Abstract
In this work, we evaluated the antioxidant properties of the eight novel silybin analogues for their capacity to scavenge free radicals including superoxide anion radicals and 1,1-diphenyl-2-picrylhydrazyl (DPPH) radicals in vitro. Compound 7d demonstrated an excellent antioxidant effect in scavenging superoxide anion free radical with an IC50 value of 26.5 μM, while the IC50 of quercetin (the reference compound) was 38.1 μM. Compounds 7b, 7e, 7h showed certain scavenging activities for both types of free radicals.
Introduction
Oxidative stress (OS), which refers to the imbalance between cellular production of free radicals and the ability of cells to defend against them, links to a variety of diseases in nervous system, such as stroke (a disease in acute central nervous system injury) [Citation1], Parkinson's disease [Citation2] and Alzheimer's disease (AD) [Citation3]. It also results in an enormous variety of pathological processes, including cardiac disease, autoimmune rheumatic disease, cancer, viral disease (such as AIDS) and aging Citation4-7. Therefore, the discovery of new antioxidants has become an efficient way to treat various diseases related to free radicals.
Superoxide anion radical () is one kind of reactive oxygen species, which may be generated by a biological process in vivo such as hypoxanthine acted on by xanthine oxidase and is extremely active due to its unpaired electron. It can quickly capture electron from other molecules and lead to the generation of hydroxide radical, the most harmful free radical so far known. Furthermore, 1,1-diphenyl-2-picrylhydrazyl (DPPH), another kind of stable free radical existing in vitro may be utilized to screen the aryl free radical scavenging activity. Therefore, it is necessary to find superoxide anion scavengers and DPPH inhibitors as new antioxidants.
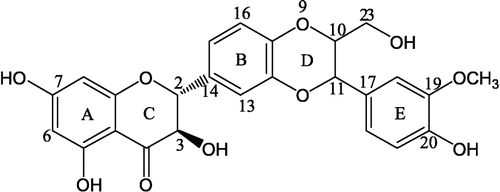
A well-known flavonolignan silybin (see ) was reported to have a variety of biological activities such as hepato-protection [Citation8], antioxidant Citation9-11 and antibacterial [Citation12]. However, its bioavailability and therapeutic efficiency are limited by its low water solubility. To explore new concepts of structure-activity relationship (SAR) of this type of flavonolignan derivatives, eight silybin analogues with a modified B ring and/or E ring of the molecule were designed and synthesized and their free radical scavenging activities on superoxide anion and DPPH free radicals determined. The present study might be beneficial for an understanding of the mechanisms of the antioxidant activity of silybin analogues and lead to a further optimizing of their application.
Materials and methods
IR spectra were recorded on a Bruker Vector-22 spectrometer as kBr discs. 1H NMR spectra were recorded on a Bruker AC2000 at 400 MHz and chemical shifts were expressed as δ (ppm). ESI were determined on a HP-5898B mass spectrometer. Column chromatography was performed on Qingdao silica gel (200–300 mesh), and thin layer chromatography (TLC) with Qingdao GF254 silica gel. Phenazine methosulfate (PMS, Sigma), nitroblue tetrazolium (NBT, Sigma), NADH (disodium, reduced, Amresco), Tris base (Gibco), quercetin (prepared in our laboratory), multi-well plates (Greiner) and multi-well plates reader (Bio-Tek Instruments, USA) were used in the experiments.
Chemistry
Synthesis of the target compounds is outlined in . Condensation of 2,4,6-tri(methoxymethoxy)acetophenone 1 with substituted benzaldehydes 2 in a methanolic solution of KOH (15.0 eq) gave the chalcones 3 in 75–80% yields. Epoxidation of 3 with H2O2 in the presence of 2 N NaOH in methanol at room temperature afforded the corresponding chalcone epoxides 4 in yields of 80 − 85%. Treatment of 4 with hydrochloric acid in methanol and THF afforded the ( ± )-dihydroflavonols 5 as white solids in 55 − 65% yields [Citation13]. Coupling of 5 with substituted cinnamyl alcohols in the presence of Ag2CO3 in dry benzene and acetone at about 60°C gave the target diastereoisomeric compounds ( ± )-7 as main products in yields of 20 − 45% [Citation14]. The relative configurations of C-2, C-3 and C-10, C-11 in 7 were both trans, based on the diagnostic 1H NMR doublets of H-2 (ca. J = 12.0 Hz) and H-11 (J = 8.0 Hz) (see ). Some physico-chemical properties and spectral data of the compounds are given in .
Figure 2 Synthetic route for the preparation of compounds 5 and 7. Conditions and reagents a: KOH, MeOH, r.t. b: 2 N NaOH, H2O2, MeOH, r.t. c: HCl, MeOH, 55°C d: Ag2CO3, dry benzene–acetone, 60°C.
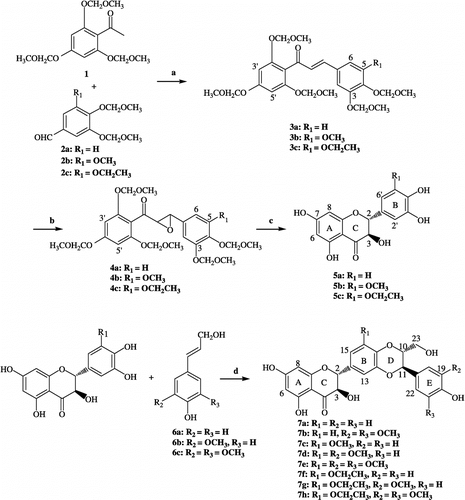
Table I. Physical and spectral data of compounds 5 and 7.
General procedure for the preparation of (±)-2,3-dihydro-2-(3,4-dihydroxy- 5-substituted)-3,5,7-trihydroxy-4H-1-benzopyran-4-one (5)
A mixture of MeOH (5 ml) and conc. HCl (1 ml) was added dropwise to a solution of 4 (1 mmol), in MeOH (4 ml) and THF (1 ml) and the reaction mixture was heated at 55°C for 15 min. After cooling, the mixture was poured into ice-water and extracted with AcOEt. The extract was washed with water, dried over Na2SO4 and evaporated. The residual oil was purified by column chromatography on silica gel using CHCl3–MeOH (10:1) as eluent to afford ( ± )-5 as a solid.
General procedure for the preparation of (±)-2-[2,3-dihydro-3-(4-hydroxy-3,5-substituted-phenyl)-2-(hydroxymethyl)-1,4-benzodioxin-6-yl]-2,3-dihydro-3,5,7-4H-1-benzopyran-4-one (7)
A mixture of 1.48 mmol of 5, 1.48 mmol of alcohol 6, 60 ml of benzene and 20 ml of acetone was placed in oil bath at 60°C and the solution was stirred for 10 min. Ag2CO3 0.408 g (1.48 mmol) was then added and the reaction mixture was stirred vigorously for 20 h. The reaction was then worked up and the solvent removed by rotary evaporation to leave a yellow powder which was subjected to column chromatography over silica gel using CHCl3–AcOEt–HCO2H (25:2:0.25) as eluent to give ( ± )-7 as a pale yellow powder.
Antioxidant activity studies
Superoxide anion scavenging activity
The superoxide anion scavenging activity of the compounds was assayed spectrophotometrically as reported with a slight modification [Citation14]. Superoxide anion radicals were generated in a non-enzymic phenazine methosulfate-NADH system by following the reduction of nitroblue tetrazolium. In this assay, the superoxide anion radicals were measured in plates which contained 78 μM of NADH, 50 μM of nitroblue tetrazolium, 5 μM of phenazine methosulfate and the test samples at different concentrations in 16 mM Tris–HCl buffer at pH 8.0. The color was monitored at 560 nm after 5 min incubation at room temperature. The blank samples did not contain phenazine methosulphate. Quercetin was used as a positive reference compound.
DPPH free radical scavenging activity
The quenching of free radicals by the compounds was assayed spectrophotometrically at 517 nm against the absorbance of the stable free DPPH radical [Citation15]. The free radical scavenging efficiency of the compounds was determined by decoloration of the DPPH radical. In brief, reaction mixtures contained various concentrations of the test compounds which were dissolved in DMSO and DPPH (0.4 mg/ml) dissolved in methanol. The methanolic solution of DPPH served as a control and quercetin was used as a reference free radical scavenger. The absorbance was measured at 517 nm after the mixture was incubated at 37°C for 30 min.
Results and discussion
Chemistry
All spectral data were in accordance with the assigned structures. Yields were not optimized in the reactions. The Ag2CO3-initiated reactions of 6a and 6b with 5a yielded 7a and 7b both as a mixture of silybin and isosilybin type products, although the normal silybin type product was the major product. Interestingly, the synthesis of 7c–7h, unlike those of 7a and 7b, did not provide the isosilybin type by-products during preparation. This regiospecific selectivity might be attributable to the presence of alkoxy groups on the B ring in the case of these compounds.
Antioxidant bioactivity
The superoxide anion radical scavenging activity was measured by the inhibition of NBT reduction and the results are summarized in . As shown in , the order of the antioxidant effect is 7d>7e ≈ 7h>7b>7g ≈ 7f>7c>7a. It is observed that compound 7d possessed the best superoxide anion radical scavenging activity in this synthetic series with an IC50 value of 26.5 μM. The reference compound, quercetin exhibited a weaker effect than that of 7d with IC50 value of 38.1 μM.
Table II. The scavenging effects of synthetic compounds 7a–7h on superoxide anion and DPPH radicals.
The effects of alkoxy groups substituted at the C-16 position of silybin were studied. It can be seen that besides 7d, compound 7e and 7h also exhibited moderate scavenging activities on superoxide anion radicals with IC50 values of 44.4 and 44.3 μM, respectively. On the contrary, compounds 7a and 7b with R1 = H showed relatively weak scavenging activities. This evidence suggests that introducing an alkoxy group at the C-16 position of silybin benefits the superoxide anion radical scavenging activity, probably due to the electron-donating properties of these groups and the subsequent modulation of the electron density. The methoxy-substituted compounds exhibited better scavenging activity than the ethoxy-substituted analogues, indicating that chain length might also play an important role.
Comparing the radical scavenging activities of 7b, 7d and 7h with a methoxy substituent on the E ring with those of 7a, 7c and 7f, it can be deduced that one or two methoxy groups attached on the E ring improves the antioxidant activity. This result is in agreement with previous findings that the alkoxy moiety substituted on the phenol ring can increase the efficacy of phenolic antioxidants [Citation16]. The enhanced activities could be attributed to the electron-donating capacity of the methoxy groups.
In addition, the DPPH radical scavenging activity of the synthesized compounds was also evaluated and the results are incorporated into . It was found that compounds 7b, 7h and 7e have a similar inhibitory activity which is higher than for other synthesized silybin analogues. It was indicated that the possession of two methoxy groups on the E ring was superior in activity to those only having one or none methoxy functionalities. Contrary to the activity of superoxide anion radical scavenging, the analogue with a H-atom on the B ring exhibited higher scavenging activity on DPPH among the three compounds 7b, 7h and 7e. To some extent, the activity for scavenging DPPH free radicals was related to the concentrations of test compounds so that in general, absorbance values decreased when the concentrations of test sample decreased. The inhibitory effects on DPPH radicals were less than 10% at concentrations of 4 and 0.4 μg/ml for most of the tested samples.
Moreover, it should be noticed that although compound 7d showed an excellent inhibitory effect on superoxide anion radical, it exhibited rather weak DPPH radical scavenging activity which suggested that there existed selectivity among the synthesized compounds towards different free radicals due to the different mechanisms involved.
In conclusion, the preliminary bio-evaluation results showed that introducing methoxy groups on the B and E rings of silybin may elevate the ability of the compounds to scavenge superoxide anion radicals, while introducing methoxy groups on the E ring improves the capacity of capturing DPPH radicals. This study afforded some useful information for further development of silybin analogues as novel antioxidants.
Acknowledgements
This work is by part financially supported by the Special 985 Foundation from Zhejiang University. The authors are grateful to Prof. Han Dong Sun and Dr. Françoise Guéritte for their encouragement on this research topic. One of the authors (Y. Zhao) would like to express his gratitude to the Chinese Ministry of Education as well as to Mr. Ka-Shing Li for the Cheung Kong Scholars Chair Professorship at Zhejiang University.
References
- Gilgun-Sherki Y, Rosenbaum Z, Melamed E, Offen D. Pharmacol Rev 2002; 54: 271–284
- Bennett JP. J Neurol Sci 1999; 170: 75–76
- Gibson GE, Huang HM. Neurobiol Aging 2005; 26: 575–578
- Lefer DJ, Granger DN. Am J Med 2000; 109: 315–323
- Sukkar SG, Rossi E. Autoimmun Rev 2004; 3: 199–206
- Toyokuni S, Okamoto K, Yodoi J, Hiai H. FEBS Lett 1995; 358: 1–3
- Stehbens WE. Exp Mol Pathol 2004; 77: 121–132
- Dvorak Z, Kosina P, Walterova D, Simanek V, Bachleda P, Ulrichova J. Toxicol Lett 2003; 137: 201–212
- Kosina P, Křen V, Gebhardt R, Grambal F, Ulrichová J, Walterová D. Phytother Res 2002; 16: S33–S39
- Varga Z, Czompa A, Kakuk G, Antus S. Phytother Res 2001; 15: 608–612
- Gažák R, Svobodová A, Psotová J, Sedmera P, Přikrylová V, Walterová D, Křen V. Bioorg Med Chem 2004; 12: 5677–5687
- Stermitz FR, Tawara-Matsuda J, Lorenz P, Mueller P, Zenewicz L, Kim L. J Nat Prod 2000; 63: 1146–1149
- Merlini L, Zanarotti A, Pelter A, Rochefort MP, Hänsel R. J Chem Soc, Perkin Trans 1980; 1: 775–778
- Robak J, Gryglewski RJ. Biochem Pharmacol 1988; 37: 837–841
- Tapia A, Rodriguez J, Theoduloz C, Lopez S, Feresin GE, Schmeda-Hirschmann G. J Ethnopharmacol 2004; 95: 155–161
- Fujisawa S, Atsumi T, Kadoma Y, Sakagami H. Toxicology 2002; 177: 39–54