Abstract
Complexes of Mn(II), Co(II), Ni(II), Cu(II), Zn(II) and Cd(II) with the Schiff bases salicylidene-o-aminothiophenol (H2L) and thiophene-o-carboxaldeneaniline (SB) have been synthesized and characterized by elemental analyses, magnetic measurements, thermogravimetric analyses as well as infrared spectra and reflectance spectra. The nature of the bonding has been discussed on the basis of IR spectral data. Magnetic susceptibility measurements and electronic spectral data suggest a six-coordinated octahedral structure for these complexes. The complexes of Mn(II), Co(II), Ni(II), Cu(II) are paramagnetic, while Zn(II) and Cd(II) are diamagnetic in nature. The complexes were tested for their antimicrobial activities against Salmonella typhi, Escherichia coli and Serratia marcescens using the “Disc Diffusion Method”. The results are compared with the standard drug (tetracycline) and show moderate activity.
Introduction
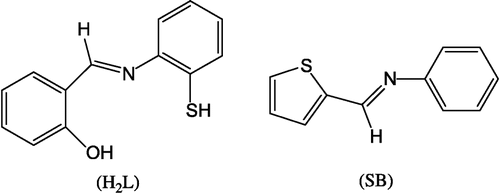
Schiff bases and their metal complexes play a key role in our understanding of the coordination chemistry of transition metal ions. They may serve as a model for biologically important species and find applications in biomimetric catalytic reactions [Citation1]. The compounds containing N, O, S, and N, S donor atoms are important owing to their significant antifungal, antibacterial and anticancer activity [Citation2]. The combination of distinctly different metal ion binding sites within one ligand can lead to materials with interesting new properties, [Citation3,Citation4] e.g. specific sensors, molecular wires, magnetic and optical devices. The activity of some drugs is increased when administered as metal complexes than as the free parent compounds [Citation5]. Zinc and copper possesses antiulcer activity and their complexes with amoxicillin, cephalexin and ciprofloxacin have anti-inflammatory and antibacterial activity Citation6-8. Several metal complexes of some Schiff bases of heterocyclic derivatives e.g. coumarins and sulfonamides have creditable antifungal, antibacterial and cytotoxic activity Citation9-11. As a continuation of our earlier work Citation12-14, in this article we report the preparation, structural features and biological screening studies of some new complexes of Mn(II), Co(II), Ni(II), Cu(II), Zn(II), and Cd(II) with tridentate and bidentate Schiff bases ().
Experimental
Methods and materials
All the chemicals used were of analytical grade. Metal chlorides and salicylaldehyde were purchased from the E. Merk (India) Ltd, Mumbai. Thiophene-o-carboxaldehyde and o-aminothiophenol were purchased from Eastgate, White Lund, Morecambe, U.K. The organic solvents were purified by recommended methods [Citation15].
Preparation of the Schiff bases
Thiophene-o-carboxaldeneaniline (SB)
An ethanolic solution (100 mL) of thiophene-o-carboxaldehyde (10 mmol, 1.12 g) and an ethanolic solution (100 mL) of aniline (10 mmol, 0.09 g) in a molar ratio of 1:1 were mixed with constant stirring and refluxing for 6 h. The solution was cooled overnight at room temperature and the formed yellow crystals were collected by filtration and dried in air. Yield: 0.73 g (60%), M.p.: >360°C.
Salicylidene-o-aminothiophenol (H2L)
The salicylidene-o-aminothiophenol (H2L) was prepared by a condensation reaction between salicylaldehyde (10 mmol, 1.22 g) and o-aminothiophenol (10 mmol, 1.25 g) in ethanol (100 mL). The obtained compound was filtered and recrystallized from dilute acetic acid. The structure was confirmed by elemental analyses and IR spectra (see later). Yield: 1.73 g (70%), M.p.: 128°C.
Synthesis of the complexes
The complexes were prepared by mixing an aqueous (100 mL) solution of metal chloride (10 mmol) with hot methanolic (100 mL) solutions of salicylidene-o-aminothiophenol (10 mmol, 2.29 g) and thiophene-o-carboxaldeneaniline (10 mmol, 1.87 g) in 1:1:1 molar ratio and heating in a water bath for 2 h at 50°C. The mixtures were kept overnight at room temperature and the obtained crystals removed by filtration, washed with water, ethanol and dried in air. It was not possible to grow good crystals of the metal complexes, due to their insolubility in common organic solvents, for X-ray diffraction studies. Elemental analyses, melting points and other data are presented in .
Table I. Analytical data for the compoundsa.
Physical measurement
Magnetic moments were obtained on a model 7304, vibrating sample magnetometer (Lake Shore, U.S.A.) which reports the total magnetic moment, m, of a sample in emu which can be converted to susceptibility units since 1 emu = 1 gauss·cm3. The susceptibility of a sample has units of volume and is defined for paramagnetic material by the equation:
The gram susceptibility, χg was calculated using the expression, χg = χ(cm3)/density, which was multiplied by the molecular weight of the sample to obtained the molar susceptibility, χM. A correction was applied for the diamagnetism of the ligands to get the corrected molar susceptibility χM′. The effective magnetic moment was calculated from the expression,
Infrared spectra (KBr pellets) were recorded on a FT-IR Nicolet 400D spectrophotometer. C, H, N and S were analyzed with a model 240 Perkin-Elmer elemental analyzer. The metal contents of the mixed-ligand complexes were analyzed by EDTA titration [Citation16] after decomposing the organic matter with a mixture of perchloric, sulfuric and nitric acids (1:1.5:2.5). Themogravimetric analyses were obtained by a model 5000\2960 SDTA, TA Instruments, U.S.A. at a heating rate of 10°C/min in a N2 atmosphere over the temperature range of 50–800°C. The reflectance spectra of the complexes were recorded in the range 1700–350 nm (as MgO discs) on a Beckman DK-2A spectrophotometer.
Biological evaluation
Preparation of stock solution
A stock solution of 250 μg/ml was made by dissolving 25 mg of each compound in dimethyl sulfoxide (100 mL).
Determination of MIC value
The biological screening of activity was estimated by the minimal inhibitory concentration (M.I.C.). The MIC was determined using the method of progressive double dilution in liquid media containing 10 μL − 50 μL of the compound being tested.
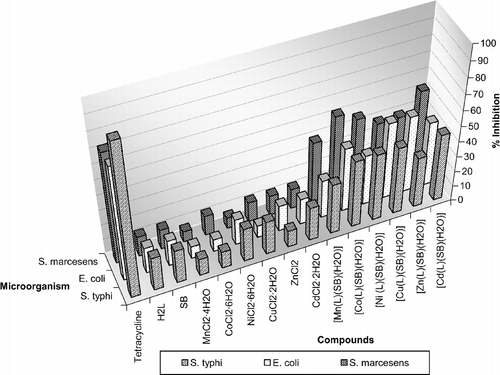
All the compounds were more effective with MIC value at 20 μL ≈ 50 μg/ml. The biological screening of all the experiment has been carried out at MIC value (50 μg/ml) and results are expressed as zone of inhibition and percentage inhibition.
The antibacterial activity of the tetracycline, ligands, metal salts, and its complexes were tested against various gram-negative bacterial cultures namely Salmonella typhi, Escherichia coli and Serratia marcescens using the Disc Diffusion Method [Citation35].
Preparation of discs
The compound (20 μL) was applied to a paper disc, (filter paper Whatmann No. 4, discs 6 mm diameter) using a micropipette. The discs were left in an incubator for 48 h at 37°C and then applied to the bacteria grown on agar plates.
Preparation of agar plates
Nutrient agar (37 g) was used for the growth of specific bacterial species. The agar plates were suspended in freshly distilled water (1 L), allowed to soak for 15 min and then boiled on a water bath until the agar was completely dissolved. The mixture was autoclaved for 15 min at 120°C and then poured into previously washed and sterilized petri discs and stored at 40°C for inoculation.
Procedure of inoculation
Inoculation was done with the help of a platinum wire loop, which was made red hot in a flame, cooled and then used for the application of the bacterial strains.
Application of discs
Sterilized forceps were used for the application of the paper disc on the previously inoculated agar plates and than the plates were incubated at 37°C for 24 h. The zone of inhibition was then measured (in mm) around the disc and the results are represented in and . Control experiments were performed where only an equivalent volume of solvent without added test compound was applied to the paper discs. All experiments were performed in triplicate and tetracycline was used as a standard drug. The complexes were soluble in DMSO so growth was compared with DMSO as the control and is expressed as zone of inhibition and percentage inhibition versus control.
Results and discussion
Chemistry
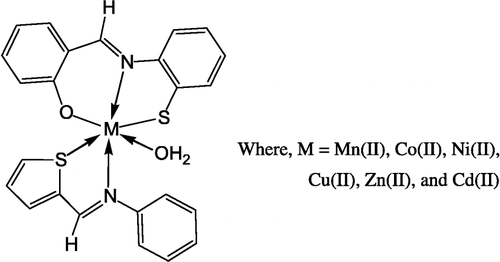
All the complexes are insoluble in water, methanol and dimethyl formamide, but are soluble in dimethyl sulfoxide. Elemental analysis of the ligands and complexes are in good agreement with theoretical expectation. They possess high melting points indicating that the complexes and Schiff bases are both stable in air. The general equations for the formation of the complex are shown below.
Infrared spectra
Infrared spectra of the Schiff bases show a new band at 1625 cm− 1, attributable to the formation of an imino group which was shifted to a lower frequency about ∼1610 cm− 1 in the complexes, indicating that the ν(C = N) group is taking part in coordination [Citation17]. This may be due to a decrease in the bond order of ν(C = N) which is further supported by the presence of a new band at ∼520 cm− 1 in the far-IR spectra of the complexes which may be assigned to the ν(M–N) mode [Citation18]. The OH stretching vibration, ν(OH), found as a medium band at 3200 cm− 1 in the Schiff base, disappeared in the spectra of the complexes indicating the participation of the OH group in chelate formation via proton displacement. This fact is further supported by the appearance of new band in all the complexes around ∼430 cm− 1, which may be assigned to the ν(M–O) mode [Citation19]. On the other hand, a band at ∼3400 cm− 1 is due to presence of water of coordination [Citation20] in the spectra of the complexes. It is difficult to obtain conclusions from the ν(OH) frequency of the OH group of the Schiff base, however, this OH vibration disappeared on drying the complexes at 200°C indicating the participation of the OH group in chelation. Another band at ∼850 cm− 1 and ∼715 cm− 1 was due to the rocking and wagging mode of the OH group [Citation21]. The ν(SH) band of salicylidene-o-aminothiophenol appears at 2600 cm− 1. The absence of a band around ∼2600 cm− 1 in the spectra of all the complexes indicates that the SH group loses the thiol proton to form a covalent bond between the sulfur and metal in all the complexes [Citation22]. The ν(C–S) Citation23-27 band of the thiophene-o-carboxaldeneaniline observed at 765 cm− 1, which is shifted to lower frequency, about 750 cm− 1, in the spectra of the complexes, indicates the participation of the sulfur atom of the thiophene ring in coordination. This fact is further supported by the appearance of a new band in all the complexes around ∼410–425 cm− 1, which may be assigned to the ν(M–S) mode [Citation28]. The sharp bands in the range 750–780 and 1525–1535 cm− 1 are due to the aromatic ν(C–H) and ν(C = C), respectively.
Magnetic moments and electronic spectra
In the high-spin, octahedrally coordinated Mn(II) complex, the lowest configuration (t2g)3(eg)2 gives rise to the ground state 6A1g. Since this is the only sextet level present, all of the absorption bands must therefore be spin-forbidden transitions. The electronic spectra of the Mn(II) complex exhibits three weak absorption bands for the transitions 6A1g → 4T1g (G) (∼15,000 cm− 1) (ν1), 6A1g → 4T2g (G) (∼20,000 cm− 1) (ν2) and 6A1g → 4Eg, 4A1g (G) (∼25,000 cm− 1) (ν3). The magnetic moment value of the Mn(II) complex is 6.04 B.M., suggesting an octahedral geometry [Citation29]. The Cu(II) complex exhibits a magnetic moment of 1.75 B.M. which is close to the spin-allowed value expected for a S = 1/2 system (1.73 B.M.) and may be indicative of an octahedral geometry around the Cu(II) ion. The Cu(II) complex displays a broad band at ∼15,000 cm− 1 due to 2Eg → 2T2g transition in an octahedral geometry [Citation30]. The Co(II) complex shows a magnetic moment value of 3.90 B.M. This high value of the magnetic moment and the stoichiometries suggest a coordination number of six for the central Co(II) ion and an octahedral geometry. The electronic spectra is also consistent with its octahedral environment around the Co(II) ion. The Co(II) complex exhibits three bands at ∼9,050 (ν1), ∼18,460 (ν2) and ∼19,000 (ν3) cm− 1, which may be assigned to the transitions 4T1g(F) → 4T2g(F), 4T1g(F) → 4A2g(F) and 4T1g(F) → 4T1g(P) expected for an octahedral structure [Citation31]. The electronic spectra of the Ni(II) complex exhibits absorption bands at ∼10,300 (ν1), ∼17,800 (ν2), and ∼25,000 (ν3) cm− 1 attributed to 3A2g(F) → 3T2g(F), 3A2g(F) → 3T1g(F) and 3A2g(F) → 3T1g(P) transitions, respectively, in an octahedral geometry [Citation32]. The Zn(II) and Cd(II) complexes are diamagnetic as expected for d10-systems. The ligand field splitting energy (10 Dq), interelectronic repulsion parameter (B) and nephelauxetic ratio (β) for the Co(II) and Ni(II) complexes have been calculated using the secular equations given by E.KÖnig [Citation33] and the results are represented in . The electronic spectra of the mixed-ligand complexes were recorded in the solid state; therefore, the ϵ values of all the complexes were not determined.
Table II. Electronic spectral data of the Co(II) and Ni(II) complexesa.
Thermal studies
The thermal stability of the complexes was investigated using thermogravimetric analyses. The TGA curves were obtained at a heating rate of 10°C/min in N2 atmosphere over the temperature range of 50–800°C. It has been observed that all the complexes show a loss in weight corresponding to one water molecule in the range of 150–180°C indicating that this water molecule is coordinated to the metal ion [Citation34]. In the temperature range 180–800°C the ligand molecules are lost. In all of the cases the final products are metal oxides. These results are in good accordance with the composition of the complexes. A suggested structure of the complex is shown in .
Biological Evaluation
The results show that the complexes are more bactericidal than their parent ligands () and metal salt against the same microorganisms and under identical experimental conditions ( and plot 1). The increase in bactericidal activity of the complexes may be due to the effect of the metal ion on the normal cellular process. A possible mode for increase in bactericidal activity may be considered in the light of Overton's concept [Citation36] and the Tweedy's chelation theory [Citation37]. According to Overton's concept of cell permeability, the lipid membrane that surrounds the cell favors the passage of only lipid-soluble materials so that liposolubility is an important factor which controls the antibacterial activity. On chelation, the polarity of the metal ion will be reduced to a greater extent due to the overlap of the ligand orbital and partial sharing of the positive charge of the metal ion with donor groups. Further, it increases the delocalization of π-electrons over the whole chelate ring and enhances the lipophilicity of the complexes. This increased lipophilicity enhances the penetration of the complexes into lipid membranes and blocks the metal binding sites in the enzymes of microorganisms. These complexes also disturb the respiration process of the cell and thus block the synthesis of proteins which restricts further growth of the organism. Furthermore, the mode of action of the compounds may involve the formation of a hydrogen bond through the azomethine group with the active centre of cell constituents, resulting in interference with the normal cellular process [Citation38]. Although there is a sufficient increase in the bactericidal activity of the complexes as compared to free ligands, metal salts and the control (DMSO), the complexes show moderate activities when compared to tetracycline the standard drug.
Table III. Antibacterial activities of the ligends, complexes and controla.
Acknowledgements
We gratefully make acknowledgment to Prof. R. M. Patel, Head, Department of Chemistry and Prof. M. Dutta, Head, Department of Biosciences, Sardar Patel University, Vallabh Vidyanagar, Gujarat, India for providing the necessary laboratory facilities.
References
- Ramadan AMJ. Inorg Biochem 1997; 65: 183
- Saxena A, Koacher JK, Tandon JP. Inorg Nucl Chem Lett 1981; 17: 229
- Vigato PA, Tamburini S. Coord Chem Rev 2004; 248: 717
- Hernandez-Molina R, Mederos A. Comprehensive Coordination Chemistry-II. Elsevier-Pergamon Press, Oxford-New York 2003; 1: 411
- Gerli A, Hogem KS, Marzilli LG. Inorg Chem 1991; 30: 4673
- Chohan ZH, Supuran CT, Scozzafava A. J Enz Inhib Med Chem 2005; 20: 303
- Mohan G, Nagar R, Agarwal SC, Mehta KA, Rao SC. J Enz Inhib Med Chem 2005; 20: 55
- Iqbal MS, Bukhari IH, Arif M. Appl Organometal Chem 2005; 19: 864
- Chohan ZH, Hassan M, Khan KM, Supuran CT. J Enz Inhib Med Chem 2005; 20: 183
- Rehman SU, Chohan ZH, Gulnaz F, Supuran CT. J Enz Inhib Med Chem 2005; 20: 333
- Chohan ZH, Pervez H, Rauf A, Khan KM, Supuran CT. J Enz Inhib Med Chem 2004; 19: 417
- Panchal PK, Parekh HM, Pansuriya PB, Patel MN. J Enz Inhib Med Chem, In Press
- Panchal PK, Patel MN. Synth React Inorg Met-Org Chem 2004; 34: 1277
- Parekh HM, Mehta SR, Patel MN. Russ J Inorg Chem 2006; 51: 67
- Vogel AI. Vogel's textbook of practical organic chemistry5th ed. Longman, London 1989
- Vogel AI. A textbook of quantitative inorganic analyses4th ed. ELBS and Longman, London 1978
- Burger K, Ruff I, Ruff F. J Inorg Nucl Chem 1965; 27: 179
- El-Asmu AA, Mabrouk HE, Al-Ansi TY, Amin RR, El-Shahat MF. Synth React Inorg Met-Org Chem 1993; 23: 1709
- Maurya RC, Mishru DD, Rao NS. Polyhedron 1992; 11: 2837
- Khalifa ME, Rakha TH, Bekheit MM. Synth React Inorg Met-Org Chem 1996; 26: 1149
- Nawar N, Hossny NM. Trans Met Chem 2000; 25: 1
- Singh NK, Kushawaha SK, Srivastava A, Sodhi A. Synth React Inorg Met-org Chem 2002; 32: 1758
- Moorlag C, Clot O, Wolf MO, Patrick BO. Chem Commn 2002; 3028
- Rincón L, Terra J, Guenzburger D, Sánchez-Delgado RA. Organometallics 1995; 14: 1292
- Harris S. Organometallics 1994; 13: 2628
- Chattopadhyay SK, Banerjee T, Roychoudhury P, Mak TCW, Ghosh S. Trans Met Chem 2002; 22: 216
- Chohan ZH, Farooq MA, Supuran CT. Metal-Based Drugs 2002; 7: 171
- Amin RR, Elgemeie GEH. Synth React Inorg Met-Org Chem 2001; 31: 431
- Figgis BN. The magnetic properties of the complex ions. Introduction to ligand fields1st ed. Wiley Interscience, New York 1966; 272–281
- El-Asmu AA, Al-Ansi TY, Amin RR, Mounir MM. Polyhedron 1990; 9: 2029
- Patel NH, Parekh HM, Patel MN. Trans Met Chem 2005; 30: 17
- Mostafa MM, Khattab MA, Ibrahim KM. Polyhedron 1983; 2: 583
- König E. The nephelauxetic effect. Structure and bonding. Springer Verlag, Berlin, Heidelberg, New York 1971; 9: 175–181
- Patel NH, Panchal PK, Patel MN. Synth React Inorg Met-Org Nano Met Chem 2005; 35: 107
- Stokes EJ, Ridgway GL. Clinical bacteriology5th ed. Edward Arnold Publisher, Baltimore, Maryland, USA 1980; 205
- Anjaneyula Y, Rao RP. Synth React Inorg Met-Org Chem 1986; 16: 257
- Tweedy BG. Phytopathology 1964; 55: 910
- Dharmaraj N, Viswanathamurthi P, Natarajan K. Trans Met Chem 2001; 26: 105