Abstract
A series of naphthalene-chalcone derivatives (3a–3t) were prepared and evaluated as tubulin polymerisation inhibitor for the treatment of breast cancer. All compounds were evaluated for their antiproliferative activity against MCF-7 cell line. The most of compounds displayed potent antiproliferative activity. Among them, compound 3a displayed the most potent antiproliferative activity with an IC50 value of 1.42 ± 0.15 µM, as compared to cisplatin (IC50 = 15.24 ± 1.27 µM). Additionally, the promising compound 3a demonstrated relatively lower cytotoxicity on normal cell line (HEK293) compared to tumour cell line. Furthermore, compound 3a was found to induce significant cell cycle arrest at the G2/M phase and cell apoptosis. Compound 3a displayed potent tubulin polymerisation inhibitory activity with an IC50 value of 8.4 µM, which was slightly more active than the reference compound colchicine (IC50 = 10.6 µM). Molecular docking analysis suggested that 3a interact and bind at the colchicine binding site of the tubulin.
1. Introduction
Microtubules (composed of α-tubulin and β-tubulin heterodimers) are essential components of the cytoskeleton of eukaryotic cells, and they play important roles in a series of cellular processes such as determination and maintenance of cell shape, regulation of motility, organisation of intracellular architecture, secretion, cellular transport, and cell divisionCitation1,Citation2. Over the past two decades, microtubule has been recognised as an attractive target for developing chemotherapeutic drugs to treat cancer due to its important roles in the life cycle of the cellCitation3–6. Therefore, discovery and development of new tubulin polymerisation inhibitors has attracted great attention in recent years.
Chalcones are an important class of natural compounds belonging to the flavonoid family, which have two aromatic rings (A-ring and B-ring) are linked by a three carbon α, β-unsaturated carbonyl system (). Chalcones and derivatives received significant attention since their have diverse and interesting biological properties such as antifungal, anti-inflammatory, antituberculosis, antihyperglycemic, antimalarial, antileishmanial, and anticancerCitation7–9. More particularly, a number of synthetic and natural chalcones exhibited potent anticancer activity against many cancer cell lines via inhibition of tubulin polymerizationCitation10–12. Many previous studies have shown that the presence of a 2′-hydroxyl group on the A-ring is important for the antitumor activity of chalcone derivativesCitation13–15. For example, Shin et al. reported the synthesis of 2-hydroxy-4-methoxy-2′,3′-benzochalcone (HymnPro) which exhibited antiproliferative activity in several human solid tumour cell lines and suppressed xenografted tumour growth in nude mice (). Additionally, mechanistic studies showed that it can induced cell cycle arrest at the G2/M phase and increased in apoptotic cell death through the inhibition of tubulin polymerizationCitation16. Lee et al reported the synthesis of 2′-hydroxy-5′,6′-naphthochalcone derivatives and the most active compound (HMNC-74) was found to be strongly inhibited the clonogenicity of SW620 colon cancer cells (). Mechanistically, HMNC-74 triggered cell cycle arrest at G2/M phase and apoptosis by disrupting the microtubular networkCitation14.
One the other hand, many researchers think that chalcones possessed significant anticancer activity due to their have a similar mode of action to the structurally related natural combretastatin A-4Citation17, and the methoxy substituent in A ring is a crucial pharmacophoric group for the anticancer potency by inhibition of tubulin polymerisation ()Citation18–23. Moreover, introduction of different substituents on aromatic rings of chalcone can bring the significant changes in anticancer activity.
Based on the studies discussed above, we decided to report the the synthesis of naphthalene-chalcone derivatives containing 2′-methoxyl in the A ring based on lead compound HMNC-74 (). The synthesised compounds were evaluated for their anticancer activity.
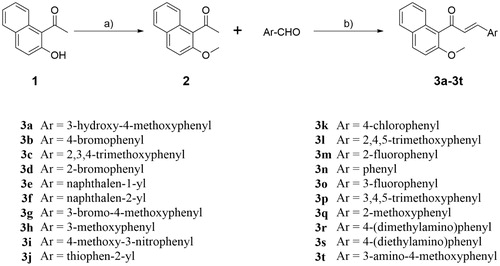
2. Chemistry
The synthesis route of naphthalene-chalcone derivatives 3a–3t was shown in Scheme 1 (see Supplementary material). Compound 2 was prepared by alkylation of 1–(2-hydroxynaphthalen-1-yl)ethan-1-one (1) in classical conditions using methyl iodide in the presence of Cs2CO3 in dry acetone. Condensation of 2 with a variety of commercially available aryl aldehydes in the presence of KOH at room temperature to provide the target compounds 3a–3t, which were characterised by 1H NMR, 13C NMR, and HRMS. The 1H NMR of compound 3a shows two doublets (δ = 6.96 and 7.21 ppm) with coupling constants J = 16.0 Hz for the olefin hydrogen of α,β-unsaturated ketone. The protons of 3-hydroxyl-4-methoxy phenyl moiety were appeared as two doublets for one proton each at δ 6.78 ppm and δ 7.16 ppm with the coupling constants J = 8.0 Hz and 2.0 Hz, respectively and a doublet of doublets of one proton at δ 6.95 ppm (J = 8.0 Hz and 2.0 Hz). The remaining aryl protons were appeared as two multiplets for two protons between δ 7.35–7.44 ppm and four doublets for one proton each at δ 7.31, 7.66, 7.80 and 7.91 ppm with the J values 8.8, 8.4, 8.0, and 8.8 Hz, respectively. The methoxyl protons of OCH3 appeared as two singlets at 3.89 and 3.91 ppm. The single peak of OH proton was observed at δ 5.64 ppm. The 13C NMR of compound 3a shows the carbonyl carbon appeared at 197.53 ppm. The signals of two methoxyls appeared at δ 56.10 and 56.75 ppm. The remaining aromatic and olefin carbons resonates around δ 110.55–154.09 ppm. The high-resolution mass spectrum of compound 3a showed a molecular ion peak at m/z 389.0149 as [M + Na]+ which also supports the proposed structure of the compound. Similar pattern was observed in 1H NMR and 13C NMR spectroscopy of all the title compounds 3a–3t. The HRMS (TOF) of all the compounds 3a–3t showed a molecular ion peak equivalent to their molecular formulae.
3. Biological evaluation
3.1. In vitro anticancer activity against breast cancer cell line (MCF-7)
All the synthesised naphthalene-chalcone derivatives 3a–3t were evaluated for their anticancer activity by 3-[4,5-dimethylthiazole-2-yl]-2,5-diphenyltetrazolium bromide (MTT) assay method against human breast carcinoma cell line MCF-7. Cisplatin was used as a reference drug. The results were expressed as the IC50 (50% inhibitory concentration). As shown in , most of the tested compounds displayed potent antiproliferative activity with IC50 values ranging from 1.42 ± 0.15 to >10 µM. Among these compounds, compounds 3c and 3j were found to be inactive toward MCF-7 cell line (IC50 > 10 µM). All other tested compounds shown low-micromole IC50 values (IC50 < 10 µM). Particularly, compounds 3a and 3t displayed the most potent antiproliferative activity with IC50 values of 1.42 ± 0.15 and 2.75 ± 0.26 µM, respectively.
Table 1. Anticancer activity of compounds 3a–3t against MCF-7 cell line.
Analysis of structure-activity relationship (SAR) of this class of compounds revealed that the substituents of aryl ring have influences on their antiproliferative activities. Introduction of halogen group (Cl or Br) at para position of phenyl ring results in a slight increase of the biological activity (3b and 3k). However, shifting these group to the 2- or 3- position decreased the biological activity (3d, 3m, and 3o). Furthermore, the replacement of the 4-Br or 4-Cl substitutes with dialkylamine group (3r and 3s) resulted in a decrease of the inhibitory activity. The replacement of the right phenyl ring with thiophene (3j) resulted in dramatically decrease of antiproliferative activity. Among the series, 3-OH-4-OMe derivative (3a) and 3-NH2-4-OMe derivative (3t) were found to be the most active compound, with IC50 values of 1.42 ± 0.15 and 2.75 ± 0.26 µM, respectively. Additionally, compound 3a (IC50 = 1.42 ± 0.15 µM) with 3-hydroxyl-4-methoxy phenyl moiety was found to be the most active compound.
In order to verify the safety profile of this class of compounds, the most potent compound 3a was selected to test its cytotoxicity against human embryonic kidney (HEK293) cell line in comparison to reference drug cisplatin. As presented in , compound 3a exhibited cytotoxic activity against normal HEK293 cells with IC50 value of 18.3 ± 1.3 µM, as compared to cisplatin (IC50 = 5.3 ± 0.4 µM). Hence, we could conclude that these compounds have good safety for potential application in the treatment of tumour cells.
Table 2. Cytotoxic activity (IC50, µM) of selected compound 3a and cisplatin against human embryonic kidney (HEK293) cell line.
3.2. In vitro tubulin polymerisation inhibitory assay
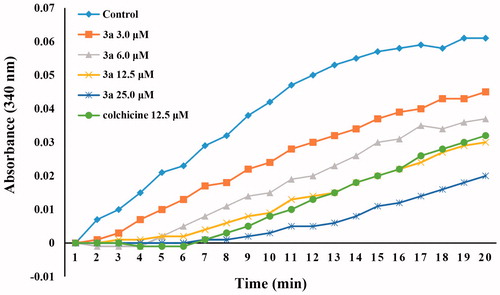
To evaluate whether this class of compounds target the tubulin–microtubule system, compound 3a, one of the most active compounds in this series of chalcone derivatives, was chosen to investigate its ability to block microtubule assembly, with colchicine as the reference compound. As shown in , compound 3a inhibited the polymerisation of tubulin in a concentration-dependent manner, which suggests that this class of compounds interfere with the microtubule polymerisation. Treatment with 3.0, 6.0, 12.5, and 25 µM of compound 3a inhibited tubulin polymerisation by 21%, 41%, 60%, and 82%, respectively. Compound 3a was slightly more active than the reference compound colchicine, with the IC50 values of 8.4 and 10.6 µM, respectively. These results indicated that compound 3a is a tubulin polymerisation inhibitor, which can bind to tubulin and induces microtubule polymerisation.
3.3. Cell cycle arrest
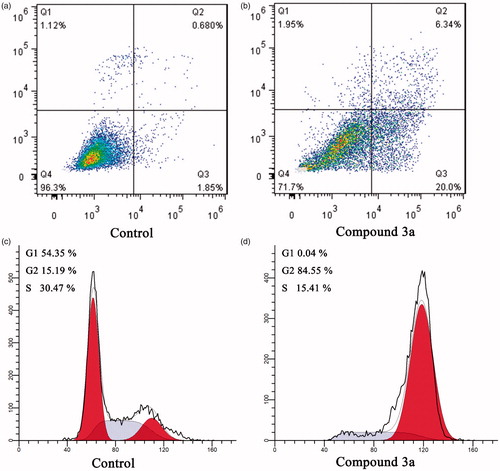
Many studies have reported that tubulin polymerisation inhibitors can arrest cancer cells in G2/M phase and lead to apoptosisCitation24,Citation25. The potent tubulin polymerisation inhibitory activity of compound 3a promoted us to further investigate its cellular mechanisms of action in MCF-7 cancer cells by using flow cytometry analysis. In order to elucidate the molecular mechanism of compound 3a, we first studied its effect on cell cycle progression in MCF-7 cellsCitation26. As shown in , control group show a typical pattern of cell cycle in G1, S and G2/M phase. In contrast, after treatment of MCF-7 cells with compound 3a at concentrations of 2.0 µM, the accumulation of cancer cells was detected at G2/M phase by 5.5 folds compared to the control group, from 15.19% in the control group to 84.55% in the compound 3a treated group (). The result indicates that compound 3a could arrest cells in G2/M phase and halt cell mitosis, which leads to inhibited MCF-7 cells proliferation.
3.4. Cell apoptosis analysis
To investigate whether compound 3a could induces apoptosis, the apoptotic effect of compound 3a and DMSO (control) were evaluated by an annexin V FITC/PI (AV/PI) dual staining assayCitation27. After treatment MCF-7 cells with compound 3a at the concentration of 2.0 µM for 24 h, the cells were labelled with the two dyes and analysed by flow cytometry. In comparison to the DMSO control group, it was observed that compound 3a could induce an increase in the late/secondary cellular apoptosis from 0.68% to 6.34%. In addition, an increase in the early/primary apoptosis was also observed from 1.85% to 20.0% (). Collectively, these results confirmed that this series of compounds could arrest cells in G2/M phase and induce apoptotic cell death.
3.5. Molecular modelling studies
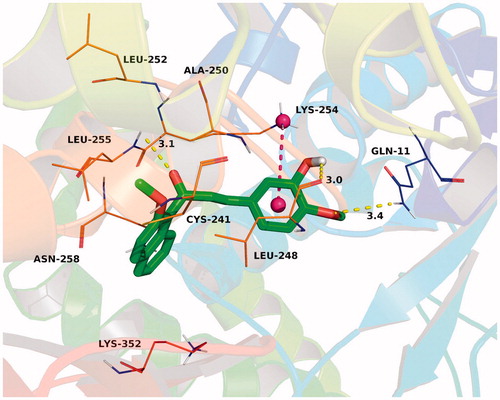
To explain the binding modes of this class of compounds with tubulin, we performed a docking study of the most active compound 3a into the colchicine binding pocket of tubulin (PDB code: 1SA0) by using the Autodock vina 1.1.2 softwareCitation28. The result was shown in and the estimated binding energy was −8.8 kcal·mol−1. Compound 3a adopted a “L-shaped” conformation in the pocket of the tubulin. The 2-methoxynaphthyl group of 3a located at the hydrophobic pocket, surrounded by the residues Cys-241, Leu-248, Ala-250, Leu-252, and Leu-255, forming a strong hydrophobic binding. Detailed analysis showed that the phenyl group in the middle of 3a formed a cation-π interaction with the residue Lys-254. It was shown that the residues Gln-11 (bond length: 3.4 Å), Leu-248 (bond length: 3.0 Å), and Leu-255 (bond length: 3.1 Å) formed three hydrogen bond interactions with 3a, which was the main interaction between 3a and tubulin.
4. Conclusion
In summary, we designed and synthesised a series of naphthalene-chalcone derivatives (3a–3t) as tubulin polymerisation inhibitors for the treatment of breast cancer. Among them, compound 3a bearing 3-hydroxyl-4-methoxy phenyl moiety was found to be the most active compound, with an IC50 value of 1.42 ± 0.15 µM against MCF-7 breast cancer cell line. The promising compound 3a demonstrated relatively lower cytotoxicity on normal cell line (HEK293) compared to tumour cell line. Additionally, in mechanistic studies, the representative compound 3a was found to induce significant cell cycle arrest at the G2/M phase and cell apoptosis in MCF-7 cell lines. Notably, compound 3a also displayed potent tubulin polymerisation inhibitory activity with an IC50 value of 8.4 µM, which was slightly more active than the reference compound colchicine (IC50 = 10.6 µM). Furthermore, molecular docking studies revealed that these compounds can bind at the colchicine binding site of the tubulin.
Supplemental Material
Download PDF (3.6 MB)Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Jordan MA, Wilson L. Microtubules as a target for anticancer drugs. Nat Rev Cancer 2004;4:253–65.
- Dumontet C, Jordan MA. Microtubule-binding agents: a dynamic field of cancer therapeutics. Nat Rev Drug Discov 2010;9:790–803.
- Kaur R, Kaur G, Gill RK, et al. Recent developments in tubulin polymerization inhibitors: an overview. Eur J Med Chem 2014;87:89–124.
- Li Q, Sham HL. Discovery and development of antimitotic agents that inhibit tubulin polymerisation for the treatment of cancer. Expert Opin Ther Pat 2002;12:1663–702.
- Shi Q, Chen K, Morris-Natschke SL, Lee KH. Recent progress in the development of tubulin inhibitors as antimitotic antitumor agents. Curr Pharm Des 1998;4:219–48.
- Jordan A, Hadfield JA, Lawrence NJ, McGown AT. Tubulin as a target for anticancer drugs: agents which interact with the mitotic spindle. Med Res Rev 1998;18:259–96.
- Bukhari SNA, Jasamai M, Jantan I. Synthesis and biological evaluation of chalcone derivatives (mini review). Mini Rev Med Chem 2012;12:1394–403.
- Gomes MN, Muratov EN, Pereira M, et al. Chalcone derivatives: promising starting points for drug design. Molecules 2017;22:1210.
- Zhuang CL, Zhang W, Sheng CQ, et al. Chalcone: a privileged structure in medicinal chemistry. Chem Rev 2017;117:7762–810.
- Mirzaei H, Emami S. Recent advances of cytotoxic chalconoids targeting tubulin polymerization: synthesis and biological activity. Eur J Med Chem 2016;121:610–39.
- Mahapatra DM, Bharti SK, Asati V. Anti-cancer chalcones: structural and molecular target perspectives. Eur J Med Chem 2015;98:69–114.
- Sharma R, Kumar R, Kodwani R, et al. A review on mechanisms of anti tumor activity of chalcones. Anticancer Agents Med Chem 2015;16:200–11.
- Loa J, Chow P, Zhang K. Studies of structure-activity relationship on plant polyphenol-induced suppression of human liver cancer cells. Cancer Chemother Pharmacol 2009;63:1007–16.
- Lee JM, Lee MS, Koh D, et al. A new synthetic 2′-hydroxy-2,4,6-trimethoxy-5′,6′-naphthochalcone induces g2/m cell cycle arrest and apoptosis by disrupting the microtubular network of human colon cancer cells. Cancer Lett 2014;354:348–54.
- Pouget C, Lauthier F, Simon A, et al. Flavonoids: structural requirements for antiproliferative activity on breast cancer cells. Bioorg Med Chem Lett 2001;11:3095–7.
- Shin SY, Kim JH, Yoon H, et al. Novel antimitotic activity of 2-hydroxy-4-methoxy-2′,3′-benzochalcone (hymnpro) through the inhibition of tubulin polymerization. J Agric Food Chem 2013;61:12588–97.
- Singh P, Raj R, Kumar V, et al. 1,2,3-triazole tethered beta-lactam-chalcone bifunctional hybrids: synthesis and anticancer evaluation. Eur J Med Chem 2012;47:594–600.
- Li L, Jiang SB, Li XX, et al. Recent advances in trimethoxyphenyl (tmp) based tubulin inhibitors targeting the colchicine binding site. Eur J Med Chem 2018;151:482–94.
- Lindamulage IK, Vu HY, Karthikeyan C, et al. Novel quinolone chalcones targeting colchicine-binding pocket kill multidrug-resistant cancer cells by inhibiting tubulin activity and mrp1 function. Sci Rep 2017;7:10298.
- Konieczny MT, Bulakowska A, Pirska D, et al. Influence of s-oxidation on cytotoxic activity of oxathiole-fused chalcones. Chem Biol Drug Des 2016;88:519–33.
- Konieczny MT, Bulakowska A, Polak J, et al. Structural factors affecting cytotoxic activity of (e)-1-(benzo d 1,3 oxathiol-6-yl)-3-phenylprop-2-en-1-one derivatives. Chem Biol Drug Des 2014;84:86–91.
- Tu HY, Huang AM, Hour TC, et al. Synthesis and biological evaluation of 2′,5′-dimethoxychalcone derivatives as microtubule-targeted anticancer agents. Bioorg Med Chem 2010;18:2089–98.
- Konieczny MT, Buɬakowska A, Pirska D, et al. Structural factors affecting affinity of cytotoxic oxathiole-fused chalcones toward tubulin. Eur J Med Chem 2015;89:733–42.
- Ji YT, Liu YN, Liu ZP. Tubulin colchicine binding site inhibitors as vascular disrupting agents in clinical developments. Curr Med Chem 2015;22:1348–60.
- Zhou ZZ, Shi XD, Feng HF, et al. Discovery of 9h-purins as potential tubulin polymerization inhibitors: synthesis, biological evaluation and structure activity relationships. Eur J Med Chem 2017;138:1126–34.
- Wang G, Li C, He L, et al. Design, synthesis and biological evaluation of a series of pyrano chalcone derivatives containing indole moiety as novel anti-tubulin agents. Bioorg Med Chem 2014;22:2060–79.
- Ettari R, Pallio G, Pizzino G, et al. Non-covalent immunoproteasome inhibitors induce cell cycle arrest in multiple myeloma mm.1r cells. J Enzyme Inhib Med Chem 2019;34:1307–13.
- Trott O, Olson AJ. Software news and update autodock vina: improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J Comput Chem 2010;31:455–61.