a state of evenly distributed uncertainty, it was then necessary to find whether the measurement was flawed, or the microscope not well calibrated, the slide preparation protocol not followed, the hypothesis incorrect, or the experiment not well designed. Markers were re-quantified, images re-examined, microscopes assessed, notebooks checked, and, sometimes, experiments repeated or re-designed.
In what lab members often described as experiments leading to a “mechanism paper” (B), the situation was one where markers, forms of measurement and protocols for preparation and quantification were known and established. The focus of their investigation, in this situation, was on modeling and setting experiments to test new causal pathways, which required visually evidencing mechanism within the structure of the cell. An example of this was how lab members planned and tinkered with, for a few weeks, on how to model the “bystander effect” – the biological response in a cell resulting from an event in an adjacent or nearby cell – and set up a “physiologically accurate” experimental model of this intercellular process. Evidencing this process required a careful tracing of the timing of the immunological response process, so that accepted staining procedures and fluorescent markers could be set up to visualize the intercellular process.
When the causal mechanisms are established and experimentally reproducible but the focus of investigation was on developing or improving specific marker of senescence, the situation is best described by what lab members called “a biomarkers paper” (C). A useful example of this was the Lab’s work on developing a marker evidencing their and others’ previous, published work on mitochondrial processes in cellular senescence. This entailed exploring the role of a specific protein on oxygen and energy metabolism, so as to be able to propose it as a necessary condition for senescence. More pressingly, it required establishing a way of accurately quantifying the protein expression in a standardized cell model frequently used in biology of ageing. This, in turn, enabled the careful establishment of equivalences between markers, evidenced by statistical correlations between known – “gold standard” – markers of senescence and the new, proposed one.
A “biomedical paper” (D) refers to situations where both mechanisms and markers are relatively well established, and the focus of the investigation is on exploring the possible clinical relevance of findings. As one lab member once put it, when “we know the mechanisms [and how to measure] them, we can do an intervention study.” This work was usually done on “model disease” tissues: tissues collected from patients with a condition associated with increased age. The choice of the condition relied on the identification of a specific process (e.g. inflammation) that biologists of ageing agree drive the ageing process. Ideally, this condition should be well documented in physiological and clinical terms, through standardized measurements that can be used to enable the lab to focus on establish channels between their lab-based work and the clinic. Drawing on collaboration with clinicians working in the nearby medical school or in research clinics in the US and Netherlands, experiments explored links between cell-based physiological markers of the condition and senescence mechanisms. Work with model organisms took these further, aiming to establish “proof of principle” in using cellular senescence eradication or modulation strategies to affect the model condition’s known “phenotypes.”
This latter experimental system is where the lab’s “translational” work was seen to happen, potentially bringing to bear their work on senescence to the management of a whole variety of conditions. That is to say that members referred to specific projects as “translational,” and implicitly, and sometime explicitly, used known models of translational research to frame, justify and present their work. This was not only a question of language but was reflexively articulated as organizing the work of the lab, institutionally and materially. Indeed, over the three years of fieldwork, the importance of this type of work was increasingly recognized by lab members, both for obtaining the necessary funds to support all the other research conducted in the lab and to position the lab in the field of biology of ageing, which was seen to be moving towards a more applied direction, as suggested above. Working on senescence eradication compounds in specific age-associated conditions aligned the laboratory with clinically or health care oriented research funding programs, and with the direction and prospect of the whole field of ageing biology. As Oscar put it more than once to other lab members, “this is the future.”
Experiments and their futures
In the previous section, I proposed a conceptual model to understand the experimental work conducted in cell biology of ageing laboratory. I suggested that the differential distribution of uncertainty across the two main epistemic cultures of biology of ageing engendered four types of experimental system. Within this model, translational research is positioned in work that relies on the robustness of knowledge claims in relation to both visualization and quantification criteria. Drawing on fieldwork data, I suggested that lab members increasingly orientated to the language, conventions and institutional arrangements of translational research, shaping not only the mix of expertise available in the lab, and its networks, but also the instruments, tissues and other materials that ordered their experimental practice. This was intimately related to the hopes embodied in a new category of therapeutic agents to manage age-associated diseases.
Work on the role of technoscientific expectations is highly pertinent to understand Oscar’s proclamation about the future, referred to at the end of the last section. The sociology of expectations analyses how future-oriented visions constitute new fields of research, playing a key function in the enrollment of support, mobilization of resources and the shaping of scientific objects and technological artifacts (e.g. Martin, Brown, and Kraft Citation2008). To do so, visions require enactment, crafting normative and material alignment between specific settings and sociotechnical promises. This crafting, as exemplified in Oscar’s assertion about the future, can be understood as a form of “anticipation work” (Clarke Citation2016), bringing prospective arrangements of research and innovation in biology of ageing to bear in the present situation. From this perspective, anticipation work aligns situations to possible, specified futures, by supporting the framing of projects, the amassing of resources, and the design, running and analysis of experiments.
Anticipation work entails durable, explicit, materialized commitment to a particular version of the future. This is reinforced if this version of the future embodies “translational” representations of innovation processes, themselves embedded in institutional programs and procedures for research funding. The strength of such conventions and procedures can be garnered from the vignette given in the beginning of this paper. They require fashioning future oriented, technology centered justifications for experiments. Resources, experimental materials and expertise are assembled to support such experiments. Projects become accountable to stated “translational” objectives, measuring success in achieving “milestones” in the path towards implementing innovation in the market or clinic. The value of experiments becomes ensnared to their capacity to bring about, even if in small ways, the envisioned future.
Concerns about the effects of such conventions and procedures on both science and society motivated Felt et al. (Citation2007) to warn about reliance on linear models of innovation in research programs and policy. Such models, programs and conventions partake on what they have labeled the regime of technoeconomic promises, where innovation hinges on a process of “linear progression from pure research, to ideas of application, to design, testing, choice and demonstration, then social diffusion” (Felt et al. Citation2007, 5), a process perhaps uniquely exemplified by the MRC translational pathway (). In this regime, Felt and colleagues suggest, research is justified by alignment with the promise to address a specific societal problem (e.g. ageing associated disease burden). It is argued that this aim is best achieved by competitive engagement between laboratories, researchers, universities, and companies, underpinned by strong intellectual property rights regulation. Expectations and models of innovation thus also partially foreclose experimental practice, while embedding it in specific economic and institutional arrangements.
As the situation described at the beginning of the paper makes clear, lab members were aware of the narrowing down of questions and experiments that the alignment with “translational” programs entailed. Lab members, and Oscar in particular, often stated that “there is something about senescence that is linked to ageing,” emphasizing the uncertainty of the nature and character of the relationship. As referred above, cellular senescence is known to be a response to a variety of conditions including development, wounds, DNA damage and oncogene activation, making it key, as one lab member once put it, not to “confuse senescence and ageing.” From this perspective, understanding the biological functions and drivers of senescence were fundamental problems in contemporary biology, linked to work on cell replication and repair, biological theories of the development and ageing processes, and to debates about the evolution of ageing. The cost of committing to “translational” programs was that those questions could not be addressed, had to be downplayed in the present, or merely postponed.
They also acknowledged that the exploration of the connection between cellular senescence and ageing is far from direct or straightforward. It was not a simple question of designing an experiment to test the effects of senescence eradication on specific biological or phenotypical markers. Although hypotheses guided imagination and the design of experiments, lab members’ interest and curiosity was provoked often by unexpected results and data. By generating unanticipated events, they would become what Stengers (Citation1997) characterized as “interesting experiments,” endowing cells and other experimental materials with the capacity to act, prompting new questions or even downright puzzlement. Fieldwork data suggests that the generating of unexpected data, which Rheinberger (Citation1997) sees as central to experimental work, linked them into “experimental chains,” reshaping not only the on-going experiment itself but also the design and conduct of future experiments, or inspiring the re-analysis of old data. Exploration of the relationship between senescence and ageing relied fundamentally on the possibility of constructing these experimental chains.
In order to build, maintain and rebuild such experimental chains, lab members navigated between the four subtypes of experimental system described in the previous section. These operated as epistemic scaffolds, recognizable “genres” of experimental activity, deploying specific epistemic criteria, linked to particular outputs.
In one instance, lab members were discussing the biomedical, translational potential of data produced by an experiment focusing on modulating the proinflammatory effects of senescent cells – Senescence-Associated Secretory Phenotype or SASP (e.g. Coppe et al. Citation2010) – in a specific, relatively unusual model disease. Despite initial optimism about the experimental data, further analysis made it look less promising as it would result in “giving the drug to [only] a few older people [who have the model disease] to eliminate the SASP” with very little therapeutic effect. Valuing the therapeutic promise of the experiment as weak prompted lab members to look more closely at the images and measurements before them, and noticing that the visual “differences (between tissues) look[ed] larger than the quantification.” Their first line of enquiry focused on whether using a different statistical test would better align the two repertoires, but this was unlikely, they concluded, as some of the markers used in the quantification were well established and routinely used in the field and in the lab. The other possibility was that the visualizations were improperly timed, that is to say, that they were made on the assumption, based on the literature, that the proinflammatory process would follow a specific temporal pattern, a conjecture that now appeared less robust. Their attention shifted to the mechanism of proinflammation itself, raising the question of whether “finding something novel (about this process was) possible” through a different experimental procedure. “I need to think a lot more before I go back to tissue culture,” concluded the lead researcher in the project.
What is striking about the situation described above is that, within a few minutes, the project shifted between three types of experimental system (). At the outset, the experiment and the data it generated was evaluated from a “translational” perspective, adequately fitting its initial emplacement as a “biomedical paper” (D) or project, where both mechanisms and markers used are relatively established. In a restricted view of experiments, the weakness of the data should have led to the reporting of a “negative” test. Instead, its failure to qualify for the biomedical “genre” of experimental system led lab members to investigate the causes of the problem. Finding some misalignment between imaging and quantified data, they first considered the possibility of there being uncertainty relating to the statistical tests or markers used. Could the project be one that should focus on the biomarkers used to measure proinflammation (C)? Using the robustness of the markers used in the experiment as a scaffold, they reallocated their probing towards the visualizations and the process through which they had been produced (B). This, in turn, opened the exciting prospect of the project pertaining to the mechanism of proinflammation, a fundamental biological regulation mechanism, and perhaps “finding something novel” about it. Shifting between types of experimental system enabled cell biologists to build an experimental chain, generatively ordering their practices and enabling the exploration of the myriad dimensions of their objects of inquiry.
Although I observed this fluidity many times during my fieldwork, this process is not unique to cell biology of ageing. Indeed, the ability to shift focus, to turn “technical objects” into “epistemic things” and vice versa, has been noted by Nelson et al. (Citation2014) in their studies of clinical trials of cancer therapies, where the initial aim to test a hypothesis about the clinical effectiveness of a compound can easily turn into an investigation about novel mechanisms of disease progression, or the development of new markers of this processes. The process is best described through the artistic metaphor of choreography, evoking movements and the often improvised shifting between routinized sequences. This is particularly true in contemporary dance, where creativity is deployed between set and spontaneous actions and arrangements. Likewise, cell biologists in the Thread Lab engendered an experimental chain by navigating freely between scaffolded sets of practice.
In STS, the metaphor of choreography is mostly associated with the work of Cussins, where the concept is used to capture the “coordinated action of many ontologically heterogeneous actors in the service” of a collective undertaking (Cussins Citation1998, 600; my emphasis; see also Moreira Citation2000). While Cussins’ concept is able to emphasize the dynamic, temporal process through which heterogeneous coordination is deployed, its focus on coordinated action is less effective to articulate the generative, creative process of stringing experimental chains. To do this, it is necessary to conceptualize such chains as effects of the unfolding intra-action (Barad Citation2007; also Pickering Citation1995; Latour Citation1988) of experimental practice. In this complementary conceptualization of choreography, the creative process is neither the product of one individual actor neither of a progressing, emergent assembling of disparate elements but, instead, a nuanced process where what might have started as a “technical object” can become problematized and the focus of the experiment, and where new entities might materialize as well as disappear.
Based on this and many other similar instances, I would suggest that without this capacity to shift between situations, experiments lose the capacity to generate the future, a capacity that Rheinberger (Citation1997, 32–33) considers unique to the experimental sciences. This is, however, a different type of future from the one engendered through “anticipation work.” While the latter enacts a spatial configuration between discrete moments – between now and the point when innovation will come to bear – again best epitomized by the pictorial arrow that often frames representations of “translational processes” – the former is deployed in a durational continuum where “the past […] gnaws into the future and […] swells as it advances” (Bergson Citation2007, 3). In this respect, the choreography deployed through experimental chains is more evident for the observer, who can trace the movement from one experimental system to another, than for the practitioner, who is knowledgeably navigating between them, each time anew. The future unfolds from the present, opening aspects and proliferating questions that could not have been foreseen or planned ahead. In this, experiments become generative.
It is from this perspective that we can understand lab members’ sense that translational models narrow their work and the generative capacity of experiments, as described above. In the spatial temporality of translational pathways, focus shifting and reformulations of the problem, and of the aims and goals of research becomes a set-back, constituting, inevitably, a move backwards in the path towards innovation. However, in practice, as I have argued, those reconfigurations are essential to knowledge making dynamic of cell biology of ageing. It could be argued that these choreographies are so central to scientific practice that the effect of translational pathways can only be the sparking of disputes about the ultimate aims of biological research and the search for compromises (Boltanski and Thevenot Citation2006) within laboratories. The vignette given at the beginning of the paper is, again, heuristic in this respect, the initial confrontation between the funder’s and Oscar’s view of biology being replaced by a negotiated outcome.
Lab members often described this compromise in two ways: one, distinguishing between “what is written in the grant” and “what is done in the lab,” and two, and more to the point, as being able to ask “interesting” biological questions within “applied” or biomedical projects. The first arrangement is familiar to most researchers. It deploys a boundary around the laboratory, secluding it from institutional accountability. It is however also recognized as a temporary arrangement, as experimental reports will have to, at some point, link to the stated aims of the project. In the latter arrangement, open experimental practice becomes nested within translational projects. Choreographing knowledge is, in these situations, constructed as a return to “basic research” (see ), allowing the pursuit of interesting questions to still possibly align with the translational pathway. This alignment, as I suggested above, relies on maintaining or re-making new links to established clinical models and standardized measurements, and collaborators. Shifts in orientation or focus are thus accountable to the network of researchers, clinicians, etc. that are involved in the project. This means that this kind of compromises are inherently unstable, because they require seeking to establish alignment or justifying why it was not possible, and risking losing allies.
An additional reason for their instability relates to the effects of valuation (Dussange, Helgesson, and Lee Citation2015). Because seeking therapeutic prospects is “the future,” as Oscar put it, translational justifications for work and experiments become more legitimate than open inquiry. This has consequences for how objects, questions, experiments and persons are evaluated. It affects “what research is about” because it entails forsaking present, at hand, lines of inquiry, with exciting, if unknown, potential for a possible, but often improbable looking, future. From an experiential point of view, this can become unsatisfying, projects structured along the “translational pathway” being more valuable – amassing more resources, time, labor, and career prospects – than open, choreographed ones. This resulted, as Oscar once put to me in an informal interview, in a thinning of “ambition” and scope in the field of biology of ageing.
Caring about biology in the future
The aim of this paper was to describe and explore how translational models, embedded in institutions and standards, interact with the epistemic and material practices of cell biologists of ageing. Departing from the notion that knowledge making in cell biology of ageing relies on two different epistemic and material cultures, I argued that these cultures combine in four different configurations or types of experimental systems, only one of which can properly be seen as pertaining to translation as usually conceived. I suggested that the legitimacy and embeddedness of this type of system within cell biology of ageing had increased because of how it articulated a future for the field: working towards the development and evaluation of therapies that delay the onset of age-related illnesses and the extension of “health span” for human populations.
I contrasted the spatially organized, linear temporality of translational research with the unfolding experimental chains where, by shifting between types of experimental system, cell biologists are able to generatively reconfigure their epistemic objects. This contrast, I argued, underpins the normative and material tension between ways of working that is visible in the episode recounted at the beginning of the paper, and in many other instances observed during fieldwork. Finally, I suggested that the arrangements biologists of ageing have deployed to manage the tension are productive but fragile. The paper thus describes translation in a temporal register, supporting the re-conceptualization of translational research that, using spatial metaphors, have characterized it as a tangled web (Keating and Cambrosio Citation2013) or a set of circuits (Lewis, Hughes, and Atkinson Citation2014).
The case of cell biology of ageing is useful to understand translational research from a social science perspective because its relationship with biomedicine has been marked by historically constituted, epistemic and normative tensions between the two fields (Moreira, Citation2017). From this perspective, the introduction of translational models and ways of working in cell biology of ageing challenges the established normative and material practices of the field. Instead of a dominance of the translation mode over open inquiry practices, what I, in line with other research on this topic (e.g. Brosnan and Michael Citation2014), described was the making of a new sociotechnical arrangement where the two temporalities and normativities can co-exist. The paper further proposes that it was this fragile arrangement that supported the growth and consolidation of the field of cell biology of ageing in the last decade, reported in the introduction.
This, however, might lead to institutional and material devaluation of the worth of aspects of work that researchers find exciting and motivating, and possibly to an impoverished understanding of the phenomena of cellular senescence. They might also be having effects on the scope of the field of research itself and its research practices, as suggested by various Tread Lab members. In this, they concur with Maienschein et al. (Citation2008) view that translational models and frameworks might be adversely reshaping scientific practice in the life sciences. Importantly, however, Maienschein and colleagues warn that this epistemic transformation pertains also to institutional, normative and ethical aspects of scientific practice. The paper supports the conclusion that this constraining effect imposed by translation models on cell biology of ageing can be related to the policy framing of technologies, tools, experiments etc. as steps in a pathway. It is constraining because it fails to publicly recognize the character of the experimental sciences and the generative capacities of experiments, as was argued in the paper. Embedding empirically robust social science understanding of scientific practice in the conventions, institutions and procedures of research programs would facilitate knowledge making not only in cell biology of ageing but biological and biomedical sciences in general.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Addison, C. 2017. “Bench, Bedside, Boardroom: Negotiating Translational Gene Therapy.” New Genetics and Society 36 (1): 22–42. doi: 10.1080/14636778.2017.1289468
- Bakalar, N. 2018. “How Long Can People Live?” New York Times, November 18.
- Barad, K. 2007. Meeting the Universe Halfway: Quantum Physics and the Entanglement of Matter and Meaning. Durham, NC: Duke University Press.
- Bergson, H. 2007. Creative Evolution. New York: Palgrave Macmillan.
- Boltanski, L., and L. Thevenot. 2006. On Justification: Economies of Worth. Princeton, NJ: Princeton University Press.
- Brosnan, C., and M. Michael. 2014. “Enacting the ‘Neuro’ in Practice: Translational Research, Adhesion and the Promise of Porosity.” Social Studies of Science 44 (5): 680–700. doi: 10.1177/0306312714534333
- Caliskan, K., and M. Callon. 2010. “Economization, Part 2: A Research Programme for the Study of Markets.” Economy and Society 39 (1): 1–32. doi: 10.1080/03085140903424519
- Campisi, J., P. Kapahi, G. J. Lithgow, S. Melov, J. C. Newman, and E. Verdin. 2019. “From Discoveries in Ageing Research to Therapeutics for Healthy Ageing.” Nature 571 (7764): 183–192. doi: 10.1038/s41586-019-1365-2
- Clarke, A. E. 2016. “Anticipation Work: Abduction, Simplification, Hope.” In Boundary Objects and Beyond, edited by G. Bowker, S. Timmermans, A. Clarke, and E. Balka, 85–120. Cambridge: MIT Press.
- Coopmans, C., J. Vertesi, M. E. Lynch, and S. Woolgar, eds. 2014. Representation in Scientific Practice Revisited. Cambridge: MIT Press.
- Coppe, J. P., P. Y. Desprez, A. Krtolica, and J. Campisi. 2010. “The Senescence-Associated Secretory Phenotype: the Dark Side of Tumor Suppression.” Annual Review of Pathology: Mechanisms of Disease 5: 99–118. doi: 10.1146/annurev-pathol-121808-102144
- Cussins, C. 1998. “Ontological Choreography: Agency for Women Patients in an Infertility Clinic.” In Differences in Medicine: Unraveling Practices, Techniques, and Bodies, edited by M. Berg and A. Mol, 166–201. Durham, NC: Duke University Press.
- Deursen, J. M. 2014. “The Role of Senescent Cells in Ageing.” Nature 509 (7501): 439–446. doi: 10.1038/nature13193
- Deursen, J. M. 2019. “Senolytic Therapies for Healthy Longevity.” Science 364 (6441): 636–637. doi: 10.1126/science.aaw1299
- Doing, P. 2008. “Give Me a Laboratory and I Will Raise a Discipline: The Past, Present, and Future of Laboratory Studies in STS.” In Handbook of Science & Technology Studies. 3rd ed., edited by E. Hackett, O. Amsterdamska, M. Lynch, and J. Wajcman, 279–295. Cambridge: MIT Press.
- Dussange, I., C. F. Helgesson, and F. Lee. 2015. Value Practices in the Life Sciences and Biomedicine. New York: Oxford University Press.
- Felt, U., B. Wynne, A. Stirling, M. Callon, and M. E. Goncalves. 2007. Taking European Knowledge Society Seriously, Report of the Expert Group on Science and Governance to the Science. Economy and Society Directorate Brussels: European Commission.
- Fleming, A. 2019. “The Science of Senolytics: How a New Pill Could Spell the End of Ageing.” The Guardian, September 13.
- Gardner, J., and A. Webster. 2017. “Accelerating Innovation in the Creation of Biovalue: The Cell and Gene Therapy Catapult.” Science, Technology, & Human Values 42 (5): 925–946. doi: 10.1177/0162243917702720
- Hayden, E. 2015. “Ageing Pushed as Treatable Condition.” Nature 522: 265–266. doi: 10.1038/522265a
- Herranz, N., and J. Gil. 2018. “Mechanisms and Functions of Cellular Senescence.” The Journal of Clinical Investigation 128 (4): 1238–1246. doi: 10.1172/JCI95148
- Katz, J. 2002. “From How to Why: On Luminous Description and Causal Inference in Ethnography.” Ethnography 3 (1): 63–90. doi: 10.1177/1466138102003001003
- Keating, P., and A. Cambrosio. 2013. “21st-Century Oncology: A Tangled Web.” The Lancet 382 (9909): e45–e46. doi: 10.1016/S0140-6736(13)62660-4
- Kirkland, J. L., T. Tchkonia, Y. Zhu, L. J. Niedernhofer, and P. D. Robbins. 2017. “The Clinical Potential of Senolytic Drugs.” Journal of the American Geriatrics Society 65 (10): 2297–2301. doi: 10.1111/jgs.14969
- Knorr-Cetina, K. K. 1997. Epistemic Cultures: How the Sciences Make Knowledge. Harvard, MA: Harvard University Press.
- Latour, B. 1988. “Irréductions.” In The Pasteurisation of France, edited by B. Latour. Cambridge: Harvard University Press.
- Lewis, J., J. Hughes, and P. Atkinson. 2014. “Relocation, Realignment and Standardisation: Circuits of Translation in Huntington’s Disease.” Social Theory & Health 12 (4): 396–415. doi: 10.1057/sth.2014.13
- Lynch, M. 1991. “Laboratory Space and the Technological Complex: An Investigation of Topical Contextures.” Science in Context 4 (1): 51–78. doi: 10.1017/S0269889700000156
- Lynch, M., and S. Woolgar. 1990. Representation in Scientific Practice. Cambridge: MIT Press.
- Maienschein, J., M. Sunderland, R. A. Ankeny, and J. S. Robert. 2008. “The Ethos and Ethics of Translational Research.” The American Journal of Bioethics 8 (3): 43–51. doi: 10.1080/15265160802109314
- Martin, P., N. Brown, and A. Kraft. 2008. “From Bedside to Bench? Communities of Promise, Translational Research and the Making of Blood Stem Cells.” Science as Culture 17 (1): 29–41. doi: 10.1080/09505430701872921
- Martin, A., and M. Lynch. 2009. “Counting Things and People: The Practices and Politics of Counting.” Social Problems 56 (2): 243–266. doi: 10.1525/sp.2009.56.2.243
- Miller, R. A. 2002. “Extending Life: Scientific Prospects and Political Obstacles.” The Milbank Quarterly 80 (1): 155–174. doi: 10.1111/1468-0009.00006
- Mittra, J. 2013. “Repairing the ‘Broken Middle’ of the Health Innovation Pathway: Exploring Diverse Practitioner Perspectives on the Emergence and Role of ‘Translational Medicine’.” Science and Technology Studies 26 (3): 103–123. doi: 10.23987/sts.55290
- Moreira, T. 2017. Science, Technology and the Ageing Society. Abingdon: Routledge.
- Moreira, T. 2000. “Translation, Difference and Ontological Fluidity: Cerebral Angiography and Neurosurgical Practice (1926–45).” Social Studies of Science 30 (3): 421–446. doi: 10.1177/030631200030003004
- Nelson, N. C., P. Keating, A. Cambrosio, A. Aguilar-Mahecha, and M. Basik. 2014. “Testing Devices or Experimental Systems? Cancer Clinical Trials Take the Genomic Turn.” Social Science & Medicine 111: 74–83. doi: 10.1016/j.socscimed.2014.04.008
- Pickering, A. 1995. The Mangle of Practice. Chicago, IL: University of Chicago Press.
- Porter, T. 1995. Trust in Numbers: The Pursuit of Objectivity in Science and Public Life. Princeton, NJ: Princeton University Press.
- Rheinberger, H.-J. 1997. Synthesizing Proteins in the Test Tube Toward a History of Epistemic Things. Stanford, CA: Stanford University Press.
- Stengers, I. 1997. Power and Invention: Situating Science. Minneapolis: University of Minnesota Press.
- Vos, R. 1991. Drugs Looking for Diseases. Dordrecht: Springer.
- Ziewitz, M., and M. Lynch. 2018. “It’s Important to Go to the Laboratory.” Engaging Science, Technology, and Society 4: 366–385. doi: 10.17351/ests2018.220

![Picture 1. Aligning visualization and quantification [Photo by author].](/cms/asset/f9657ec9-9446-42da-b27b-6a07fe03967a/cngs_a_1825932_f0003_oc.jpg)