Abstract
Reactivators of phosphorylated acetylcholinesterase (oximes) are substances used as a human antidotal therapy for organophosphate poisoning. The objective of our study was to examine if juveniles of freshwater microcrustacean Daphnia magna could be employed as test animals in early screen toxicity tests of those substances as a first step for further experiments with daphnids intoxicated by organophosphates. For this purpose, seven different oximes were investigated. It was found that toxicity of all tested oximes increased with time. Mono-quaternary oximes were approximately ten fold (EC50, 14.9 mg.l− 1) more toxic in 24 hour tests and five fold (EC50 was 79.46 mg.l− 1) more toxic in 48 hour tests than bis-quaternary oximes. Tests with daphnids were shown to be easy to carry out at low cost and provided valuable results which could be used as a starting point for further research.
Introduction
Human antidotal therapy for organophosphate intoxication is generally a combination of an anticholinergic substance (atropine mainly) together with a so called acetylcholinesterase (AChE; EC 3.1.1.7) reactivator. The most active reactivators are oximes with a pyridine group. Oximes specifically restore the AChE blocked by organophosphates to its normal function [Citation1]. Atropine protects against the excess of acetylcholine formed during nerve agent poisoning. The mixture of atropine and the reactivator is injected into an individual using a device called an auto-injector.
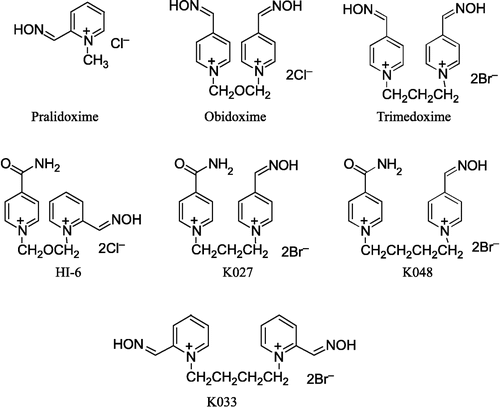
From a structural view, AChE reactivators are mono- (e.g. pralidoxime) or bis-quaternary (e.g. obidoxime and HI-6) pyridinium aldoximes with a functional oxime group. These compounds break down the bond between an inhibitor (organophosphorus compound residue) and the enzyme (AChE) by removing the organophosphoryl moiety resulting in a reactivated enzyme which is able to fulfil its physiological role in the organism again [Citation2].
The efficacy of oximes to reactivate the organophosphate-inhibited AChE differs. A process of losing an alkyl group from the inhibited enzyme called “aging” plays an important role as well as oxime affinity for the inhibited enzyme and its chemical structure [Citation3,Citation4]. Consequently, there is a continually search for new and more efficient reactivators. One of the first steps in research of a newly synthesized substance is to evaluate its toxicity and to compare it to other oximes.
In our research, we focused in finding an animal model which could be used in early screen toxicity tests of those substances as well as for further experiments with organophosphate-intoxicated animals. We found the water flea Daphnia magna [Citation5] a suitable candidate. This choice was made because daphnids are already used in acute and chronic toxicity testing in aquatic ecotoxicology and several standards describing tests methodologies exists [Citation6], however they have not yet been employed in experimental toxicology. The use of acute toxicity tests with daphnids aimed to prescreen the toxicity of AChE reactivators is a new approach in military toxicology.
The methodology described in the standard EN ISO 6341 [Citation7] was chosen for purposes of our study. We decided to evaluate its applicability by testing the toxicity of seven different AChE reactivators, one of them mono-quaternary and six bis-quaternary compounds. Four of these substances are already commercially known and three are newly synthesized ones.
Materials and methods
Chemicals and reagents
Pralidoxime (2-PAM; 2-hydroxyiminomethyl-1-methylpyridinium chloride), trimedoxime (TMB-4; 1,3-bis(4-hydroxyiminomethylpyridinium)-propane dibromide) were purchased from Leciva (Czech Republic), whereas obidoxime (Toxogonin®; 1,3-bis(4-hydroxyiminomethylpyridinium)-2-oxa-propane dichloride), HI-6 (1-(2-hydroxyiminomethylpyridinium)-3-(4-carbamoylpyridinium)–2-oxa-propane dichloride) were purchased from Merck (Germany). The other three oxime reactivators: K027 (1-(4-hydroxyiminomethylpyridinium)-3-(4-carbamoylpyridinium) propane dibromide), K033 (1,4-bis(2-hydroxyiminomethylpyridinium) butane dibromide) and K048 (1-(4-hydroxyiminomethylpyridinium)-4-(4-carbamoylpyridinium) butane dibromide) were prepared in our laboratory [Citation8,Citation9,Citation10] (). More than 95% pure oximes were used; their purities were tested using an HPLC technique prior to the experiment.
Stock solutions of individual oximes were prepared in ultra pure water (conductivity less than 5μS.cm− 1) immediately before the start of the test. Nominal test concentrations were subsequently prepared by adding an appropriate aliquot of the stock solution to the synthetic ISO medium [Citation7]. Chemicals used for preparing ISO medium (CaCl2.2H2O, MgSO4.7H2O, NaHCO3, KCl) were purchased from Lachner – Lachema (Czech Republic) and were analytical grade. The ultra pure water used was the same as for the stock solutions.
Culture of maternal animals
To minimize maternal effects [Citation11] only neonates that originated from a third to sixth brood of Daphnia magna clone HK (clone a sensu Baird et al. [Citation11]) were used as maternal animals. Ten animals were kept in 1 litre glass beakers. As culture medium, water, taken from a small pond, filtered through a 40 μm sieve was used to which daily 2 mg of carbon per litre of the laboratory cultured green alga Scenedesmus acutus MEYEN was added. We used a light regime of 16 h light: 8 h dark, and a culture temperature of 20 ± 1°C.
Design of daphnids experiments
For all tests female neonates from a third to sixth brood not older than 24 h were used. Tests were carried out for 24 and 48 h. For methodology and conditions of the tests see EN ISO [Citation7]. Temperature and photoperiod were the same as used for the stock cultures. Per treatment 21 animals divided into three groups of 7 animals were used.
The aim of the tests was to determine the median effective concentration, EC50, defined as the concentration at which 50% of the exposed organisms are affected by a measured effect [Citation12]. In our tests, incapacitancy was chosen as the effect where daphnids as a result of the toxic action of the tested substance were so immobilized, that they were not able to start to swim within 15 s after gentle shaking of the medium in the test beaker [Citation7].
Data analysis
EC50 (24 h, 48 h) values and dose-response curves were calculated by nonlinear regression using a four parameter logistic equation [Citation13] by the computer program GraphPad PRISM, version 4.0.
Results
Relationship between oximes concentration at the beginning of the tests and percentage inhibition of daphnids in tests lasting 24 and 48 h is shown in . This figure is divided into seven graphs. In each graph, fitted dose-response curves for one oxime in both tests are shown. For all tested oximes except for K027 (after 24 h) sigmoid curves were obtained. The curve for K027 (after 24 h) could not be fitted because of the lack of data. The largest difference between slopes of 24 and 48 h curves was found for HI-6, followed by K048, K033 and pralidoxime. For obidoxime and trimedoxime, the difference in slopes was minimal.
Figure 2 Relationship between oxime concentration at the beginning of the test and percentage inhibition in Daphnia magna in tests lasting 24 (▪) and 48 h (▵) for seven different oximes. Error bars represent+1SD.
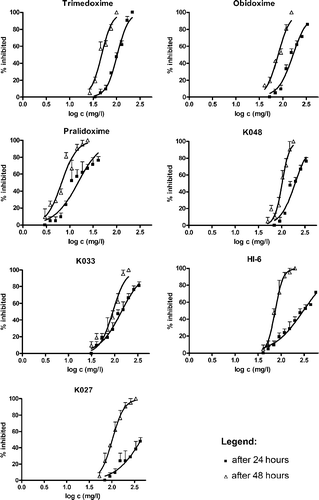
The tests results are summarized in . The highest EC50 value (after 24 h) was obtained for HI-6, the lowest for pralidoxime, whereas K048, obidoxime, K033 and trimedoxime showed intermediate values. There was a problem in determining EC50 (after 24 h) for the oxime K027. At concentrations up to 429 mg.l− 1 the proportion of immobilized daphnids increased with increasing concentration of oxime, but from 570 mg.l− 1 onwards, up to a concentration of 741 mg.l− 1, all test animals were swimming actively around the beaker. The highest values of EC50 in tests lasting 48 h were observed for oxime K027 and K048, the lowest for pralidoxime, whereas K033, obidoxime, HI-6 and trimedoxime showed intermediate values.
Table I. EC50 values using Daphnia magna as test animal after 24 and 48 h for seven different AChE reactivators.
According to the different molecular weight of the oximes tested, EC50's expressed as molar concentrations (M) are a little bit different in comparison with those expressed as mg.l− 1. Their values for EC50 (after 24 h) are as follows: trimedoxime (2.58. 10− 4 M), obidoxime (4.61. 10− 4 M), pralidoxime (5.66. 10− 5 M), HI-6 (8.08. 10− 4 M), K048 (4.23. 10− 4 M), K027 (not obtained) and K033 (3.0. 10− 4 M). Values for EC50 (after 48 h) are as follows: trimedoxime (1.19. 10− 4 M), obidoxime (2.21. 10− 4 M), pralidoxime (2.54. 10− 5 M), HI-6 (1.97. 10− 4 M), K048 (2.19. 10− 4 M), K027 (2.29. 10− 4 M) and K033 (1.98. 10− 4 M).
EC50 of all tested oximes decreased with time (, ). The highest decrease, about three quarters of the EC50 value was observed for HI-6, the EC50 values of the other oximes being approximately halved.
Discussion
Ecotoxicological tests are a common tool used in aquatic toxicology. They are mainly employed to determine the critical amount of toxicants and their mixtures for water organisms and ecosystems, to predict their influence and their fate [Citation14].
Employing daphnids in tests has several advantages. They are easy to breed at low cost in any laboratory [Citation15]. They reproduce by parthenogenesis, so that all tests animals of the same genotype can be used. Large number of animals can be used in one test making it possible to fit the whole dose-response curve. Neonates of Daphnia magna are of a convenient size (about 0.8 mm) and therefore relatively small amounts of test substance and space are required.
Daphnids have a cholinergic nervous system and their cholinesterase shows characteristics of a pseudocholinesterase, since it prefers propionylthiocholine to acetylthiocholine. However, like mammalian AChE, it is inhibited by high concentrations of substrate [Citation16].
Pyridinium oximes bind to the active surface of AChE and are weak inhibitors of this enzyme [Citation17]. If applied in high doses they cause an inhibition of AChE and neuromuscular blocking [Citation18,Citation19]. Their toxicity increases with exposure time. They are metabolized largely by the liver and breakdown products are excreted by the kidney [Citation20]. In water fleas, the organ that similarly functions like mammalian liver is gut diverticula located at the anterior midgut region [Citation21].
According to our results, all the tested oximes were toxic to juveniles of Daphnia magna at low concentrations (tens of milligrams per litre for mono-quaternary compound, hundreds of milligrams per litre for bis-quaternary ones). An increase in slope of dose-response curves with time for all oximes, except trimedoxime and obidoxime, was observed. This is caused by accumulation of the toxic effect of oximes with time and by the different ability of daphnids to eliminate oximes of different structure.
Owing to the fact that all AChE reactivators have in their molecule the same structural factors, such as quaternary nitrogen, presence of the oxime group and the chain between two quaternary pyridinium rings in bis-quaternary compounds, their difference in acute toxicity to daphnia is caused by specific differences in structure such as the quaternary nitrogens, position of the oxime group and the length of linking chain between both pyridinium rings. The dependence of the reactivation potency of tested AChE reactivators on their chemical structure was observed also for AChE reactivation in vitro [Citation22]. Probably the attainable AChE reactivators concentration in vivo is 10− 4 M and lower. Owing to this fact, oximes with good reactivation potency at high concentrations (10− 3 M and higher), could not be used as promising AChE reactivators [Citation3].
The most toxic oxime amongst the tested substances was the mono-quaternary, pralidoxime. This is probably caused by the small size of its molecule in comparison with the larger and double charged bis-quaternary compounds, which enables pralidoxime to penetrate more easily through barriers. A recent study showed that it is in small amounts able to penetrate the blood brain barrier [Citation23].
According to our results, oxime HI-6, which is currently the most promising AChE reactivator, seems to be the least toxic to Daphnia magna according to its EC50 value after 24 h. This result is in a good agreement with data obtained from in vivo tests using mice [Citation24]. On the other hand, in tests lasting 48 h, the EC50 value of this oxime was not the highest one, better results being found for oximes K027 and K048. Unfortunately, because of the unexpected behaviour of daphnids at higher oxime concentrations in the tests with K027, we were not able to determine its EC50 value after 24 hours. Although tests were repeated several times, the same effect was always observed. Our explanation is that the toxic action of this oxime on the daphnid's body initially causes partial paralysis then the critical concentration in the organism causes convulsions, expressed by abnormally intensive swimming in the upper part of the media in the test beaker, followed by death after 48 h. This explanation however needs additional research.
As it can be seen from the results, tests with juveniles of Daphnia magna provide valuable information. They are a good method for use in early screen toxicity testing of newly synthesized oximes as well as substances others than AChE reactivators. Daphnids proved to be a useful animal model. They might also be used for further experiments on the treatment of organophosphate poisoning in military toxicology.
Conclusion
The possibility of using juveniles of the freshwater microcrustacean Daphnia magna in tests aimed to prescreen the toxicity of newly synthesized oximes was examined. Those tests showed that they are easy to carry out at low cost, the results fitted dose-response curves and resulting EC50 values can be satisfactorily used in evaluating and comparing the toxicity of existing oximes as well as newly synthesized ones. They can be also used for early screen testing of other substances.
Acknowledgements
We thank Koos (J.) Vijverberg for critically commenting on earlier versions of the manuscript. The experiments described in this work were carried out in compliance with the current law of the Czech Republic. The work was supported by the grant of Ministry of Defence, No.OBVRSVZDR200301.
References
- Bajgar J. Organophosphates nerve agents poisoning: mechanism of action, diagnosis, prophylaxis and treatment. Adv Clin Chem 2004; 38: 151–216
- Sidell FR. Medical aspects of chemical and biological warfare. FR Sidell, ET Takafuji, DR Franz. Borden Institute, Washington 1997; 129–179
- Kuca K, Kassa J. A comparison of the ability of a new bispyridinium oxime - 1-(4-hydroxyiminomethylpyridinium)-4-(4-carbamoylpyridinium) butan dibromide and currently used oximes to reactivate nerve agent-inhibited rat brain acetylcholinesterase by in vitro methods. J Enz Inhib Med Chem 2003; 18: 529–535
- Worek F, Thiermann H, Szinicz L, Eyer P. Kinetic analysis of interactions between human acetylcholinesterase, structurally different organophosphorous compounds and oximes. Biochem Pharmacol 2004; 68: 2237–2248
- Benzie JAH. Cladocera: The genus Daphnia (including Daphniopsis). Guides to the identification of the microinvertebrates of the continental waters of the world. Kenobi Productions, Ghent 2005; Vol. 21
- Cooney JD. Freshwater tests. Fundamentals of aquatic toxicology, GM Rand. Taylor & Francis, New York 2003; 71–102
- EN ISO 6341. Water quality - Determination of the inhibition of the mobility of Daphnia magna Straus (Cladocera, Crustacea) - Acute toxicity test. EN ISO 6341:1996/AC 1998. Brussels: European Committee for Standardization; 1998.
- Kuca K, Bielavsky J, Cabal J, Kassa J. Synthesis of a new reactivator of tabun inhibited acetylcholinesterase. Bioorg. Med. Chem. Lett. 2003; 13: 3545–3547
- Kuca K, Bielavsky J, Cabal J, Bielavska M. Synthesis of a potential reactivator of acetylcholinesterase 1-(4-hydroxyiminomethylpyridinium)-3-(carbamoylpyridinium)-propane dibromide. Tetrahedron Lett. 2003; 44: 3123–3125
- Kuca K, Cabal J, Patocka J, Kassa J. Synthesis of bisquaternary symmetric -χ,δ-bis(2-hydroxyiminomethylpyridinium)alkane dibromides and their reactivation of cyclosarin-inhibited acetylcholinesterase. Lett. Org. Chem. 2004; 1: 84–86
- Baird DJ, Barber I, Bradley M, Calow P, Soares AMVM. The Daphnia bioassay: a critique. Hydrobiologia 1989; 188/189: 403–406
- Newman C. Quantitative methods in aquatic toxicology. Lewis Publishers, Florida 1995
- Motulsky HJ, Christopoulos A. Fitting models to biological data using linear and nonlinear regression. A practical guide to curve fitting. GraphPad Software Inc, San Diego CA 2003
- Rand GM, Wells PG, McCarty LS. Introduction to aquatic toxicology. Fundamentals of aquatic toxicology, GM Rand. Taylor & Francis, New York 2003; 3–67
- Peters RH. Daphnia culture. Daphnia, RH Peters, R De Bernardi, Mem Ist Ital Idrobiol 1987;45:483–495
- Diamantino TC, Almeida E, Soares AMVM, Guilhermino L. Characterization of cholinesterases from Daphnia magna Straus and their inhibition by zinc. Bull Environ Contam Toxicol 2003; 71: 219–225
- Skrinjaric-Spoljar M, Burger N, Lovric J. Inhibition of acetylcholinesterase by three new pyridinium compounds and their effect on phosphonylation of the enzyme. J Enz Inhib Med Chem 1999; 14: 331–341
- Lundy PM, Tremblay KP. Ganglion blocking properties of some bispyridinium soman antagonists. Eur. J. Pharmacol. 1979; 60: 47–53
- Caratsch CG, Waser PG. Effects of obidoxime chloride on native and sarin-poisoned frog neuromuscular junctions. Pflugers Arch 1984; 401: 84–90
- Taylor P. Anticholinesterase agents. JG Hardman, LE Limbird, A Goodman – Gillman. McGraw Hill, New York 2001; 175–191, Goodman & Gillman's The pharmacological basis of therapeutics. International ed.
- Peters RH. Metabolism in Daphnia. Daphnia, RH Peters, R De Bernardi, Mem Ist Ital Idrobiol 1987;45:193–243
- Kuca K, Patocka J, Cabal J. Reactivation of organophosphate inhibited acetylcholinesterase activity by α,ω-bis-(4-hydroxyiminomethylpyridinium) alkanes in vitro. J. Appl. Biomed. 2003; 1: 207–211
- Sakurada K, Matsubara K, Shimizu K, Shionom H, Seto Y, Tsuge K, Yoshino M, Sakai I, Mukoyamam H, Takatori T. Pralidoxime iodide (2-PAM) penetrates across the blood-brain barrier. Neurochem Res 2003; 28: 1401–1407
- Sevelova L, Kuca K, Krejcova-Kunesova G. Antidotal treatment of GF-agent intoxication in mice with new bispyridinium oximes. Toxicology 2005; 207: 1–6