Abstract
Oxidative stress has been implicated in the development of many neurodegenerative diseases and also responsible from aging and some cancer types. Indolic compounds are a broad family of substances present in microorganisms, plants and animals. They are mainly related to tryptophan metabolism, and present particular properties that depend on their respective chemical structures. Due to free radical scavenger and antioxidant properties of indolic derivatives such as indolinic nitroxides and melatonin, a series of 2-phenyl indole derivatives were prepared and their in vitro effects on rat liver lipid peroxidation levels, superoxide formation and DPPH stable radical scavenging activities were determined against melatonin, BHT and α-tocopherol. The compounds significantly inhibited (72–98%) lipid peroxidation at 10− 3 M. These values were similar to that observed with BHT (88%). Possible structure–activity relationships of the compounds were discussed.
Introduction
Reactive oxygen species (ROS) play an important role in physiological processes, but when in excess, ROS cause oxidative damage to molecules. Under physiological conditions, the production and detoxification of ROS are more-or-less balanced [Citation1]. However, any internal or external pathological factor may disrupt this balance, leading to conditions referred to as oxidative stress, playing a significant role in the pathogenesis of several diseases [Citation2]. Free radicals are chemically reactive species that can attack and damage several biomolecules such as DNA and enzymes. Oxidative damage to DNA by ROS is a continuous problem that cells must guard against to survive. ROS include singlet oxygen, superoxide anion, hydrogen peroxide and hydroxyl radical. Oxidative stress has been implicated in the development of neurodegenerative diseases like Parkinson's, Alzheimer's and Huntington's diseases, epileptic seizures, stroke and is also responsible from aging and some cancer types. In the last decade, melatonin and related indole derivatives have been widely studied as a scavenger of ROS, a secondary product of the aerobic metabolism within cells Citation3-7.
Indolic compounds are a broad family of substances present in microorganisms, plants and animals [Citation8]. N-Substituted indole-2-carboxamide and 3-acetamide derivatives show promising antioxidant activity against [Citation9,Citation10]. Also N-H and N-substituted indole ester derivatives [Citation11] have anti-superoxide formation activity at a concentration of 10− 4 M.
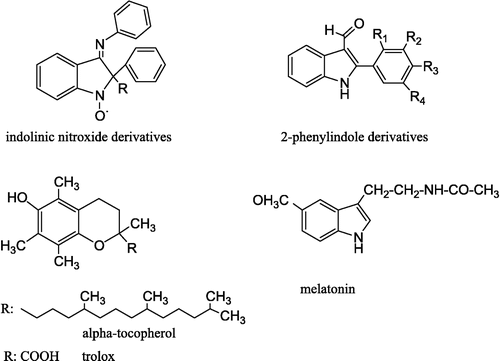
Comparison of indolinic nitroxides with vitamin E and Trolox (a hydrophilic analogue of vitamin E), showed that where the only difference between the nitroxides was the length of the hydrocarbon chain in the 2-position of indole () then all the nitroxides were effective in preventing oxidation of bovine serum albumin. Also the results clearly demonstrate that indolinic compounds are efficient antioxidants, protecting both lipids and proteins from peroxidation. The indole structure influences the antioxidant efficacy in biological systems [Citation12].
A series of 2-phenylindole (2PI) 1a–g and 2-phenylindole-3-aldehyde (2PIA) 2a–f derivatives were prepared in order to evaluate their in vitro antioxidant properties by determining superoxide anion formation, DPPH free radical scavenging activity and lipid peroxidation (LP) on rat liver homogenate. Also possible structure–activity relationships of the compounds were discussed by comparing with melatonin, alpha-tocopherol, BHT and previously published antioxidant indole derivatives.
Materials and methods
Uncorrected melting points were determined with a Büchi SMP-20 apparatus. The 1H NMR spectra were measured with a Varian 400 MHz using TMS internal standart and DMSO-d6. ESI Mass spectra were determined on a Waters micromass ZQ. Chromatography was carried out using Merck silica gel 60 (230–400 mesh ASTM). Xanthine, xanthine oxidase, cytochrome c, 2,2,diphenyl-1-picrylhydrazyl (DPPH), butylated hydroxytoluene, α-tocopherol and thiobarbituric acid (TBA) were purchased from Sigma Chemical Co. (St Louis, MO, USA). The chemical reagents used in synthesis were purchased from Sigma (Germany) and Aldrich (USA).
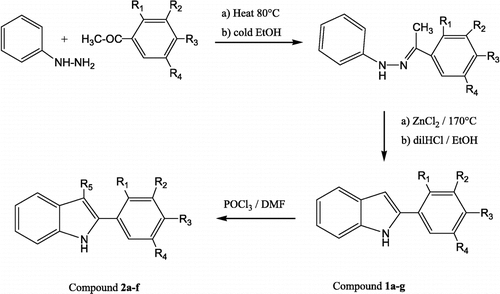
For the preparation of the 2PI derivatives, the Fischer indole synthesis [Citation13] was used. Phenyl hydrazine and acetophenone derivatives were reacted to give hydrazones. Intramolecular cyclization of the hydrazones was performed by ZnCl2. The 2PI 1a–g then were treated with POCl3 and DMF to give 2-PIA 2a–f [Citation14] ().
General procedure for the preparation of the 2-phenyl indole-3-aldehydes (2a–f)
POCl3 (6 mmol) was added dropwise to DMF (21 mmol) at − 15°C under nitrogen. After addition of 2PI derivative (6 mmol), the mixture was stirred at r.t for 0.5 h. The solution was then poured into ice and NaOH (40%) was added until the mixture became basic. The crystals were filtered and recrystallised from ethanol. The synthesis and physical data for compounds 2d, 2e and 2f that were not stated in the literature are given below.
Compound 2d
yield 88%, m.p: 256–258°C. 1H NMR (d6-DMSO) δ: (7.21, 2H, m; 7.50, 1H, s; 7.80, 2H, dd; 8.08, 1H, s; 8.18, 1H, d: aromatic-H), 9.95 (1H, s, CHO); MS: m/z (%) 292 (63.38) (M+2), 290 (98.16) (M(+, 289 (16.14), 264 (28.38), 262 (42.65), 227 (45.19), 165 (28.14).
Compound 2e
yield 61%, m.p: 149–250°C. 1H NMR (d6-DMSO) δ: (7.12, 1H, d; 7.23, 2H, m; 7.47, 1H, d; 7.63, 2H, d; 7.90, 1H, s; 8.18, 1H, d: aromatic-H), 9.97 (1H, s, CHO). MS: m/z (%) 237 (6.29) (M+1), 205 (12.16), 169 (18.10), 147 (100.00), 137 (12.15), 119 (39.08).
Compound 2f
yield 79%, m.p: 255–256°C. 1H NMR (d6-DMSO) δ: (7.44, 2H, m; 7.65, 1H, m; 8.03, 1H, d; 8.14, 1H, d; 8.20, 1H, d; 8.54, 1H, s: aromatic-H), 10.01 (1H, s, CHO); MS: m/z (%) 302 (22.27) (M+2), 301 (72.26) (M+1(, 231 (36.35), 182 (28.13), 158 (100.00), 137 (29.19), 129 (68.59).
Antioxidant activity studies
Superoxide radical scavenging activity
The ability of 2PI and 2PIA derivatives to scavenge superoxide anion formation was determined spectrophotometrically on the basis of inhibition of cytochrome c reduction according to the modified method of McCord et al. [Citation15]. Superoxide anion was generated in the xanthine/xanthine oxidase system. The activity of the enzyme was detected by its ability to reduce cytochrome c which causes an increase in absorbance at 550 nm. The incubation mixture (1 ml, total volume) consisted of phosphate buffer (pH 7.8, 0.05M), xanthine oxidase (0.32 Units/ml), xanthine (50 μM), cytochrome c (60 mM) and different concentrations of 2PI and 2PIA derivatives in 100 μl. The reaction was started by the addition of xanthine oxidase to this mixture. The absorbance was measured spectrophotometrically at 550 nm for 3 min for cytochrome c reduction. Each experiment was in triplicate, and the results are expressed as a percentage of the control.
DPPH free radical scavenging activity
The free radical scavenging activities of 2PI and 2PIA derivatives were tested by their ability to bleach the stable radical 2,2,diphenyl-1-picrylhydrazyl (DPPH) [Citation16]. This assay has often been used to estimate the anti-radical activity of antioxidants. When DPPH√ reacts with an antioxidant compound, which can donate hydrogen, it is reduced. The changes in colour (from violet to yellow) were measured at 517 nm on a visible spectrophotometer. The reaction mixture contained 100 μM DPPH in methanol and different concentrations of synthesized compounds. Absorbance at 517 nm was determined after 30 min at room temperature and the scavenging activity were calculated as a percentage of the radical reduction. Each experiment was performed in triplicate and BHT was used as a reference compound. The radical scavenging activity was obtained from the equation:
where: ODcontrol:absorption of the blank sample; OD sample:absorption of tested extract solution.
Assay of lipid peroxidation
The effect of the 2PI and 2PIA derivatives on rat liver homogenate induced with FeCl2-ascorbic acid was determined. LP was examined by the method of Mihara et al. [Citation17]. Procedures involving the animals and their care conformed to institutional guidelines, in compliance with National and International laws and guidelines for the use of animals in biomedical research. Animals were starved for 24 h. prior to sacrifice and then sacrificed by decapitation under anaesthesia. The livers were immediately removed, washed in ice-cold distilled water then immediately homogenized with a Teflon homogenizer. LP was measured spectrophotometrically by estimation of thiobarbituric acid reactants (TBARS). Amounts of TBARS were expressed in terms of nmol malondialdehyde (MDA/g tissue). A typical optimized assay mixture contained 0.5 ml of liver homogenate, 0.1 ml of Tris–HCl buffer (pH 7.2), 0.05 ml of 0.1 mM ascorbic acid, 0.05 ml of 4 mM FeCl2 and 0.05 ml of various concentrations of indole derivatives, melatonin or α-tocopherol, and was incubated for 1 h at 37°C. After incubation, 3.0 ml of H3PO4 and 1 ml of 0.6% TBA were added and the mixture shaken vigorously. The mixture was boiled for 30 min. After cooling the mixture to room temperature, n-butanol was added and mixed vigorously. The n-butanol phase was separated at 3000 rpm for 10 min. The absorbance of the supernatant was read at 532 nm against a blank, which contained all reagents except the liver homogenate.
Results
In the present study, we have investigated the antioxidant capacity of the synthesized 2PI and 2PIA derivatives in three different in vitro assays; superoxide anion DPPH stable radical scavenging activity and LP ().
Table I. Effects of the 2-phenylindole derivatives on LP levels and scavenging activity of superoxide and DPPH radicala.
The inhibitory effects of the compounds on LP levels were determined by measuring the formation of 2-thiobarbituric acid reactive substances. All the compounds were evaluated against melatonin which was chosen as a reference compound because of its high anti-oxidant capacity. As seen in , compounds 1a–f, 2e, 2f significantly inhibited (72–98%) LP at 10− 3 M and 10− 4 M concentrations. These values were similar to those observed with BHT and melatonin at 10− 3 M concentration. Additionally, compound 2b showed slight inhibition by about 12% at the 10− 3 M concentration. However the remainder of the compounds, especially 2PIA derivatives, had no effects on LP.
The superoxide anion radical scavenging activities of 2PI and 2PIA derivatives at different concentration were investigated as presented in . The results showed that compounds 1f and 1 g have a strong scavenger effect on superoxide anion at 10− 3 M concentration. Additionally, these compounds had comparable scavenger effects on superoxide radical as that of SOD (89% inhibitor at 45 IU). Compounds 1b, 1c, 1d and 1e also decreased the level of superoxide anion by about 16%, 25%, 51%, 51% at 10− 3 M concentration, respectively. Compounds 1a, 2a–f had no scavenging effect on superoxide anion at 10− 3 M and 10− 4 M concentrations. Compound 1e had a scavenging effect on superoxide anion at 10− 4 M concentration by about 31%. Compounds 1f and 1g have a promising antioxidant activity by scavenging superoxide radical. However, compounds 2a, 2b, 2c, 2d which are 2PIA derivatives increased the superoxide anion level at 10− 4 and 10− 3 M concentrations.
The scavenging effect of 2PI and 2PIA derivatives on the DPPH radical was also examined. As seen in , compounds 1e and 1f had the highest DPPH scavenger activity at 10− 3 M and 10− 4 M concentrations (88–96%). These compounds were found to be as effective as BHT, a well known antioxidant used as positive control. Compound 1g showed a slight scavenger effect on DPPH by about 38%. Compounds 1f and 1g scavenged superoxide and DPPH radical and inhibited lipid peroxidation at 10− 3 M concentrations. The remainder of the compounds showed different patterns of effect on these parameters. The inhibitory effects of compounds were noted at the level of superoxide radical but not DPPH radical. Such contradictory results have also been found in previous studies [Citation18,Citation19]. Therefore, the observation of different effects of synthetic compounds on superoxide anion and DPPH radical scavenger activity was not surprising since the mechanism of production of oxidative stress by these methods were different Citation20-22.
Discussion
Due to its free radical scavenging and antioxidant properties, all the melatonin-related compounds are under investigation to discover the ideal antioxidant compound showing the highest antioxidant activity with the lowest side effects. Indole derivatives have a heterocyclic aromatic ring structure with high resonance stability and several different substituents on the ring, and this led the researchers to suspect antioxidant activity in these compounds on theoretical founds ().
The mechanism of melatonin's interaction with reactive species probably involves donation of an electron to form the melatoninyl cation radical or through a radical addition at the site C3. Other possibilities include hydrogen donation from the nitrogen atom or substitution at position C2, C4 and C7 and nitrosation [Citation23]. The mechanisms by which melatonin protects against lipid peroxidation most likely involve direct or indirect antioxidant and free radical scavenging activities of this indoleamine The most important among them seems to be the ability of melatonin to scavenge such toxic species, like ONOO− , which is sufficiently reactive to initiate the breakdown of lipids, and NO√ [Citation24,Citation25]. 2PI derivatives have redox properties because of the presence of an electron-rich aromatic ring system which allows the indoleamine to easily function as an electron donor. However, the mechanisms by which the indole ring interacts with free radicals is still only partially understood. For 2PI derivatives the possible antioxidant mechanism may be toward carbon-centered radicals as shown in that is adopted from Antosiewicz et al. [Citation12]. Compounds bearing electron-withdrawing groups such as F, Cl, NO2 (1b, 1c, 1d, 1f) had the highest reduction in LP values since these groups are inductive electron withdrawing due to their electronegativity and they help indole ring to easily interacts with free radicals.
Figure 3 Proposed antioxidant mechanism of 2PI derivatives (adapted from Antosiewicz et al., Free Rad Biol Med 1997;22:249–55).
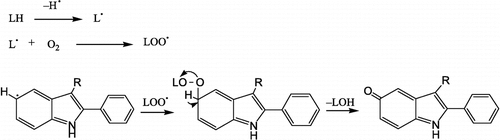
All of the examined compounds, and melatonin, had no significant inhibitory effect on superoxide anion formation except compounds 1f and 1g. This shows that melatonin and 2PI derivatives act as potent hydroxyl radical scavengers in vitro. The different effects of the compounds on LP and superoxide anion radical formation is not surprising, since the mechanism of production of oxidative stress (or reactive oxygen species) in these methods are different Citation20-22. The results suggest that the antioxidant properties of 2PI derivatives in part, may involve a direct effect on the scavenging of hydroxyl radicals.
We suggest that hydroxyl radical can react with 2PI derivatives by abstraction of an electron. The resulting indolyl cation radical may combines with an , a reaction by which the electrons are paired and electrical charges are neutralized, so that the radical reaction chain may be terminated. The occurrence of carbon centered radicals may explain the possible antioxidant mechanism of indole derivatives [Citation26] that was proved by electroanalytical studies in our laboratory [Citation27]. There was no significant activity pattern obtained from the LP experiments. The scavenging activity of the compounds against hydroxyl radicals was greater than against superoxide anion radicals.
During the oxidation of indolic compounds, it was observed that an electron was removed from the nitrogen atom (–NH) of the pyrrole ring, generating a radical cation Citation28-30. Due to this capacity of the pyrrole ring, the antioxidant activity of the 3-substituted indolic compounds was affected by both functional groups present in the molecule [Citation8]. Thus, the carbonyl group bound to C3 of the indolic ring might be responsible for the absence of antioxidant activity being found for 2-PIA (except for 2e–f) derivatives, the only compounds that do not show significant antioxidant activity.
The in vitro experimental findings for the indole derivatives show that there are many derivatives that had good or in some cases better antioxidant properties than melatonin. It should be considered to synthesise more derivatives to discover their free radical scavenging activities and biologically evaluate their synthesised compounds. Further investigations should be done to reveal the exact mechanism of action and cytotoxic effects of new indole derivatives.
Acknowledgements
This work was supported by Ankara University Research and Development Grant 99-03-00-03.
References
- Karbownik M, Lewinski A. The role of oxidative stress in physiological and pathological processes in the thyroid gland; possible involvement in pineal-thyroid interactions. Neuro Endocrinol Lett 2003; 24: 293–303
- Dröge W. Free radicals in the physiological control of cell function. Physiol Rev 2002; 82: 47–95
- Reiter RJ, Acuna CD, Tan DX. Free radical-mediated molecular damage. Mechanisms for the protective actions of melatonin in the central nervous system. Ann N Y Acad Sci USA 2001; 939: 200–215
- Reiter RJ, Tan DX, Burkhardt S. Reactive oxygen and nitrogen species and cellular and organismal decline: Amelioration with melatonin. Mech Ageing Dev 2002; 123: 1007–1019
- Hardeland R, Zsizsik BK, Poeggeler B, Fuhrberg B, Holst S, Coto-Montes A. Indole-3-pyruvic and -propionic acids, kynurenic acid, and related metabolites as luminophores and free-radical scavengers. Adv Exp Med Biol 1999; 467: 389–395
- Bendheim PE, Poeggeler B, Neria E, Ziv V, Pappolla M, Chain DG. Development of indole-3-propionic acid (OXIGON) for Alzheimer's disease. J Mol Neurosci 2002; 19: 213–217
- Andreadou I, Tasouli A, Bofilis E, Chrysselis M, Rekka E, Tsantili-Kakoulidou A, Iliodromitis E, Siatra T, Kremastinos DT. Antioxidant activity of novel indole derivatives and protection of the myocardial damage in rabbits. Chem Pharm Bull 2002; 50: 165–168
- Cano A, Alcaraz O, Arnao MB. Free radical-scavenging activity of indolic compounds in aqueous and ethanolic media. Anal Bioanal Chem 2003; 376: 33–37
- Dong Y, Venkatchalam TK, Narla RK, Trieu VN, Sudbeck EA, Uckun F. Antioxidant function of phenethyl-5-bromo-pyridyl thiourea compounds with potent anti-HIV activity. Free Rad Biol Med 2000; 10: 87–90
- Olgen S, Coban T. Synthesis and antioxidant properties of novel N-substituted indole-2-carboxamide and indole-3-acetamide derivatives. Arch Pharm Pharm Med Chem 2002; 7: 331–338
- Olgen S, Coban T. Antioxidant evaluations of novel N-H and N-substituted indole esters. Biol Pharm Bull 2003; 26: 736–738
- Antosiewicz J, Damiani E, Jassem W, Wozniak M, Orena M, Greci L. Influence of structure on the antioxidant activity of indolinic nitroxide radicals. Free Rad Biol Med 1997; 22: 249–255
- Shiriner RL, Ashies WC, Welch E. Synthesis of 2-phenylindole. Org Synthesis 1955; 3: 98–100
- Shabica AC, Howe EE, Zeigler JB, Tishler M. Improved synthesis of indole-3-aldehyde. J Am Chem Soc 1946; 68: 1156
- McCord J, Fridowich I. An enzymic function for erythrocuprein. J Biol Chem 1969; 243: 6049–6055
- Blois MS. Antioxidant determination by the use of stable free radical. Nature 1958; 181: 1199–1200
- Mihara M, Uchiyama M, Fukuzawa K. Thiobarbituric acid value of rat as a parameter of lipid peroxidation in aging, CCl4 intoxication, and vitamin E deficiency. Biochem Med 1980; 23: 303–311
- Dundar B, Bozdağ-Dündar O, Can-Eke B, Çoban T, Iscan M, Büyükbingöl E. Synthesis and antioxidative properties of novel thiazolidinedione/imidazolidinedione compounds as retinoids. Die Pharmazie 2002; 57: 438–441
- Ohsugi M, Fan W, Hase K, Xiong Q, Tezuka Y, Komatsu K, Namba T, Saitoh T, Tazawa K, Kadota S. Active-oxygen scavenging activity of traditional nourishing-tonic herbal medicines and active constituents of Rhodiola sacra. J Ethnopharmacol 1999; 67: 111–119
- Kombrust DJ, Mavis RD. Microsomal lipid peroxidation. II. Stimulation by carbon tetrachloride. Mol Pharmacol 1980; 17: 408–414
- Parke DV, Ionnides C, Lewis DFV. The 1990 pharmaceutical manufacturers association of Canada keynote lecture. The role of the cytochromes P450 in the detoxication and activation of drugs and other chemicals. Can J Physiol Pharmacol 1991; 69: 537–549
- Dix TA, Aikens J. Mechanisms and biological relevance of lipid peroxidation initiation. Chem Res Toxicol 1993; 6: 2–18
- Tan DX, Reiter RJ, Manchester LC, Yan MT, El-Sawi M, Sainz RM, Mayo JC, Kohen R, Allegra M, Hardeland R. Chemical and physical properties and potential mechanisms: Melatonin as a broad spectrum antioxidant and free radical scavenger. Curr Top Med Chem 2002; 2: 181–197
- Reiter RJ, Tan D-X, Qi W, Manchester LC, Karbownik MB, Calvo JR. Pharmacology and physiology of melatonin in the reduction of oxidative stress in vivo. Biol Signals Recept 2000; 9: 160–171
- Tan DX, Manchester LC, Hardeland R, Lopez-Burillo S, Mayo JC, Sainz RM, Reiter RJ. Melatonin: A hormone, a tissue factor, an autocoid, a paracoid, and an antioxidant vitamin. J Pineal Res 2003; 34: 75–78
- Electroanalytical techniques in clinical chemistry and laboratory medicine, J Wang. VCH Publisher Inc., New York 1988
- Suzen S, Demircigil BT, Buyukbingol E, Ozkan SA. Electroanalytical evaluation and determination of 5-(3-indolyl)-2-thiohydantoin derivatives by voltammetric studies: Possible relevance to in vitro metabolism. New J Chem 2003; 27: 1007–1011
- Bromme HJ, Morke W, Peschke D, Ebelt H, Peschke D. Scavenging effect of melatonin on hydroxyl radicals generated by alloxan. J Pineal Res 2000; 29: 201–208
- Goyal RN, Kumar N, Singhal NK. Oxidation chemistry and biochemistry of indole and effect of its oxidation product in albino mice. Biolectrochem Bioenerg 1998; 45: 47–53
- Suzen S, Ateş-alagöz Z, Demircigil T, Ozkan SA. Synthesis and analytical evaluation by voltammetric studies of some new indole-3-propionamide derivatives. Il Farmaco 2001; 56: 835–840